Abstract
This is a cross-sectional study to assess the presence of antibodies in ruminants against selected pathogens associated with reproductive disorders in cattle in four Brazilian states, including the zoonotic agent Coxiella burnetii. The used tests were Virus Neutralization Assay for IBR and BVD, Microscopic Agglutination Test for Leptospira spp., Indirect Fluorescent Antibody Test (IFAT) for C. burnetii and Toxoplasma gondii, and Enzyme-Linked Immunosorbent Assay for Neospora caninum and Trypanosoma vivax. Seropositivity for C. burnetii was 13.7% with titers from 128 to 131,072; 57.8% for BoHV-1, with titers between 2 and 1,024; 47.1% for BVDV-1a, with titers from 10 to 5,120; 89.2% for N. caninum; 50% for T. vivax; and 52.0% for Leptospira spp., with titers between 100 to 800 (the following serovars were found: Tarassovi, Grippotyphosa, Canicola, Copenhageni, Wolffi, Hardjo, Pomona and Icterohaemorrhagiae); 19.6% for T. gondii with titer of 40. This is the first study that has identified C. burnetii in cattle associated with BoHV and BVDV, N. caninum, Leptospira spp., T. gondii and T. vivax. Thus, future studies should be conducted to investigate how widespread this pathogen is in Brazilian cattle herds.
Keywords:
Coxiella burnetii; abortion; Q Fever; cattle
Resumo
Este é um estudo transversal para avaliar a presença de anticorpos em ruminantes contra patógenos selecionados e associados a distúrbios reprodutivos em bovinos de quatro estados brasileiros, incluindo o agente zoonótico Coxiella burnetii. Os testes utilizados foram Teste de Vírus-Neutralização para BoHV e BVDV, teste de Aglutinação Microscópica para Leptospira spp., Reação de Imunofluorescência Indireta for C. burnetii e Toxoplasma gondii, e Ensaio de Imunoabsorção Enzimática para Neospora caninum e Trypanosoma vivax. A soropositividade para C. burnetii foi de 13,7% com títulos de 128 a 131.072; 57,8% para BoHV-1, com títulos entre 2 a 1.024; 47,1% para BVDV-1a, com títulos de 10 a 5.120; 89,2% para N. caninum; 50% para T. vivax; e 52,0% para Leptospira spp., com títulos entre 100 a 800 (sorovares encontrados: Tarassovi, Grippotyphosa, Canicola, Copenhageni, Wolffi, Hardjo, Pomona e Icterohaemorrhagiae) 19,6% para T. gondii com título de 40. Este é o primeiro estudo que evidencia a participação de C. burnetii em bovinos associada ao Vírus da Rinotraqueíte bovina infecciosa e da diarreia viral bovina, N. caninum, Leptospira spp., T. gondii e T. vivax em bovinos. Desta forma, futuros estudos devem ser conduzidos a fim de investigar o quão disseminado se encontra este patógeno em rebanhos bovinos brasileiros.
Palavras-chave:
Coxiella burnetii; abortamento; Febre Q; gado
Introduction
Both Brazilian beef and dairy cattle production are prominent in economic scenario worldwide. However, reproductive disorders are a serious obstacle to excellence in production (LUCY, 2001; ROYAL, et al., 2000), and abortion is considered to be one of the biggest causes of economic losses in cattle farming (REICHEL et al., 2013). Considering that infections involving the reproductive system can reduce fertility, the pathogenicity involved infectious agents has been associated with both intrinsic and extrinsic factors (PELLERIN et al., 1994; STENLUND et al., 2003; GROOMS, 2004), including management practices, such as vaccination and introduction of new animals into the herd (CHI et al., 2002).
In the center-west of the United States, it was found that approximately 30% of abortions were caused by infectious agents; half of them involved pathogenic bacteria as etiologic agents (KIRKBRIDE, 1992). Infectious-parasitic agents associated with reproductive disorders in ruminants include Coxiella burnetii, Bovine Herpesvirus (BoHV) and bovine viral diarrhea (BVD) virus, Neospora caninum (KIRKBRIDE, 1992), Leptospira spp., (MURRAY, 1990), Toxoplasma gondii (BÁRTOVÁ et al., 2009) and Trypanosoma vivax (SILVA et al., 1996, 1998).
Coxiella burnetii, a Gram-negative obligatory intracellular bacterium (VAN SCHAIK et al, 2013; ABDEL-MOEIN & HAMZA, 2017), was reported for the first time in 1930 and has been detected worldwide ever since, except in New Zealand (MAURIN & RAOULT, 1999; ELDIN et al., 2017). This parasite is the zoonotic agent that causes Q fever (MAURIN & RAOULT, 1999). It was also considered as a potential bioterrorism agent by the Center for Disease Control and Prevention (CDC, 2018). Although the main route of infection for humans is via aerosols (MAURIN & RAOULT, 1999; PARKER et al., 2006; TISSOT-DUPONT & RAOULT, 2008), ingestion of contaminated food and infected ticks may also represent alternative routes of transmission of the parasite (ELDIN et al., 2017).
In humans, this agent has been associated with pneumonia, a syndrome similar to influenza and hepatitis; in some cases, pruritus, pericarditis, myocarditis, encephalitis, and osteomyelitis have also been observed (MAURIN & RAOULT, 1999; TISSOT-DUPONT & RAOULT, 2008). The chronic form of the infection can cause endocarditis, which is usually associated with valvular heart disease and immunosuppression. Although less commonly, individuals can develop granulomatous lesions in the bones, joints, liver, lungs, testicles and other tissues (RALPH et al. 2007; TISSOT-DUPONT & RAOULT, 2008).
The first serological evidence of exposure to C. burnetii infection in Brazil occurred in the 1950s in humans in the state of São Paulo (BRANDÃO et al., 1953) and in cattle in the state of Rio de Janeiro (TRAVASSOS et al., 1954 apud OLIVEIRA et al., 2018). Ever since, serological and molecular evidence of circulation of the parasite has been found in humans in São Paulo (BRANDÃO et al., 1953; VALLE et al., 1955; SICILIANO et al., 2015), Rio de Janeiro (LAMAS et al., 2009, 2013; LEMOS et al., 2011, 2018; ROZENTAL et al., 2012, 2018; MARES-GUIA et al., 2016), Minas Gerais (RIEMANN et al., 1974; COSTA et al., 2005; 2006) and Bahia (SICILIANO et al., 2008).
The main clinical signs associated with this infection in ruminants are infertility, abortion, stillbirth, endometritis and mastitis (TISSOT-DUPONT & RAOULT, 2008). Although C. burnetii is a zoonotic agent associated with the occurrence of abortion in ruminants all over the world, there are few studies about the occurrence of this agent in ruminants in Brazil (TRAVASSOS et al., 1954 apud OLIVEIRA et al., 2018; MARES-GUIA et al., 2014; GUIMARÃES et al., 2017; OLIVEIRA et al., 2018; SOUZA et al., 2018). Therefore, the objective of this study was to investigate the frequency of antibodies against ruminant-selected pathogens, namely C. burnetii, BoHV-1, BVDV, N. caninum, Leptospira spp., T. gondii and T. vivax in serum samples from cattle with a history of reproductive problems from four Brazilian states.
Materials and Methods
Sample selection
This is a cross-sectional study to assess the presence of antibodies to ruminant-selected pathogens associated to reproductive disorders in cattle from four Brazilian states, including the zoonotic C. burnetii. The total of 12 proprieties (Supplementary Material Supplementary Material Supplementary material accompanies this paper. Supplementary Table. Detailed serological results for selected bovine pathogens associated to abortion in cattle in Brazil. This material is available as part of the online article from Revista Brasileira de Parasitologia Veterinária (RBPV, 2019) - Home Page - SciELO. ) were conveniently selected from the Reproductive Viruses Center (São Paulo State University, Unesp, Jaboticabal - São Paulo, Brazil). The total of 102 serum samples were obtained from cows showing a history of reproductive disorders. All sample sets were located in the central-western and southeastern regions of Brazil.
Eligibility criteria
Study animals consisted of adult cows (> 24 months) from productions (milk and breeding) (i.e., commercial cattle production). All samples were obtained from the sampled farms between December, 2014 and November, 2015.
Bovine serum samples
Between 2014 and 2015, serum samples collected from 102 cattle presenting reproductive disorders were selected by convenience from four Brazilian states, São Paulo (José Bonifácio, Urânia and Torrinha), Minas Gerais (Espera Feliz, Patos de Minas, Lagoa Grande and Coromandel), Mato Grosso do Sul (Anastácio) and Goiás (Nova Caixás and Goiatuba). While the proprieties A, B, G and L were composed by beef cattle, the proprieties C, D, E, F, H, I, J and K were composed by dairy cattle (Suplementary Table). Serum samples were stored at -20°C until all serological assays were performed.
Virus Neutralization (VN) assay for detection of BVDV-1 and BoHV-1
The selected bovine serum samples were tested by virus neutralization (VN) assay for the detection of antibodies to BVDV-1 and BoHV-1, as recommended by the “Manual of Diagnostics Tests and Vaccines for Terrestrial Animals” (OIE, 2018a,b), with modifications. For this purpose, “Madin-Darby bovine kidney (MDBK)” cell line and cytopathic strains of BVDV-1a (Singer) and BoHV-1 (Nebraska), were used. All seropositive samples were tested twice in order to estimate the geometric mean of titer (GMT) values. In addition to the internal controls, positive and negative controls were also used (GATTO et al., 2018). A sample was considered positive when the total neutralization of 100 TCID50 occurred in the serum and no cytopathic effect (CPE) was observed in the cell layer in serum dilutions higher than 1:10 (BVDV) and 1:2 (BoHV-1) (OIE, 2018a,b).
BVDV-1
The serum samples were heat-inactivated for 30 minutes at 56°C. All serum samples were tested in duplicate at the same time and were subjected to successive dilutions ranging from 1:10 to 1:5120, using cell culture medium as diluent. At each dilution of serum, for each sample one well was left without virus, aiming at monitoring for evidence of sample toxicity that could mimic viral cytopathology or interfere with virus replication. An equal volume (50 μL) of a stock of cytopathic strain of BVDV, which contained 100 TCID50 (50%) tissue culture infective doses, was added to each well. The plate was incubated for 1 hour at 37°C. Fifty microliters of the cell suspension at 2 × 105 /mL were added to each well. The plate was incubated at 37°C for 4–5 days, either in a 5% CO2 atmosphere.
BoHV-1
The serum samples were heat-inactivated for 30 minutes at 56°C. All serum samples were tested in duplicate and were subjected to successive dilutions ranging from 1:2 to 1:1024, using cell culture medium as diluent. At each dilution of serum, for each sample, one well was also left without virus in order to monitor the evidence of sample toxicity that could mimic viral cytopathology or interfere with virus replication. An equal volume (50 μL) of a stock of cytopathic strain of BoHV-1, which contained 200 TCID50 (50%) tissue culture infective doses, was added to each well. The plate was incubated for 24 hours at 37°C. One hundred microliters of the cell suspension at 3 × 105 cells per well were added to each well. The plate is incubated at 37°C for 3–5 days, either in a 5% CO2 atmosphere.
Microscopic Agglutination Test (MAT) for detection of IgG antibodies to Leptospira spp.
The Leptospira spp. antigens used in serological tests were obtained from bacteria subcultured weekly in liquid EMJH culture medium (Ellighausen, McCullough, Johnson and Harris), with 10% of the medium volume used to seed cultures that were maintained in a bacteriological incubator at 28°C ± 1°C (OIE, 2018c).
The MAT was used to identify the serogroups/serovars. The 24 Leptospira serovars used can be found in Table 1. Serum samples were diluted in saline, at an initial dilution of 1:50. Aliquots (25 μL) of serum were placed in polystyrene plates with a flat bottom, with an equal quantity of antigens, resulting in a dilution of 1:100. The serum-antigen mixture was homogenized gently and incubated in an environmental incubator at a temperature of 28 °C for 40 to 120 minutes.
Results were read by dark field microscopy with 10× objective, directly from the plate wells. Samples shoeing 50% agglutination were considered to be reactive. Samples reactive at the initial dilution were assayed with serial twofold dilutions from the original 1:100 dilution. Positive tests were defined as MAT results ≥1:100 for at least one of the 24 serovars (Table 1).
Indirect Fluorescent Antibody Test (IFAT) for detection of IgG anti-C. burnetii and anti-T. gondii antibodies
The detection of anti-C. burnetii IgG antibodies was performed with crude antigen of strain At12 of C. burnetii, phase-1 reactive (PACHECO et al., 2013). The serum samples were first diluted at 1:64 in phosphate-buffered saline solution, PBS pH 7.4 (130 mM NaCl; 2.7 mM KCl; 5.6 mM Na2HPO4; 1.0 mM KH2PO4; 0.8 mM NaH2PO4). After that, 20 μL of each diluted serum sample was deposited in wells of slides containing the antigen of C. burnetii. The slides were incubated at 37°C for 30 minutes, in a moist chamber. Afterwards, they were washed with Washing Buffer solution (phosphate-buffered saline solution, PBS pH 7.4 + 1% Triton) and then dried. Each slide received 20 μL conjugate (bovine anti-IgG diluted at 1:200) marked by fluorescein isothiocyanate (Sigma-Aldrich®, St. Louis - Missouri, USA) for test samples as well as positive and negative controls. The slides were then incubated for a further 30 minutes at 37°C in a moist chamber. After being washed (this time was added 1.5 ml of Evans Blue on each wash in the washing buffer solution) and dried again, the slides were evaluated through ultraviolet light microscopy. The reaction was considered to be positive when cells were fluorescent at the dilution of 1:64 (PACHECO et al., 2013), according to the protocol previously described by Reeves et al. (2006). Bovine serum samples previously tested for C. burnetii and considered non-reactive and reactive were used as negative and positive controls, respectively (GUIMARÃES et al., 2017).
For T. gondii, the tachyzoites of the RH strain were used as an antigen, according to a protocol previously described (OLIVEIRA et al., 2008; ANDRÉ et al., 2010). Samples were considered positive when titration was above 40. The used protocol was similar to that one used for detection of IgG antibodies for C. burnetii, with some modifications, such as following: i.) PBS pH 7.4 was used in all washing procedures; ii.) Evans Blue at 10% to the conjugate solution was used instead of the washing solution. IFAT used a serial dilution of the test sera to the log base 2 for titration of antibodies. The positive and negative controls used in this serological assay were serum samples from cattle known to be positive and negative for T. gondii, provided by IMUNODOT Diagnósticos (Jaboticabal - São Paulo, Brazil).
Indirect Immunoassay (iELISA) for detection of IgG antibodies anti-Neospora caninum and anti-Trypanosoma vivax
The detection of IgG antibodies anti-T. vivax was performed with the indirect ELISA (iELISA), according to the protocol established by Machado et al. (1997) and Aquino et al. (1999), with minor adaptations. After purification of the trypomastigote forms, as described by González et al. (2005), and sonication in a 750w Ultrasonic Processor (Coleparmer – Montréal – Quebec, Canada), the protein concentration of the soluble antigen was determined by the bicinchoninic acid method (Pierce BCA Protein Assay Kit, Thermo Fisher Scientific, San Diego – California, USA). The total antigen of T. vivax was diluted in carbonate-bicarbonate buffer 0.5M and pH 9.6 and its optimal concentration was adjusted to 0.1 μg/mL. After incubation for 12 hours at 8 °C, blocking was performed with PBS Tween 20 (pH 7.2), and 6% normal rabbit serum was added. The plates (Nunc Maxisorp®, Thermo Fisher Scientific, São Paulo - São Paulo, Brazil) sensitized with T. vivax antigen were incubated for 90 minutes at 37°C, in a moist chamber. After three washes with PBS-Tween 20 buffer, reference positive and negative sera (FIDELIS et al., 2016) and test sera were added to the ELISA plate. All serum samples were diluted 1:400 solution in PBS-Tween 20 plus 5% of skimmed milk powder (Molico®, Nestlé, São Paulo - São Paulo, Brazil). The plates were incubated again at 37°C for 90 minutes. After three washes with PBS-Tween 20 buffer, the ELISA plate received the anti-bovine IgG antibody conjugate linked to alkaline phosphatase (Sigma®, St. Louis - Missouri, USA) at a dilution of 1:30000 in PBS-Tween 20 plus 5% of skimmed milk powder (Molico®, Nestlé, São Paulo - São Paulo Brazil), with subsequent incubation and washing. Finally, the substrate of the enzyme alkaline phosphatase, p-nitrophenyl phosphate (Sigma®, St. Louis - Missouri, USA), diluted to 1 mg/mL at pH 9.8 buffer diethanolamine (Sigma®, St. Louis - Missouri USA) was added.
The total antigen of N. caninum (IMUNODOT®, Jaboticabal - São Paulo, Brazil), at optimal concentration of 0.1 μg/mL, was diluted in carbonate-bicarbonate buffer 0.5M and pH 9.6. After incubation for 12 hours at 8 °C, blocking was performed with PBS Tween 20 (pH 7.2), and 6% of skimmed milk powder (Molico®, Nestlé, São Paulo - São Paulo, Brazil). The plates (Nunc Maxisorp®, Thermo Fisher Scientific, São Paulo - São Paulo, Brazil) were sensitized with the antigen of this parasite and then were incubated for 60 minutes at 37°C in a moist chamber. After three washes with PBS-Tween 20 buffer, the ELISA plate received the reference positive and negative sera, supplied with the kit, and tested sera, which were diluted at 1:200 in PBS-Tween 20 solution. The plates were incubated again at 37°C for 60 minutes. After three washes with PBS-Tween 20, the ELISA plate received the anti-bovine IgG antibody conjugate linked to alkaline phosphatase (Sigma®, St. Louis - Missouri, USA) at a dilution of 1:30000 in PBS-Tween 20, with subsequent incubation and washing. Finally, the substrate of the enzyme alkaline phosphatase, p-nitrophenyl phosphate (Sigma®, St. Louis - Missouri, USA), diluted at 1 mg/mL at pH 9.8 diethanolamine buffer (Sigma®, St. Louis - Missouri, USA) was added.
The plates were sealed with aluminum foil and incubated for 45 minutes at ambient temperature. Reading was performed in ELISA plate reader (B.S.-100; Embrabio, São Paulo - São Paulo, Brazil), with a 405nm filter. The cut-off point was calculated as 2.5 times the average absorbance of negative control sera (MACHADO et al., 1997).
Results
The seropositivity for C. burnetii was 13.7% (14/102), with titers ranging from 128 to 131072; 57.8% (59/102) for BoHV-1, with titers ranging from 2 to 1024; 47.1% (48/102) for BVDV-1a, with titers ranging from 10 to 5120; 19.6% (20/102) for T. gondii, with titers of 40; 89.2% (91/102) for N. caninum, with optical densities ranging from 0.309 to 1.646 (cutoff point: 0.309); 50% (51/102) for T. vivax, with optical densities ranging from 0.406 to 2.597 (cutoff point: 0.393); and 52.0% (53/102) for Leptospira spp., with titers ranging from 100 to 800 (serovars found: Tarassovi, Grippotyphosa, Canicola, Copenhageni, Wolffi, Hardjo, Pomona and Icterohaemorrhagiae).
Antibodies for C. burnetii were found in two animals in the state of Goiás (10%, 2/20), four animals in the state of São Paulo (12.5% 4/32), seven animals in the state of Minas Gerais (17.9%, 7/40) and only one animal in Mato Grosso do Sul (10%, 1/10). All seropositive animals for C. burnetii also showed antibodies to at least one more agents. There were significant differences for the presence of antibodies against Leptospira spp., T. gondii and BVDV-1 between the animals sampled in the four selected states (Table 2 and Table 3).
Seropositive samples for selected bovine pathogens associated to abortion in cattle in Brazil.
Seropositivity for parasites associated with abortion in cattle in Brazil according to place of origin.
Among the selected animals presenting reproductive disorders in the present study, a higher seropositivity for N. caninum was found. In this sense, antibodies to N. caninum were found in 100% (10/10) of the animals sampled in the state of Mato Grosso do Sul, 85% (17/20) of the animals sampled in the state of Goiás, 93.7% (30/32) of the animals sampled in the state of São Paulo, and 85% (34/40) of the animals sampled in the state of Minas Gerais. Six animals showed to be seropositive only for N. caninum (Table 2 and Figure 1).
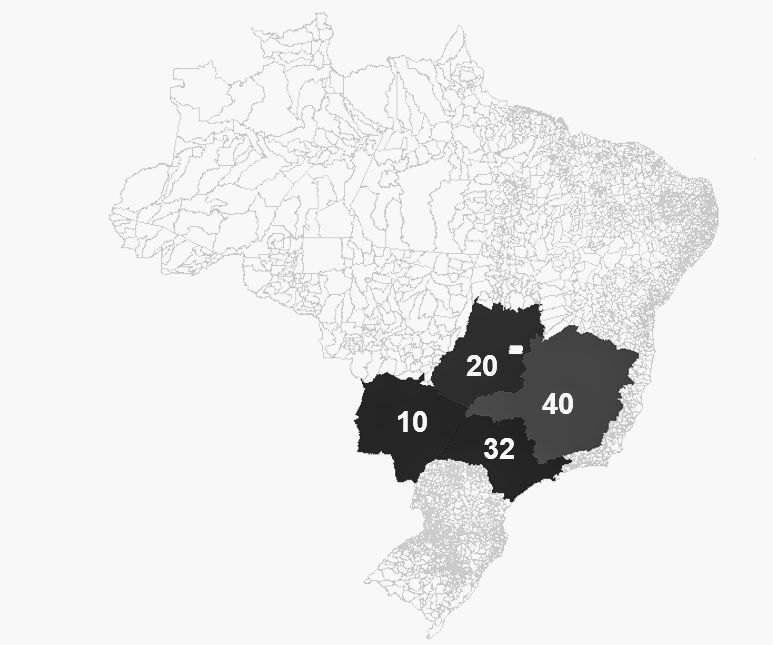
Selected sampling States: Goiás, Minas Gerais, Mato Grosso do Sul, São Paulo. This map was generated using MapInfo software.
Discussion
Although several pathogens interfere with the reproductive performance of cattle, e.g., the bovine viral diarrhea virus (BVDV), bovine herpesvirus (BoHV), Brucella abortus (occasionally B. melitensis), N. caninum, C. burnetii, Campylobacter fetus venerealis or Campylobacter fetus fetus, Leptospira spp., Tritrichomonas foetus and Chlamydia abortus (AGERHOLM, 2013; BUTZLER, 2004; DAMMAN et al., 2015; DÍAZ APARICIO, 2013; CERQUEIRA-CÉZAR et al., 2017), there is still not enough information on the occurrence and the epidemiological impact of some pathogens on reproductive disorders in ruminants.
Despite the low rate of seropositivity found for C. burnetii (13.7%, 14/102) among the sampled bovine population with reproductive disorders, it should be noted that most (71.4%, 10/14) of the seropositive animals showed high antibody titers, ranging from 512 to 131072. Even though high antibody titers are suggestive of recent infection (GUIMARÃES et al., 2017; OLIVEIRA et al., 2018), the antigen used in the present study is only reactive to phase-1 antibodies, found only in the chronic phase of the disease (PEACOCK et al., 1983). Therefore, it is possible that the seropositive animals were in the chronic phase of the disease at the time of sampling. Additionally, despite the high titers found in the present study, we can’t rule out the occurrence of serological cross-reaction with other agents, such as Legionella sp. and Bartonella sp. (MUSSO & RAOULT, 1997; SCOLA & RAOULT, 1996). Although a previous study reported the occurrence of antibodies to C. burnetii in cattle in Brazil (BRANDÃO et al., 1953), the present work showed high antibody titers against this agent in this country.
Although serological evidence of exposure to C. burnetii has already been reported in cattle in Europe (CZAPLICKI et al., 2009; AGGER et al., 2010; BOTTCHER et al., 2011; GACHE et al., 2017; RYAN et al., 2018; VARELA-CASTRO et al., 2018; VIDAL et al., 2017; SOFTIC et al., 2018), Asia (CETINKAYA et al., 2000; NOKHODIAN et al., 2016), North America (MCQUISTON et al., 2005) and Africa (KAMGA-WALADJO et al., 2010; SCOLAMACCHIA et al., 2010), there are few data on seroprevalence to this agent in ruminants in Brazil. In this regard, while Guimarães et al. (2017) found a seropositivity rate of 2% for C. burnetii among sheep sampled in the state of Piauí, northeast of Brazil, Oliveira et al. (2018) found a seroprevalence of 55.1% in a herd of goats with history of reproductive disorders in the state of Alagoas, Northeast of Brazil. Recently, Souza et al. (2018) found 2.2% of the goats and 2.1% of the sheep seropositive to C. burnetii in the state of Pernambuco, also in northeastern Brazil. In southeastern Brazil, Mares-Guia et al. (2014) found 66.6% seropositivity in sheep and 50% seropositivity in goats in the state of Rio de Janeiro.
According to Mori & Roest (2018), vaginal swabs samples from cows that aborted within 8 days should preferably be collected aiming at the direct detection of C. burnetii by PCR. However, if the abortion occurred a longer time ago or if there is any history of reproductive problem in the herd, serologic tests can be used to verify if there was any contact with C. burnetii in the herd. This approach justifies the use of IFAT on cattle serum samples for investigating the occurrence of C. burnetii in the cattle showing reproductive disorders in the present study. Unfortunately, additional information regarding the abortion in each sampled property was not available.
In the present study, the seropositive samples were originated from both beef and dairy cattle herds. The ELISA test in milk storage tanks has proved very effective in sorting lactating animals seropositive for C. burnetii (VAN DEN BROM et al., 2012; GUATTEO et al., 2007) as well as have produced results concordant with those found from individual serum samples because of the transfer of immunoglobulins from blood to milk (NIELSEN et al., 2011). Thus, future large-scale studies should be conducted in Brazil using this approach aiming at investigating the exposure of dairy cows to the agent under study. The detection of DNA of C. burnetii, but not of viable bacteria in dairy products (cheese, yoghurt, butter and creams) in France (ELDIN et al., 2013) emphasizes the need for additional studies in Brazil to investigate the prevalence of this agent in dairy cattle.
Although the main clinical signs associated with Q fever in ruminants are linked to reproduction (infertility, abortion, stillbirth, endometritis and mastitis) (TISSOT-DUPONT & RAOULT, 2008; ELDIN et al., 2017), seronegative cows that do not have medical abortion are also capable of spreading the parasite through vaginal discharges (RODOLAKIS et al., 2007). Considering that C. burnetii can be dispersed via aerosols at distances greater than 30 Km (ELDIN et al., 2016) and survives for long periods in the environment as a form of resistance (Small Cell Variant) (ELDIN et al., 2017), this agent is very likely to be widely distributed in regions close to the areas sampled in this study. Because C. burnetii is an agent that can infect a wide range of hosts, from unicellular beings (such as amoeba) to invertebrates, reptiles, birds and mammals (CUTLER et al., 2007; NASPHV, 2013; ELDIN et al., 2017), the dispersion of this pathogen in extensive areas seems to be easily achieved. In this sense, the data presented in this study were relevant, both from an economic point of view and for public health, considering that cattle are one of the main reservoirs for human infection (GEORGIEV et al., 2013; NOKHODIAN et al., 2016). An outbreak of Q fever has been recently described among cadets in the state of Rio de Janeiro, Southeastern Brazil, whose diagnosis was confirmed by serologic tests (LEMOS et al., 2018). Additionally, future studies should be conducted about the participation of ticks in the transmission of C. burnetii between animals and humans in Brazil.
In the present study, we have also reported the occurrence of antibodies against BoHV-1, BVDV and Leptospira spp. In Brazil, previous seroepidemiological studies have indicated a significant dissemination of BVDV, BoHV-1 (AFFONSO et al., 2010; FINO et al., 2012; FLORES et al., 2013), and Leptospira sp. in herds (FÁVERO et al., 2017). While prevalence rates ranging from 22.2% to 85.4% (FLORES et al., 2013; QUINCOZES et al., 2007; ALMEIDA et al., 2013) have been reported for BVDV, seropositivity rates between 18% and 90% were found for BoHV-1 in non-vaccinated herds in different geographic regions of Brazil (LOVATO et al., 1995; TAKIUCHI et al., 2001; DIAS et al., 2013). The Leptospira spp. serovars Hardjo, Wolffi and Grippotyphosa, are the most frequently associated with the occurrence of abortions in cattle (MINEIRO et al., 2007), which corroborates the results of the present study. The serological results of this study for BVDV, BoHV-1 and Leptospira sp. support the evidence that these three etiological agents are disseminated in Brazil and may directly favor the occurrence of reproductive disorders in cattle, thus participating as primary infectious agents or worsening clinical co-infection conditions.
The highest rate of seropositivity (89.2%, 91/102) in the present study was found for the apicomplexan N. caninum. Our data corroborate those found previously in other Brazilian states, in which N. caninum has been reported as the main causative agent of abortion in cattle. In this sense, seropositivity rates of 23-91.7% to this agent were described in cattle presenting abortions in the states of Rio Grande do Sul, Minas Gerais, Paraná, Rondônia, Mato Grosso and Amazonas (CERQUEIRA-CÉZAR et al., 2017). In Brazil, seroprevalence to N. caninum in cattle ranges from 6.7 to 91.7%, depending on the geographic region and cattle breed. Cattle can be infected by ingesting oocysts of the parasite eliminated through feces of Canidae (horizontal transmission) or via the placenta (vertical transmission) (CERQUEIRA-CÉZAR et al., 2017). Transplacental transmission was incriminated the main N. caninum’s route of transmission in cattle in the state of Pernambuco, northeastern Brazil, allowing the maintenance of this agent in production systems in the study region (RAMOS et al., 2016). In this sense, farms that use embryo recipients should take preventive measures to avoid economic losses and perpetuation of the parasite on farms.
While N. caninum stands out as one of the most important parasites associated with reproductive problems in cattle, such animals are considered to be resistant to T. gondii (CERQUEIRA-CÉZAR et al., 2017), in spite of its worldwide distribution and its association with abortions in small ruminants (WYROSDICK & SCHAEFER, 2015). Toxoplasmosis in animal production is serious because it poses potential risks to public health, as a result of the consumption of non-pasteurized milk and raw or underdone beef, associated with economic losses caused by abortion in small ruminants (TENTER et al., 2000; WYROSDICK & SCHAEFER, 2015). Herein, a seroprevalence of 19.2% (20/102) was found to T. gondii, which is lower than that others reported in previous studies (29.1-32.9%) in Brazil (TILAHUN et al., 2018). This fact corroborates results of previous studies that indicate low pathogenicity of T. gondii in cattle, with little or no influence on the occurrence of abortion in herds (CERQUEIRA-CÉZAR et al., 2017).
Trypanosoma vivax infects a large number of species of domestic and wild ungulates and, in South America, it is the main etiologic agent of trypanosomiasis in cattle, hence it causes great economic losses (JONES & DÁVILA, 2001). In Brazil, the transmission occurs mechanically by Tabanidae (horseflies) and Stomoxys calcitrans (stable fly) (PAIVA et al., 2000; RODRIGUEZ-BATISTA et al., 2005; CADIOLI et al., 2012), or iatrogenically by fomites (CADIOLI et al., 2012). The present study reported a seroprevalence of 50% (51/102) for this agent among the cattle population with reproductive disorders. This protozoan is currently associated with the occurrence of abortions in different Brazilian regions (DÁVILA & SILVA, 2000; LINHARES et al., 2006; CADIOLI et al., 2012). Previously considered as an endemic agent in cattle herds in the Pantanal region in the Brazilian states of Mato Grosso (SILVA et al., 1996) and Mato Grosso do Sul (PAIVA et al., 1997, 2000), T. vivax has been recently causing outbreaks of the disease in several Brazilian states, e.g., Maranhão (FEITOSA et al., 2004), Tocantins (LINHARES et al., 2006), Paraíba (BATISTA et al., 2007), Minas Gerais (CARVALHO et al., 2008), Rio Grande do Sul (SILVA et al., 2009), Pernambuco (PIMENTEL et al., 2012), São Paulo (CADIOLI et al., 2012), Alagoas (ANDRADE et al., 2015), Santa Catarina (FÁVERO et al., 2016), Goiás (BASTOS et al., 2017), Sergipe (VIEIRA et al., 2017) and Piauí (LOPES et al., 2018). Our study did not find significant differences regarding the presence of antibodies against T. vivax across the cows sampled in the four states, which suggests that the parasite is being scattered across the country. Such expansion of its geographical distribution in Brazil is mainly due to the movement of cattle from endemic regions to those where there is a favorable epidemiological situation (climatic conditions favoring the presence of hematophagous dipterans, animals without prior immunity to the parasite in question and long-standing practice of sharing needles contaminated with blood [fomites] during vaccination and application of oxytocin) (CADIOLI et al., 2012; BASTOS et al., 2017).
Although the found results presented information about serological evidence of exposure of cattle to the selected pathogens, it is not possible to determine which one was the real causative agent of the abortion, or if there was an association of agents that could possibly have potentialized the final outcome of a reproductive disorder. The present study was the first to show C. burnetii as a pathogen associated with bovine rhinotracheitis and bovine viral diarrhea viruses, N. caninum, Leptospira spp., T. gondii and T. vivax in cattle with abortion in Brazil. Future studies should be conducted to investigate how widespread C. burnetii is in Brazilian cattle herds and its real role in the etiology of reproductive problems in cattle in South America, as the single agent causing abortion or in co-infections with other agents.
Supplementary Material
Supplementary material accompanies this paper.
Supplementary Table. Detailed serological results for selected bovine pathogens associated to abortion in cattle in Brazil.
This material is available as part of the online article from Revista Brasileira de Parasitologia Veterinária (RBPV, 2019) - Home Page - SciELO.
Acknowledgements
This research was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Zanatto has received scholarship under the master’s program in Agricultural Microbiology from de Faculdade de Ciências Agrárias e Veterinárias (FCAV), Universidade Estadual Paulista Júlio de Mesquita Filho (UNESP). We appreciate Dr. Samara for kindly supplied the cattle serum samples, Dr. Labruna for the Coxiella burnetii antigen and IMUNODOT Diagnósticos Ltda. (Jaboticabal, SP) for N. caninum, T. gondii, T. vivax antigens and serological kits. MRA is a fellowship researcher of “Conselho Nacional de Desenvolvimento Científico e Tecnólogico” (CNPq Process number #302420/2017-7).
References
-
Abdel-Moein KA, Hamza DA. The burden of Coxiella burnetii among aborted dairy animals in Egypt and its public health implications. Acta Trop 2017; 166: 92-95. http://dx.doi.org/10.1016/j.actatropica.2016.11.011 PMid:27845064.
» http://dx.doi.org/10.1016/j.actatropica.2016.11.011 -
Affonso IB, Amoril JG, Alexandrino B, Buzinaro MDG, Medeiros ASR, Samara SI. Anticorpos contra o Herpesvírus Bovino Tipo 1 (BoHV-1) nas dez regiões de planejamento do estado de Goiás, Brasil. Cienc Anim Bras 2010; 11(4): 892-898. http://dx.doi.org/10.5216/cab.v11i4.6318
» http://dx.doi.org/10.5216/cab.v11i4.6318 -
Agerholm JS. Coxiella burnetii associated reproductive disorders in domestic animals-a critical review. Acta Vet Scand 2013; 55(1): 13. http://dx.doi.org/10.1186/1751-0147-55-13 PMid:23419216.
» http://dx.doi.org/10.1186/1751-0147-55-13 -
Agger JF, Christoffersen AB, Rattenborg E, Nielsen J, Agerholm JS. Prevalence of Coxiella burnetii antibodies in Danish dairy herds. Acta Vet Scand 2010; 52(1): 5. http://dx.doi.org/10.1186/1751-0147-52-5 PMid:20092653.
» http://dx.doi.org/10.1186/1751-0147-52-5 -
Almeida LL, Miranda ICS, Hein HE, Santiago WS No, Costa EF, Marks FS, et al. Herd-level risk factors for bovine viral diarrhea virus infection in dairy herds from southern Brazil. Res Vet Sci 2013; 95(3): 901-907. https://doi.org/10.1016/j.rvsc.2013.08.009 PMid:24079841.
» https://doi.org/10.1016/j.rvsc.2013.08.009 - Andrade AQA No, Afonso JAB, Mendonça CL, Souto RJC, André MR, Machado RZ. Surtos de tripanossomíase em bovinos leiteiros no agreste dos estados de Pernambuco e Alagoas. Biologico 2015; 77(S2): 143.
-
André MR, Adania CH, Teixeira RH, Silva KF, Jusi MM, Machado ST, et al. Antibodies to Toxoplasma gondii and Neospora caninum in captive neotropical and exotic wild canids and felids. J Parasitol 2010; 96(5): 1007-1009. http://dx.doi.org/10.1645/GE-2502.1 PMid:20950109.
» http://dx.doi.org/10.1645/GE-2502.1 -
Aquino LP, Machado RZ, Alessi AC, Marques LC, Castro MB, Malheiros EB. Clinical, parasitological and immunological aspects of experimental infection with Trypanosoma evansi in dogs. Mem Inst Oswaldo Cruz 1999; 94(2): 255-260. http://dx.doi.org/10.1590/S0074-02761999000200025 PMid:10224539.
» http://dx.doi.org/10.1590/S0074-02761999000200025 -
Bártová E, Sedlák K, Literák I. Toxoplasma gondii and Neospora caninum antibodies in sheep in the Czech Republic. Vet Parasitol 2009; 161(1-2): 131-132. http://dx.doi.org/10.1016/j.vetpar.2008.12.022 PMid:19181450.
» http://dx.doi.org/10.1016/j.vetpar.2008.12.022 -
Bastos TSA, Faria AM, Madrid DMC, Bessa LC, Linhares GFC, Fidelis OL Jr, et al. First outbreak and subsequent cases of Trypanosoma vivax in the state of Goiás, Brazil. Rev Bras Parasitol Vet 2017; 26(3): 366-371. http://dx.doi.org/10.1590/s1984-29612017019 PMid:28678894.
» http://dx.doi.org/10.1590/s1984-29612017019 -
Batista JS, Riet-Correa F, Teixeira MMG, Madruga CR, Simões SDV, Maia TF. Trypanosomiasis by Trypanosoma vivax in cattle in the Brazilian semiarid: Description of an outbreak and lesions in the nervous system. Vet Parasitol 2007; 143(2): 174-181. http://dx.doi.org/10.1016/j.vetpar.2006.08.017 PMid:16965857.
» http://dx.doi.org/10.1016/j.vetpar.2006.08.017 -
Böttcher J, Vossen A, Janowetz B, Alex M, Gangl A, Randt A, et al. Insights into the dynamics of endemic Coxiella burnetii infection in cattle by application of phase-specific ELISAs in an infected dairy herd. Vet Microbiol 2011; 151(3-4): 291-300. http://dx.doi.org/10.1016/j.vetmic.2011.03.007 PMid:21482042.
» http://dx.doi.org/10.1016/j.vetmic.2011.03.007 -
Brandão H, Vale LAR, Christovão DA. Investigações sôobre a febre “Q” em São Paulo. I - Estudo sorológico em operários de um frigorífico. Arq Fac Hig Saude Publica Univ Sao Paulo 1953; 7(1): 127-131. http://dx.doi.org/10.11606/issn.2358-792X.v7i1p127-131
» http://dx.doi.org/10.11606/issn.2358-792X.v7i1p127-131 -
Butzler JP. Campylobacter, from obscurity to celebrity. Clin Microbiol Infect 2004; 10(10): 868-876. http://dx.doi.org/10.1111/j.1469-0691.2004.00983.x PMid:15373879.
» http://dx.doi.org/10.1111/j.1469-0691.2004.00983.x -
Cadioli FA, Barnabé PA, Machado RZ, Teixeira MCA, André MR, Sampaio PH, et al. First report of Trypanosoma vivax outbreak in dairy cattle in São Paulo state, Brazil. Rev Bras Parasitol Vet 2012; 21(2): 118-124. http://dx.doi.org/10.1590/S1984-29612012000200009 PMid:22832751.
» http://dx.doi.org/10.1590/S1984-29612012000200009 -
Carvalho AU, Abrão DC, Facury EJ Fo, Paes PRO, Ribeiro MFB. Occurrence of Trypanosoma vivax in Minas Gerais state, Brazil. Arq Bras Med Vet Zootec 2008; 60(3): 769-771. http://dx.doi.org/10.1590/S0102-09352008000300037
» http://dx.doi.org/10.1590/S0102-09352008000300037 -
Centers for Disease Control and Prevention – CDC. Bioterrorism agents/diseases [online]. 2018 [cited 2018 Dec 22]. Available from https://emergency.cdc.gov/agent/agentlist.asp
» https://emergency.cdc.gov/agent/agentlist.asp -
Cerqueira-Cézar CK, Calero-Bernal R, Dubey JP, Gennari SM. All about neosporosis in Brazil. Rev Bras Parasitol Vet 2017; 26(3): 253-279. http://dx.doi.org/10.1590/s1984-29612017045 PMid:28876360.
» http://dx.doi.org/10.1590/s1984-29612017045 -
Cetinkaya B, Kalender H, Ertas HB, Muz A, Arslan N, Ongor H, et al. Seroprevalence of coxiellosis in cattle, sheep and people in the east of Turkey. Vet Rec 2000; 146(5): 131-136. http://dx.doi.org/10.1136/vr.146.5.131 PMid:10706331.
» http://dx.doi.org/10.1136/vr.146.5.131 -
Chi J, VanLeeuwen JA, Weersink A, Keefe GP. Management factors related to seroprevalences to bovine viral-diarrhoea virus, bovine-leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum in dairy herds in the Canadian Maritimes. Prev Vet Med 2002; 55(1): 57-68. http://dx.doi.org/10.1016/S0167-5877(02)00067-3 PMid:12324207.
» http://dx.doi.org/10.1016/S0167-5877(02)00067-3 -
Costa PSG, Brigatte ME, Greco DB. Antibodies to Rickettsia rickettsii, Rickettsia typhi, Coxiella burnetii, Bartonella henselae, Bartonella quintana, and Ehrlichia chaffeensis among healthy population in Minas Gerais, Brazil. Mem Inst Oswaldo Cruz 2005; 100(8): 853-859. http://dx.doi.org/10.1590/S0074-02762005000800006 PMid:16444416.
» http://dx.doi.org/10.1590/S0074-02762005000800006 -
Costa PSG, Brigatte ME, Greco DB. Questing one Brazilian query: reporting 16 cases of Q fever from Minas Gerais, Brazil. Rev Inst Med Trop São Paulo 2006; 48(1): 5-9. http://dx.doi.org/10.1590/S0036-46652006000100002 PMid:16547572.
» http://dx.doi.org/10.1590/S0036-46652006000100002 -
Cutler SJ, Bouzid M, Cutler RR. Q fever. J Infect 2007; 54(4): 313-318. http://dx.doi.org/10.1016/j.jinf.2006.10.048 PMid:17147957.
» http://dx.doi.org/10.1016/j.jinf.2006.10.048 - Czaplicki G, Houtain JY, Mullender C, Manteca C, Saegerman C. Bulk tank milk, reliable tool for diagnosing Q Fever in dairy herds? Epidémiol Santé Anim 2009; 56: 117-127.
-
Damman A, Viet AF, Arnoux S, Guerrier-Chatellet MC, Petit E, Ezanno P. Modelling the spread of bovine viral diarrhea virus (BVDV) in a beef cattle herd and its impact on herd productivity. Vet Res 2015; 46(1): 12. http://dx.doi.org/10.1186/s13567-015-0145-8 PMid:25828555.
» http://dx.doi.org/10.1186/s13567-015-0145-8 -
Dávila AM, Silva RAM. Animal trypanosomiasis in South America: current status, partnership, and information technology. Ann N Y Acad Sci 2000; 916(1): 199-212. http://dx.doi.org/10.1111/j.1749-6632.2000.tb05291.x PMid:11193622.
» http://dx.doi.org/10.1111/j.1749-6632.2000.tb05291.x -
Dias JA, Alfieri AA, Ferreira-Neto JS, Gonçalves VS, Muller EE. Seroprevalence and Risk factors of bovine herpesvirus 1 infection in cattle herds in the state of Parana. Transbound Emerg Dis 2013; 60(1): 39-47. http://dx.doi.org/10.1111/j.1865-1682.2012.01316.x PMid:22364224.
» http://dx.doi.org/10.1111/j.1865-1682.2012.01316.x -
Díaz Aparicio E. Epidemiology of brucellosis in domestic animals caused by Brucella melitensis, Brucella suis and Brucella abortus. Rev Sci Tech 2013; 32(1): 43-60. http://dx.doi.org/10.20506/rst.32.1.2187 PMid:23837364.
» http://dx.doi.org/10.20506/rst.32.1.2187 -
Eldin C, Mélenotte C, Mediannikov O, Ghigo E, Million M, Edouard S, et al. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev 2017; 30(1): 115-190. http://dx.doi.org/10.1128/CMR.00045-16 PMid:27856520.
» http://dx.doi.org/10.1128/CMR.00045-16 -
Eldin C, Mélenotte C, Million M, Cammilleri S, Sotto A, Elsendoorn A, et al. 18F-FDG PET/CT as a central tool in the shift from chronic Q fever to Coxiella burnetii persistent focalized infection: A consecutive case series. Medicine 2016; 95(34): e4287. http://dx.doi.org/10.1097/MD.0000000000004287 PMid:27559944.
» http://dx.doi.org/10.1097/MD.0000000000004287 -
Eldin C, Renvoisé A, Raoult D, Angelakis E. Coxiella burnetii DNA, but not viable bacteria, in dairy products in France. Am J Trop Med Hyg 2013; 88(4): 765-769. http://dx.doi.org/10.4269/ajtmh.12-0212 PMid:23382158.
» http://dx.doi.org/10.4269/ajtmh.12-0212 -
Fávero JF, Araújo HL, Lilenbaum W, Machado G, Tonin AA, Baldissera MD, et al. Bovine leptospirosis: Prevalence, associated risk factors for infection and their cause-effect relation. Microb Pathog 2017; 107: 149-154. http://dx.doi.org/10.1016/j.micpath.2017.03.032 PMid:28351712.
» http://dx.doi.org/10.1016/j.micpath.2017.03.032 -
Fávero JF, Silva AS, Biazus AH, Volpato A. Trypanosoma vivax infection in goat in west of Santa Catarina state, Brazil. Comp Clin Pathol 2016; 25(2): 497-499. http://dx.doi.org/10.1007/s00580-015-2216-7
» http://dx.doi.org/10.1007/s00580-015-2216-7 - Feitosa AB Jr, Guerra RMSNC, Santos HP, Abreu-Silva AL. Registro e morfometria de Trypanosoma vivax em esfregaço sanguíneo de bovino no município de Itapecuru-Mirim, Maranhão. Rev Bras Parasitol Vet 2004; 13(Supl. 1): 232.
-
Fidelis OL Jr, Sampaio PH, Machado RZ, André MR, Marques LC, Cadioli FA. Evaluation of clinical signs, parasitemia, hematologic and biochemical changes in cattle experimentally infected with Trypanosoma vivax. Rev Bras Parasitol Vet 2016; 25(1): 69-81. http://dx.doi.org/10.1590/S1984-29612016013 PMid:27007249.
» http://dx.doi.org/10.1590/S1984-29612016013 - Fino TCM, Melo CB, Ramos AF, Leite RC. Diarréia bovina a vírus (BVD) - uma breve revisão. Rev Bras Med Vet 2012; 34(2): 131-140.
-
Flores EF, Weiblen R, Cargnelutti JF, Bauermann V, Spilki FR, Mori E, et al. Emerging animal viruses: real threats or simple bystanders? Pesq Vet Bras 2013; 33(10): 1161-1173. http://dx.doi.org/10.1590/S0100-736X2013001000001
» http://dx.doi.org/10.1590/S0100-736X2013001000001 -
Gache K, Rousset E, Perrin JB, De Cremoux R, Hosteing S, Jourdain E, et al. Estimation of the frequency of Q fever in sheep, goat and cattle herds in France: results of a 3-year study of the seroprevalence of Q fever and excretion level of Coxiella burnetii in abortive episodes. Epidemiol Infect 2017; 145(15): 3131-3142. http://dx.doi.org/10.1017/S0950268817002308 PMid:29039279.
» http://dx.doi.org/10.1017/S0950268817002308 -
Gatto IRH, Linhares DCL, Souza Almeida HM, Mathias LA, Medeiros ASR, Poljak Z, et al. Description of risk factors associated with the detection of BVDV antibodies in Brazilian pig herds. Trop Anim Health Prod 2018; 50(4): 773-778. http://dx.doi.org/10.1007/s11250-017-1493-3 PMid:29264821.
» http://dx.doi.org/10.1007/s11250-017-1493-3 - Georgiev M, Afonso A, Neubauer H, Needham H, Thiery R, Rodolakis A, et al. Q fever in humans and farm animals in four European countries, 1982 to 2010. Euro Surveill 2013; 18(8): 20407. PMid:23449232.
-
González A, Gómez BL, Diez S, Hernández O, Restrepo A, Hamilton AJ, et al. Purification and partial characterization of a Paracoccidioides brasiliensis protein with capacity to bind to extracellular matrix proteins. Infect Immun 2005; 73(4): 2486-2495. http://dx.doi.org/10.1128/IAI.73.4.2486-2495.2005 PMid:15784595.
» http://dx.doi.org/10.1128/IAI.73.4.2486-2495.2005 -
Grooms DL. Reproductive consequences of infection with bovine viral diarrhea virus. Vet Clin North Am Food Anim Pract 2004; 20(1): 5-19. http://dx.doi.org/10.1016/j.cvfa.2003.11.006 PMid:15062471.
» http://dx.doi.org/10.1016/j.cvfa.2003.11.006 -
Guatteo R, Beaudeau F, Joly A, Seegers H. Coxiella burnetii shedding by dairy cows. Vet Res 2007; 38(6): 849-860. http://dx.doi.org/10.1051/vetres:2007038 PMid:17903418.
» http://dx.doi.org/10.1051/vetres:2007038 - Guimarães MF, Araujo AC, Freire DP, Machado DM, Martins NN, Moraes-Filho J, et al. Infection survey of Rickettsia rickettsii and Coxiella burnetii in sheep and goats from National Park of Serra das Confusões, Piauí. Pesq Vet Bras 2017; 37(6): 555-560.
-
Jones TW, Dávila AM. Trypanosoma vivax–out of Africa. Trends Parasitol 2001; 17(2): 99-101. http://dx.doi.org/10.1016/S1471-4922(00)01777-3 PMid:11228017.
» http://dx.doi.org/10.1016/S1471-4922(00)01777-3 -
Kamga-Waladjo AR, Gbati OB, Kone P, Lapo RA, Chatagnon G, Bakou SN, et al. Seroprevalence of Neospora caninum antibodies and its consequences for reproductive parameters in dairy cows from Dakar–Senegal, West Africa. Trop Anim Health Prod 2010; 42(5): 953-959. http://dx.doi.org/10.1007/s11250-009-9513-6 PMid:19997972.
» http://dx.doi.org/10.1007/s11250-009-9513-6 -
Kirkbride CA. Etiologic agents detected in a 10-year study of bovine abortions and stillbirths. J Vet Diagn Invest 1992; 4(2): 175-180. http://dx.doi.org/10.1177/104063879200400210 PMid:1616982.
» http://dx.doi.org/10.1177/104063879200400210 -
Lamas CC, Ramos RG, Lopes GQ, Santos MS, Golebiovski WF, Weksler C, et al. Bartonella and Coxiella infective endocarditis in Brazil: molecular evidence from excised valves from a cardiac surgery referral center in Rio de Janeiro, Brazil, 1998 to 2009. Int J Infect Dis 2013; 17(1): e65-e66. http://dx.doi.org/10.1016/j.ijid.2012.10.009 PMid:23219032.
» http://dx.doi.org/10.1016/j.ijid.2012.10.009 -
Lamas CC, Rozental T, Bóia MN, Favacho ARM, Kirsten AH, Silva APM, et al. Seroprevalence of Coxiella burnetii antibodies in human immunodeficiency virus-positive patients in Jacarepaguá, Rio de Janeiro, Brazil. Clin Microbiol Infect 2009; 15(Supl. 2): 140-141. http://dx.doi.org/10.1111/j.1469-0691.2008.02144.x PMid:19298403.
» http://dx.doi.org/10.1111/j.1469-0691.2008.02144.x -
Lemos ERS, Rozental T, Mares-Guia MAM, Almeida DN, Moreira N, Silva RG, et al. Q fever as a cause of fever of unknown origin and thrombocytosis: first molecular evidence of Coxiella burnetii in Brazil. Vector Borne Zoonotic Dis 2011; 11(1): 85-87. http://dx.doi.org/10.1089/vbz.2009.0261 PMid:20569012.
» http://dx.doi.org/10.1089/vbz.2009.0261 -
Lemos ERS, Rozental T, Siqueira BN, Pessoa AA Jr, Joaquim TE, Silva RG, et al. Q Fever in Military Firefighters during Cadet Training in Brazil. Am J Trop Med Hyg 2018; 99(2): 303-305. http://dx.doi.org/10.4269/ajtmh.17-0979 PMid:29943714.
» http://dx.doi.org/10.4269/ajtmh.17-0979 - Linhares GFC, Dias FC Fo, Fernandes PR, Duarte SC. Tripanossomíase em bovinos no município de Formoso do Araguaia, Tocantins. Cienc Anim Bras 2006; 7(4): 455-460.
- Lopes STP, Silva Prado B, Martins GHC, Esmeraldo H, Beserra A, Sousa MAC Fo, et al. Trypanosoma vivax em bovino leiteiro. Acta Sci Vet 2018; 46(Supl. 1): 287.
-
Lovato LT, Weiblen R, Tobias FL, Moraes MP. Herpesvírus bovino tipo 1 (HVB 1): inquérito soro-epidemiológico no rebanho leiteiro do Estado do Rio Grande do Sul, Brasil. Cienc Rural 1995; 25(3): 425-430. http://dx.doi.org/10.1590/S0103-84781995000300017
» http://dx.doi.org/10.1590/S0103-84781995000300017 -
Lucy MC. Reproductive loss in high-producing dairy cattle: where will it end? J Dairy Sci 2001; 84(6): 1277-1293. http://dx.doi.org/10.3168/jds.S0022-0302(01)70158-0 PMid:11417685.
» http://dx.doi.org/10.3168/jds.S0022-0302(01)70158-0 -
Machado RZ, Montassier HJ, Pinto AA, Lemos EG, Machado MRF, Valadão IFF, et al. An enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against Babesia bovis in cattle. Vet Parasitol 1997; 71(1): 17-26. http://dx.doi.org/10.1016/S0304-4017(97)00003-4 PMid:9231985.
» http://dx.doi.org/10.1016/S0304-4017(97)00003-4 -
Mares-Guia MA, Rozental T, Guterres A, Ferreira MS, Botticini RG, Terra AK, et al. Molecular identification of Q fever in patients with a suspected diagnosis of dengue in Brazil in 2013–2014. Am J Trop Med Hyg 2016; 94(5): 1090-1094. http://dx.doi.org/10.4269/ajtmh.15-0575 PMid:26928831.
» http://dx.doi.org/10.4269/ajtmh.15-0575 -
Mares-Guia MAM, Rozental T, Guterres A, Gomes R, Almeida DN, Moreira NS, et al. Molecular identification of the agent of Q fever – Coxiella burnetii – in domestic animals in State of Rio de Janeiro, Brazil. Rev Soc Bras Med Trop 2014; 47(2): 231-234. http://dx.doi.org/10.1590/0037-8682-0076-2013 PMid:24861300.
» http://dx.doi.org/10.1590/0037-8682-0076-2013 -
Maurin M, Raoult DF. Q fever. Clin Microbiol Rev 1999; 12(4): 518-553. http://dx.doi.org/10.1128/CMR.12.4.518 PMid:10515901.
» http://dx.doi.org/10.1128/CMR.12.4.518 -
McQuiston JH, Nargund VN, Miller JD, Priestley R, Shaw EI, Thompson HA. Prevalence of antibodies to Coxiella burnetii among veterinary school dairy herds in the United States, 2003. Vector Borne Zoonotic Dis 2005; 5(1): 90-91. http://dx.doi.org/10.1089/vbz.2005.5.90 PMid:15815154.
» http://dx.doi.org/10.1089/vbz.2005.5.90 -
Mineiro ALBB, Bezerra EEA, Vasconcellos SA, Costa FAL, Macedo NA. Infecção por leptospira em bovinos e sua associação com transtornos reprodutivos e condições climáticas. Arq Bras Med Vet Zootec 2007; 59(5): 1103-1109. http://dx.doi.org/10.1590/S0102-09352007000500003
» http://dx.doi.org/10.1590/S0102-09352007000500003 -
Mori M, Roest HJ. Farming, Q fever and public health: agricultural practices and beyond. Arch Public Health 2018; 76(1): 2. http://dx.doi.org/10.1186/s13690-017-0248-y PMid:29321921.
» http://dx.doi.org/10.1186/s13690-017-0248-y - Murray RD. A field investigation of causes of abortion in dairy cattle. Vet Rec 1990; 127(22): 543-547. PMid:2281595.
- Musso D, Raoult D. Serological cross-reactions between Coxiella burnetii and Legionella micdadei. Clin Diagn Lab Immunol 1997; 4(2): 208-212. PMid:9067657.
-
National Association of State Public Health Veterinarians – NASPHV. Prevention and control of Coxiella burnetii infection among humans and animals: guidance for a coordinated public health and animal health response [online]. Des Moines: National Assembly of State Animal Health Officials. 2013 [cited 2019 Apr 24]. Available from: http://www.nasphv.org/Documents/Q_Fever_2013.pdf
» http://www.nasphv.org/Documents/Q_Fever_2013.pdf -
Nielsen KT, Nielsen SS, Agger JF, Christoffersen AB, Agerholm JS. Association between antibodies to Coxiella burnetii in bulk tank milk and perinatal mortality of Danish dairy calves. Acta Vet Scand 2011; 53(1): 64. http://dx.doi.org/10.1186/1751-0147-53-64 PMid:22136406.
» http://dx.doi.org/10.1186/1751-0147-53-64 -
Nokhodian Z, Feizi A, Moradi A, Yaran M, Hoseini SG, Ataei B, et al. Detection and risk factors of Coxiella burnetii infection in dairy cattle based on bulk tank milk samples in center of Iran. Prev Vet Med 2016; 134: 139-144. http://dx.doi.org/10.1016/j.prevetmed.2016.10.003 PMid:27836035.
» http://dx.doi.org/10.1016/j.prevetmed.2016.10.003 -
Office International des Epizooties – OIE. Manual of diagnostics tests and vaccines for terrestrial animals [online]. Paris: OIE; 2018a [cited 2019 Apr 23]. Chapter 3.4.7, Bovine Viral Diarrhoea; p. 1075-1096. Available from: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.04.07_BVD.pdf
» http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.04.07_BVD.pdf -
Office International des Epizooties – OIE. Manual of diagnostics tests and vaccines for terrestrial animals [online]. Paris: OIE; 2018b [cited 2019 Apr 23]. Chapter 3.4.11, Infectious bovine rhinotracheitis/infectious pustular vulvovaginitis; p. 1139-1157. Available from: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.04.11_IBR_IPV.pdf
» http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.04.11_IBR_IPV.pdf -
Office International des Epizooties – OIE. Manual of diagnostics tests and vaccines for terrestrial animals [online]. Paris: OIE; 2018c [cited 2019 Apr 23]. Chapter 3.4.12, Leptospirosis; p. 503-516. Available from: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.01.12_LEPTO.pdf
» http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.01.12_LEPTO.pdf -
Oliveira JMB, Rozental T, Lemos ERS, Forneas D, Ortega-Mora LM, Porto WJN, et al. Coxiella burnetii in dairy goats with a history of reproductive disorders in Brazil. Acta Trop 2018; 183: 19-22. http://dx.doi.org/10.1016/j.actatropica.2018.04.010 PMid:29621535.
» http://dx.doi.org/10.1016/j.actatropica.2018.04.010 -
Oliveira TMFS, Furuta PI, Carvalho D, Machado RZ. A study of cross-reactivity in serum samples from dogs positive for Leishmania sp., Babesia canis and Ehrlichia canis in enzyme-linked immunosorbent assay and indirect fluorescent antibody test. Rev Bras Parasitol Vet 2008; 17(1): 7-11. http://dx.doi.org/10.1590/S1984-29612008000100002 PMid:18554433.
» http://dx.doi.org/10.1590/S1984-29612008000100002 -
Pacheco RC, Echaide IE, Alves RN, Beletti ME, Nava S, Labruna MB. Coxiella burnetii in ticks, Argentina. Emerg Infect Dis 2013; 19(2): 344-346. http://dx.doi.org/10.3201/eid1902.120362 PMid:23343647.
» http://dx.doi.org/10.3201/eid1902.120362 - Paiva F, Lemos RAA, Nakazato L, Mori AE, Brum KB, Bernardo KC. Trypanosoma vivax em bovinos no Pantanal do Estado do Mato Grosso do Sul, Brasil: I.-Acompanhamento clínico, laboratorial e anatomopatológico de rebanhos infectados. Rev Bras Parasitol Vet 2000; 9(2): 135-141.
- Paiva F, Lemos RAA, Oshiro AE, Salvador SC, Nakazato L. Ocorrência de Trypanosoma vivax em bovinos do Estado de Mato Grosso do Sul. Rev Bras Parasitol Vet 1997; 6(Supl. 1): 349.
-
Parker NR, Barralet JH, Bell AM. Q fever. Lancet 2006; 367(9511): 679-688. http://dx.doi.org/10.1016/S0140-6736(06)68266-4 PMid:16503466.
» http://dx.doi.org/10.1016/S0140-6736(06)68266-4 - Peacock MG, Philip RN, Williams JC, Faulkner RS. Serological evaluation of O fever in humans: enhanced phase I titers of immunoglobulins G and A are diagnostic for Q fever endocarditis. Infect Immun 1983; 41(3): 1089-1098. PMid:6885155.
-
Pellerin C, Van Den Hurk J, Lecomte J, Tijssen P. Identification of a new group of bovine viral diarrhea virus strains associated with severe outbreaks and high mortalities. Virology 1994; 203(2): 260-268. http://dx.doi.org/10.1006/viro.1994.1483 PMid:8053150.
» http://dx.doi.org/10.1006/viro.1994.1483 -
Pimentel DS, Ramos CA, Ramos RA, Araújo FR, Borba ML, Faustino MA, et al. First report and molecular characterization of Trypanosoma vivax in cattle from state of Pernambuco, Brazil. Vet Parasitol 2012; 185(2-4): 286-289. http://dx.doi.org/10.1016/j.vetpar.2011.10.019 PMid:22054681.
» http://dx.doi.org/10.1016/j.vetpar.2011.10.019 -
Quincozes CG, Fisher G, Hübner SO, Vargas GDA, Vidor T, Brod CS. Prevalence and factors associated with bovine viral diarrhea virus infection in south of Rio Grande do Sul. Semina: Ciênc Agrár 2007; 28(2): 269-276. http://dx.doi.org/10.5433/1679-0359.2007v28n2p269
» http://dx.doi.org/10.5433/1679-0359.2007v28n2p269 - Ralph A, Markey P, Schultz R. Q fever cases in the Northern Territory of Australia from 1991 to 2006. Commun Dis Intell Q Rep 2007; 31(2): 222-227. PMid:17724999.
-
Ramos IAS, Silva RJ, Maciel TA, Silva JABA, Fidelis OL Jr, Soares PC, et al. Assessment of transplacental transmission of Neospora caninum in dairy cattle in the Agreste region of Pernambuco. Rev Bras Parasitol Vet 2016; 25(4): 516-522. http://dx.doi.org/10.1590/s1984-29612016055 PMid:27737368.
» http://dx.doi.org/10.1590/s1984-29612016055 -
Reeves WK, Loftis AD, Sanders F, Spinks MD, Wills W, Denison AM, et al. Borrelia, Coxiella and Rickettsia in Carios capensis (Acari: Argasidae) from a brown pelican (Pelecanus occidentalis) rookery in South Carolina, USA. Exp Appl Acarol 2006; 39(3-4): 321-329. http://dx.doi.org/10.1007/s10493-006-9012-7 PMid:16821092.
» http://dx.doi.org/10.1007/s10493-006-9012-7 -
Reichel MP, Ayanegui-Alcérreca MA, Gondim LF, Ellis JT. What is the global economic impact of Neospora caninum in cattle – the billion dollar question. Int J Parasitol 2013; 43(2): 133-142. http://dx.doi.org/10.1016/j.ijpara.2012.10.022 PMid:23246675.
» http://dx.doi.org/10.1016/j.ijpara.2012.10.022 -
Revista Brasileira de Parasitologia Veterinária – RBPV [online]. 2019 [cited 2019 Apr 23]. Available from: http://www.scielo.br/rbpv
» http://www.scielo.br/rbpv -
Riemann HP, Brant PC, Franti CE, Reis R, Buchanan AM, Stormont C, et al. Antibodies to Toxoplasma gondii and Coxiella burnetii among students and other personnel in veterinary colleges in California and Brazil. Am J Epidemiol 1974; 100(3): 197-208. http://dx.doi.org/10.1093/oxfordjournals.aje.a112028 PMid:4606228.
» http://dx.doi.org/10.1093/oxfordjournals.aje.a112028 -
Rodolakis A, Berri M, Héchard C, Caudron C, Souriau A, Bodier CC, et al. Comparison of Coxiella burnetii shedding in milk of dairy bovine, caprine, and ovine herds. J Dairy Sci 2007; 90(12): 5352-5360. http://dx.doi.org/10.3168/jds.2006-815 PMid:18024725.
» http://dx.doi.org/10.3168/jds.2006-815 -
Rodríguez-Batista Z, Leite RC, Oliveira PR, Lopes CML, Borges LMF. Populational dynamics of Stomoxys calcitrans (Linneaus) (Diptera: Muscidae) in three biocenosis, Minas Gerais, Brazil. Vet Parasitol 2005; 130(3-4): 343-346. http://dx.doi.org/10.1016/j.vetpar.2005.03.006 PMid:15908125.
» http://dx.doi.org/10.1016/j.vetpar.2005.03.006 -
Royal MD, Darwash AO, Flint APF, Webb R, Woolliams JA, Lamming GE. Declining fertility in dairy cattle: changes in traditional and endocrine parameters of fertility. Anim Sci 2000; 70(3): 487-501. http://dx.doi.org/10.1017/S1357729800051845
» http://dx.doi.org/10.1017/S1357729800051845 -
Rozental T, Mascarenhas LF, Rozenbaum R, Gomes R, Mattos GS, Magno CC, et al. Coxiella burnetii, the agent of Q fever in Brazil: its hidden role in seronegative arthritis and the importance of molecular diagnosis based on the repetitive element IS1111 associated with the transposase gene. Mem Inst Oswaldo Cruz 2012; 107(5): 695-697. http://dx.doi.org/10.1590/S0074-02762012000500021 PMid:22850965.
» http://dx.doi.org/10.1590/S0074-02762012000500021 -
Rozental T, Silva ASV, Oliveira RC, Favacho ARM, Oliveira MLA, Bastos FI, et al. Seroprevalence of Bartonella spp., Coxiella burnetii, and Hantavirus among people who inject drugs in Rio de Janeiro, Brazil: a retrospective assessment of a biobank. Rev Inst Med Trop São Paulo 2018; 60(0): e31. http://dx.doi.org/10.1590/s1678-9946201860031 PMid:30043935.
» http://dx.doi.org/10.1590/s1678-9946201860031 -
Ryan ED, Wrigley K, Hallinan A, McGrath G, Clegg TA. Antibodies to Coxiella burnetii in Irish bulk tank milk samples. Vet Rec 2018; 182(19): 550. http://dx.doi.org/10.1136/vr.104663 PMid:29445016.
» http://dx.doi.org/10.1136/vr.104663 - Scola B, Raoult D. Serological cross-reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J Clin Microbiol 1996; 34(9): 2270-2274. PMid:8862597.
-
Scolamacchia F, Handel IG, Fèvre EM, Morgan KL, Tanya VN, Bronsvoort BMC. Serological patterns of Brucellosis, Leptospirosis and Q Fever in Bos indicus Cattle in Cameroon. PLoS One 2010; 5(1): e8623. http://dx.doi.org/10.1371/journal.pone.0008623 PMid:20098670.
» http://dx.doi.org/10.1371/journal.pone.0008623 -
Siciliano RF, Castelli JB, Mansur AJ, Santos FP, Colombo S, Nascimento EM, et al. Bartonella spp. and Coxiella burnetii associated with community-acquired, culture-negative endocarditis, Brazil. Emerg Infect Dis 2015; 21(8): 1429-1432. http://dx.doi.org/10.3201/eid2108.140343 PMid:26197233.
» http://dx.doi.org/10.3201/eid2108.140343 -
Siciliano RF, Ribeiro HB, Furtado RHM, Castelli JB, Sampaio RO, Santos FCP, et al. Endocardite por Coxiella burnetii (febre Q): doença rara ou pouco diagnosticada? Relato de caso. Rev Soc Bras Med Trop 2008; 41(4): 409-412. http://dx.doi.org/10.1590/S0037-86822008000400017 PMid:18853017.
» http://dx.doi.org/10.1590/S0037-86822008000400017 -
Silva AS, Costa MM, Polenz MF, Polenz CH, Teixeira MMG, Lopes STA, et al. Primeiro registro de Trypanosoma vivax em bovinos no Estado do Rio Grande do Sul, Brasil. Cienc Rural 2009; 39(8): 2550-2554. http://dx.doi.org/10.1590/S0103-84782009005000189
» http://dx.doi.org/10.1590/S0103-84782009005000189 -
Silva RAMS, Eguez A, Morales G, Eulert E, Montenegro A, Ybañez R, et al. Bovine Trypanosomiasis in Bolivian and Brazilian Lowlands. Mem Inst Oswaldo Cruz 1998; 93(1): 29-32. http://dx.doi.org/10.1590/S0074-02761998000100006 PMid:9698839.
» http://dx.doi.org/10.1590/S0074-02761998000100006 -
Silva RAMS, Silva JA, Schneider RC, Freitas J, Mesquita D, Mesquita T, et al. Outbreak of trypanosomiasis due to Trypanosoma vivax (Ziemann, 1905) in bovines of the Pantanal, Brazil. Mem Inst Oswaldo Cruz 1996; 91(5): 561-562. http://dx.doi.org/10.1590/S0074-02761996000500005 PMid:9137742.
» http://dx.doi.org/10.1590/S0074-02761996000500005 -
Softic A, Asmare K, Granquist EG, Godfroid J, Fejzic N, Skjerve E. The serostatus of Brucella spp., Chlamydia abortus, Coxiella burnetii and Neospora caninum in cattle in three cantons in Bosnia and Herzegovina. BMC Vet Res 2018; 14(1): 40. http://dx.doi.org/10.1186/s12917-018-1361-z PMid:29394895.
» http://dx.doi.org/10.1186/s12917-018-1361-z -
Souza EAR, Castro EMS, Oliveira GMB, Azevedo SS, Peixoto RDM, Labruna MB, et al. Serological diagnosis and risk factors for Coxiella burnetii in goats and sheep in a semi-arid region of Northeastern Brazil. Rev Bras Parasitol Vet 2018; 27(4): 514-520. http://dx.doi.org/10.1590/s1984-296120180086 PMid:30517422.
» http://dx.doi.org/10.1590/s1984-296120180086 -
Stenlund S, Kindahl H, Uggla A, Björkman C. A long-term study of Neospora caninum infection in a Swedish dairy herd. Acta Vet Scand 2003; 44(1-2): 63-71. http://dx.doi.org/10.1186/1751-0147-44-63 PMid:14650545.
» http://dx.doi.org/10.1186/1751-0147-44-63 -
Takiuchi E, Alfieri AF, Alfieri AA. Bovine herpervirus type 1: infection and diagnosis methods. Semina: Ciênc Agrár 2001; 22(2): 203-209. http://dx.doi.org/10.5433/1679-0359.2001v22n2p203
» http://dx.doi.org/10.5433/1679-0359.2001v22n2p203 -
Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol 2000; 30(12-13): 1217-1258. http://dx.doi.org/10.1016/S0020-7519(00)00124-7 PMid:11113252.
» http://dx.doi.org/10.1016/S0020-7519(00)00124-7 -
Tilahun B, Tolossa YH, Tilahun G, Ashenafi H, Shimelis S. Seroprevalence and risk factors of Toxoplasma gondii infection among domestic ruminants in East Hararghe zone of Oromia Region, Ethiopia. Vet Med Int 2018; 2018: 4263470. http://dx.doi.org/10.1155/2018/4263470 PMid:29887984.
» http://dx.doi.org/10.1155/2018/4263470 -
Tissot-Dupont H, Raoult D. Q fever. Infect Dis Clin North Am 2008; 22(3): 505-514, ix. http://dx.doi.org/10.1016/j.idc.2008.03.002 PMid:18755387.
» http://dx.doi.org/10.1016/j.idc.2008.03.002 - Travassos J, Ubatuba A, Silva NP, Mello MT. Febre Q no Rio de Janeiro. Cienc Cult 1954; 6: 199-200.
-
Valle LAR, Brandão H, Christovão DDA, D´Apice M. Investigações sôbre a Febre Q em São Paulo. Arq Fac Hig Saude Publica Univ Sao Paulo 1955; 9(1-2): 167-180. http://dx.doi.org/10.11606/issn.2358-792X.v9i1-2p167-180
» http://dx.doi.org/10.11606/issn.2358-792X.v9i1-2p167-180 -
Van den Brom R, Van Engelen E, Luttikholt S, Moll L, Van Maanen K, Vellema P. Coxiella burnetii in bulk tank milk samples from dairy goat and dairy sheep farms in The Netherlands in 2008. Vet Rec 2012; 170(12): 310. http://dx.doi.org/10.1136/vr.100304 PMid:22351793.
» http://dx.doi.org/10.1136/vr.100304 -
Van Schaik EJ, Chen C, Mertens K, Weber MM, Samuel JE. Molecular pathogenesis of the obligate intracellular bacterium Coxiella burnetii. Nat Rev Microbiol 2013; 11(8): 561-573. http://dx.doi.org/10.1038/nrmicro3049 PMid:23797173.
» http://dx.doi.org/10.1038/nrmicro3049 -
Varela-Castro L, Zuddas C, Ortega N, Serrano E, Salinas J, Castellà J, et al. On the possible role of ticks in the eco-epidemiology of Coxiella burnetii in a Mediterranean ecosystem. Ticks Tick Borne Dis 2018; 9(3): 687-694. http://dx.doi.org/10.1016/j.ttbdis.2018.02.014 PMid:29478883.
» http://dx.doi.org/10.1016/j.ttbdis.2018.02.014 -
Vidal S, Kegler K, Greub G, Aeby S, Borel N, Dagleish MP, et al. Neglected zoonotic agents in cattle abortion: tackling the difficult to grow bacteria. BMC Vet Res 2017; 13(1): 373. http://dx.doi.org/10.1186/s12917-017-1294-y PMid:29197401.
» http://dx.doi.org/10.1186/s12917-017-1294-y -
Vieira OLE, Macedo LOD, Santos MAB, Silva JABA, Mendonça CLD, Faustino MAG, et al. Detection and molecular characterization of Trypanosoma (Duttonella) vivax in dairy cattle in the state of Sergipe, northeastern Brazil. Rev Bras Parasitol Vet 2017; 26(4): 516-520. http://dx.doi.org/10.1590/s1984-29612017048 PMid:29091120.
» http://dx.doi.org/10.1590/s1984-29612017048 -
Wyrosdick HM, Schaefer JJ. Toxoplasma gondii: history and diagnostic test development. Anim Health Res Rev 2015; 16(2): 150-162. http://dx.doi.org/10.1017/S1466252315000183 PMid:26568360.
» http://dx.doi.org/10.1017/S1466252315000183

 Coxiella burnetii associated with BVDV (Bovine Viral Diarrhea Virus), BoHV (Bovine Herpesvirus), Leptospira spp., Neospora caninum, Toxoplasma gondii and Trypanosoma vivax in reproductive disorders in cattle
Coxiella burnetii associated with BVDV (Bovine Viral Diarrhea Virus), BoHV (Bovine Herpesvirus), Leptospira spp., Neospora caninum, Toxoplasma gondii and Trypanosoma vivax in reproductive disorders in cattle