Abstracts
OBJECTIVE:
Unaccustomed exercise causes transient Delayed Onset Muscle Soreness (DOMS); creatine-kinase and DOMS are indirect markers of muscle damage. Heat pack treatment increases blood flow and relieves pain. We determined the effects of heat pack treatment on DOMS, Creatine-Kinase, pain and jumping following maximum calf-raise exercises.
METHODS:
Exercise (3 days): calf-raise, 1 movement every 3 seconds until subjects could not maintain movement speed, Recovery: monitored for 7 days. Subjects: 14 female collegiate students (age: 20-22) with previous regular moderate exercise history, divided into heat pack treatment (n = 7; 40ºC, 20-min on both calf muscles immediately after exercise) vs. no treatment (n = 7). Measured parameters: number of daily movements, Creatine-Kinase, one-leg long jumping (JUMP) and perceived pain (PAIN). Maximum dorsiflexion, calf maximum circumference and isometric muscle strength were also measured, but showed no significant variation.
RESULTS:
There were no differences between groups regarding the number of the calf-raise repetitions; Creatine-Kinase increased significantly from day 3 of the Exercise-period to day 5 of the recovery period and peaked on Recovery day 2 in both groups; it was higher in the treated-group vs. controls; PAIN significantly decreased immediately after the heat pack treatment; DOMS peaked in both groups on day 3 of the Exercise-period, and recovered by day 4 of the recovery period. JUMP values decreased significantly after the initial exercise and recovered to initial values by Day 4 of the recovery period.
CONCLUSION:
Heat pack treatment for 20 minutes did not minimize DOMS following the maximum calf-raise exercise, but reduced immediate muscle soreness.
KEYWORDS:
Creatine kinase; Long jumping; Muscle soreness; Pain
OBJETIVO:
Exercícios desacostumados causam Dor Muscular Transitória Tardia (DMT); a creatina quinase e a DMT são marcadores indiretos de lesão muscular. Tratamento térmico aumenta o fluxo sanguíneo e alivia a dor. Determinamos os efeitos do tratamento de calor sobr a DMT, a creatina quinase, e a dor causada exercícios máximos de elevação da panturillha.
MÉTODOS:
Exercício (3 dias): elevação da panturrilha, um movimento cada 3 segundos até que os participantes não puderam manter a velocidade de movimento; esta fase foi seguida por sete dias de recuperação monitorada. Participantes: 14 estudantes universitários do sexo feminino (idade: 20-22) com história anterior exercícios moderados e regulares, divididos em participantes tratados com calor (n = 7; 40 º C, 20 min em ambos os músculos da panturrilha imediatamente após o exercício) vs. nenhum tratamento (n = 7). Os parâmetros medidos foram: número de movimentos diários, creatina quinase, slato em distância com uma única perna (JUMP) e dor percebida (DOR). Foram também medidos a dorsiflexão máxima, circunferência máxima da panturrilha e a força muscular isométrica, que não exibiram variações significativas.
RESULTADOS:
Não houve diferenças entre os grupos quanto ao número de repetições de elevação de panturrilha; a creatina quinase aumentou significativamente desde o dia 3 do período de exercício até o dia 5 do período de recuperação e chegou ao máximo no dia 2 de recuperação em ambos os grupos; seu valor foi mais elevado no grupo tratado versus controles; a DOR diminuiu significativamente imediatamente após o tratamento de calor; DMT atingiu o pico em ambos os grupos aos 3 dias do período de exercício e desapareceu no dia 4 do período de recuperação. Os valores de JUMP diminuíram significativamente após o exercício inicial e recuperam aos valores iniciais no Dia 4 do período de recuperação.
CONCLUSÃO:
O tratamento térmico por 20 minutos não minimizou a DMT após o exercício máximo de elevação de panturrilha, mas reduziu a dor muscular imediata.
PALAVRAS-CHAVE:
creatina quinase; salto em distância; dor muscular; dor
INTRODUCTION
It is well known that unaccustomed or strenuous eccentric exercises result in delayed onset muscle soreness (DOMS).11 Clarkson PM, Teemblay I. Exercise-induced muscle damage, repair, and adaptation in humans. J Appl Physiol 1988;65:1-6.,22 Newham DJ, McPhail G, Mills KR, Edwards RHT. Ultrastructual changes after concentric and eccentric contractions of human muscle. J Neurol Sci. 1983;61:109-22. It is first reported between 8 to 24 hours after exercise, peaks in intensity between 24 to 72 hours33 Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fiber injury. Sports Med. 1991;12(3):184-207.,44 Balnave CD, Thompson MW. Effects of training on eccentric exercise-induced muscle damage. J Appl Physiol. 1993;75(4):1545-51. and disappears by 5 days.55 Bobbert MF, Hollander AP, Huinjing PA. Factors in delayed onset muscular soreness of man. Med Sci Sports Exerc. 1986;18(1):75-81. After repeated bouts of the same exercise, the indicators of muscle damage show modest changes. This response is known as "repeated bout effect".11 Clarkson PM, Teemblay I. Exercise-induced muscle damage, repair, and adaptation in humans. J Appl Physiol 1988;65:1-6.,66 Chen TC, Gsieh SS. The effects of repeated maximal voluntary isokinetic eccentric exercise on recovery from muscle damage. RQES. 2000;71(3):260-6. Such altered response shows that the changes in the indictors of muscle damage after a second bout exercise are significantly smaller than those after the initial exercise, and the length of the adaptation effect is 3 days to 6 months to take place and to erase, respectively.77 Nosaka K, Clarkson PM, McGuiggin ME, Byrne JM. Time course of muscle adaptation after high force eccentric exercise. Eur J Appl Physiol Occup Physiol. 1996;63(1):70-6. The adaptation has been examined prior to full recovery of muscle damage indicators.66 Chen TC, Gsieh SS. The effects of repeated maximal voluntary isokinetic eccentric exercise on recovery from muscle damage. RQES. 2000;71(3):260-6.,88 Nosaka K, Newton M. Repeated eccentric exercise bouts do not exacerbate muscle damage and repair. J Strength Cond Res. 2002;16(1):117-22.
9 Sakamoto A, Maruyama T, Naito H, Sinclair PJ. Effects of exhaustive dumbbell exercise after isokinetic eccentric damage: Recovery of static and dynamic muscle performance. J Strength Cond Res. 2009;23(9):2467-76.-1010 Hiruma E, Umimura M, Naito H, Katamoto S. Effects of massage and compression treatment on performance in three consecutive days. MedicalExpress. 2014;1(6):328-35. The direct and indirect indicators of muscle damage include disruption of contractile tissue,1111 Friden J, Sjostrom M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med. 1983;4(3):170-6. cellular Ca++ accumulation,33 Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fiber injury. Sports Med. 1991;12(3):184-207. cellular infiltration,1212 Jones DA, Newham DJ, Round JM, Tolfree SEJ. Experimental human muscle damage: morphological changes in relation to other indices of damage. J Physiol. 1986;375:435-43. changes in ultrasound images,22 Newham DJ, McPhail G, Mills KR, Edwards RHT. Ultrastructual changes after concentric and eccentric contractions of human muscle. J Neurol Sci. 1983;61:109-22. release of specific muscular enzymes into the serum,11 Clarkson PM, Teemblay I. Exercise-induced muscle damage, repair, and adaptation in humans. J Appl Physiol 1988;65:1-6.
2 Newham DJ, McPhail G, Mills KR, Edwards RHT. Ultrastructual changes after concentric and eccentric contractions of human muscle. J Neurol Sci. 1983;61:109-22.
3 Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fiber injury. Sports Med. 1991;12(3):184-207.
4 Balnave CD, Thompson MW. Effects of training on eccentric exercise-induced muscle damage. J Appl Physiol. 1993;75(4):1545-51.
5 Bobbert MF, Hollander AP, Huinjing PA. Factors in delayed onset muscular soreness of man. Med Sci Sports Exerc. 1986;18(1):75-81.
6 Chen TC, Gsieh SS. The effects of repeated maximal voluntary isokinetic eccentric exercise on recovery from muscle damage. RQES. 2000;71(3):260-6.
7 Nosaka K, Clarkson PM, McGuiggin ME, Byrne JM. Time course of muscle adaptation after high force eccentric exercise. Eur J Appl Physiol Occup Physiol. 1996;63(1):70-6.
8 Nosaka K, Newton M. Repeated eccentric exercise bouts do not exacerbate muscle damage and repair. J Strength Cond Res. 2002;16(1):117-22.
9 Sakamoto A, Maruyama T, Naito H, Sinclair PJ. Effects of exhaustive dumbbell exercise after isokinetic eccentric damage: Recovery of static and dynamic muscle performance. J Strength Cond Res. 2009;23(9):2467-76.
10 Hiruma E, Umimura M, Naito H, Katamoto S. Effects of massage and compression treatment on performance in three consecutive days. MedicalExpress. 2014;1(6):328-35.
11 Friden J, Sjostrom M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med. 1983;4(3):170-6.
12 Jones DA, Newham DJ, Round JM, Tolfree SEJ. Experimental human muscle damage: morphological changes in relation to other indices of damage. J Physiol. 1986;375:435-43.-1313 Nosaka K, Clarkson PM. Muscle damage following repeated bouts of high force eccentric exercise. Med Sci Sports Exerc. 1995;27(9):1263-9. changes in voluntary isometric muscle strength and range of motion.11 Clarkson PM, Teemblay I. Exercise-induced muscle damage, repair, and adaptation in humans. J Appl Physiol 1988;65:1-6.,1313 Nosaka K, Clarkson PM. Muscle damage following repeated bouts of high force eccentric exercise. Med Sci Sports Exerc. 1995;27(9):1263-9. Skin temperature significantly increases 24 h after an exercise capable of inducing DOMS.1414 Al-Nakhli HH, Petrofsky JS, Laymon MS, Arai D, Holland K, Berk LS. The use of thermal infrared imaging to assess the efficacy of a therapeutic exercise program in indivuduals with doabetes. Diabetes Technol Ther. 2012;14(2):159-67.
Many previous studies examined prevention or attenuation of DOMS by microcurent treatment,1515 Allen JD, Mattacola CG, Perrin DH. Effects of microcurrent stimulation on delayed-onset muscle soreness: a double-blind comparison. J Athl Train. 1999;34(4):334-7. compression garment,1616 Jakeman JR, Byrne C, Eston RG. Efficacy of lower limb compression and combined treatment of manual massage and lower limb compression on symptoms of exercise-induced muscle damage in women. J Strength Cond Res. 2010;24(11):3157-65. hyperbaric oxygen,1717 Mekjavic IB, Exner JS, Tesch PA, Eiken O. Hyperbaric oxygen therapy does affect recovery from delayed onset muscle soreness. Med Sci Sports Exerc. 2000;32(3):558-63. massage,1010 Hiruma E, Umimura M, Naito H, Katamoto S. Effects of massage and compression treatment on performance in three consecutive days. MedicalExpress. 2014;1(6):328-35.,1818 Best TM HR, Wilcox A, Haq F. Effectiveness of sports massage for recovery of skeletal muscle from strenuous exercise. Clin J Sport Med. 2008;18(5):446-60.
19 Andersen LL, Jay K, Andersen CH, Jakobsen MD, Sundstrup E, Topp R, et al. Acute effects of massage or active exercise in relieving muscle soreness: randomized controlled trial. J Strength Cond Res. 2013;27(12):3352-9.-2020 Hilbert JE, Sforzo GA, Swensen T. The effects of massage on delayed onset muscle soreness. Br J Sports Med. 2003;37(1):72-5. cold water immersion2121 Glasgow PD, Ferris R, Bleakley CM. Cold water immersion in the management of delayed-onset muscle soreness: Is dose important? A randomised controlled trial. Phys Ther Sport. 2014;15(4):228-33. and ultrasound treatment.22 Newham DJ, McPhail G, Mills KR, Edwards RHT. Ultrastructual changes after concentric and eccentric contractions of human muscle. J Neurol Sci. 1983;61:109-22. Heat is an easy and popular therapeutic procedure for soft tissues damage as well as joint and pain relief.2222 Mayer JM, Mooney V, Matheson LN, Erasala GN, Verna JL, Udermann BE, et al. Continuous low-level heat wrap therapy for the prevention and early phase treatment of delayed-onset muscle soreness of the low back: a randomized controlled trial. Arch Phys Med Rehabil. 2006;87(10):1310-7.,2323 Petrofsky J, Batt J, Bollinger JN, Jensen MC, Maru EH, Al-Nakhli HH. Comparison of different heat modalities for treating delayed-onset muscle soreness in people with diabetes. Diabetes Technol Ther. 2011;13(6):645-55. The effect of heat is to increase circulation, metabolism, and elasticity of connective tissues and thereby to reduce pain.2424 Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and clod therapies for musculoskeletal injury. Postgrad Med. 2015;127(1):57-65. Heat modalities are commonly classified as superficial or deep heating agents. The popular modalities for deep heating are ultrasound and microwave diathermy. The most traditional methods of providing superficial heating are moist or dry hot packs. Moist heat penetrates deep tissues better than dry heat for warming because moist heat transfers heat faster.2323 Petrofsky J, Batt J, Bollinger JN, Jensen MC, Maru EH, Al-Nakhli HH. Comparison of different heat modalities for treating delayed-onset muscle soreness in people with diabetes. Diabetes Technol Ther. 2011;13(6):645-55. Moist heat immediately following an exercise session which is strenuous enough to induce DOMS preserves muscle strength.2525 Petrofsky J, Berk L, Bains G, Khowailed IA, Hui T, Granado M, et al. Moist Heat or Dry Heat for Delayed Onset Muscle Soreness. J Clin Med Res. 2013;5(6):416-25. However, moist heat does damage muscle fibers when applied 24 hours after the exercise.2525 Petrofsky J, Berk L, Bains G, Khowailed IA, Hui T, Granado M, et al. Moist Heat or Dry Heat for Delayed Onset Muscle Soreness. J Clin Med Res. 2013;5(6):416-25. To attenuate muscle damage, passive hyperthermia treatment by microwave diathermy applied one day before eccentric exercising exhibited a prophylactic effect.2626 Nosaka K, Muthalib M, Lavender A, Laursen PB. Attenuation of muscle damage by preconditioning with muscle hyperthermia 1-day prior to eccentric exercise. Eur J Appl Physiol. 2007;99(2):183-92.
27 Brock ST, Clasey JL, Gater DR, Yates JW. Effects of deep heat as a preventative mechanism on delayed onset muscle soreness. J Strength Cond Res. 2004;18(1):155-61.-2828 Saga N, Katamoto S, Naito H. Effect of heat preconditioning by microwave hyperthermia on human skeletal muscle after eccentric exercise. Journal of Sports Science and Medicine. 2008;7:176-83.
As previously noted after repeated strenuous exercise, the modest damage effect known as "repeated bout effect" occurs,11 Clarkson PM, Teemblay I. Exercise-induced muscle damage, repair, and adaptation in humans. J Appl Physiol 1988;65:1-6.,66 Chen TC, Gsieh SS. The effects of repeated maximal voluntary isokinetic eccentric exercise on recovery from muscle damage. RQES. 2000;71(3):260-6. but the mechanics underlying it are unclear. Repeated bouts of strenuous exercise applied to the damaged muscle exercised in the initial bout, did not influence the repair or the recovery process. As far as we know, no study has been published on the effect of heat treatment on the repair process of DOMS in continuous exercises.
It should also be noted that the efficacy of moist heating is mostly anecdotal and, to our knowledge, no controlled trials have yet been performed to determine the effects of heat pack treatment on muscle soreness. We hypothesized that in comparison to control conditions, the analgesic effects of the heat modality would reduce DOMS and maintain performance through the days of continuous exercises.
Therefore, the purpose of this study was to determine the effects of heat pack treatment on DOMS following maximum calf-raise exercises during three consecutive days.
METHODS
Subjects
Fourteen female college students (age 21.1 ± 1.3 years, height 158.22 ± 6.22cm, weight 56.29 ± 10.13kg) volunteered to participate in the present study (Table 1) and signed the informed consent documents in accordance with the ethical standards of American College of Sports Medicine and approved by Ethical Committee of Osaka International University. They had regularly performed moderate exercise, but had not practiced weight training or received supplementation for at least 6 months before the present study. They were requested to and agreed not to attend any exercise and weight training, to refrain from any ingesting of alcohol, supplements, and nonsteroidal anti-inflammatory drugs, and to avoid therapeutic treatment and stretching in the present study period. Seven randomly selected subjects received heat pack treatment immediately after the maximum calf-raise exercise (treatment group; T-group). For the control condition, seven other subjects received no treatment (control group; C-group).
Exercise bout
Subjects stood on the step board (30cm height) with both feet; the spacing between feet was as wide as shoulder width. Both forefeet were on the step board, with midfoot and heels in the air. Subjects performed the maximum calf raise exercise at 1 movement per 3 seconds until they could not maintain speed of movement. During the first second of each movement, the subjects raised their heels as high as possible and positioned the ankles at maximal planterflexion and supination. During the next two seconds, the subjects lowered the heels so that they ended at a level lower than the toes, in order to allow a greater stretch of the working muscles. The movement was desgned to control the speed of the eccentric contraction during lowering the heels. Both groups exercised for three days consecutively (Exercise-period). During the seven days of recovery period (Recovery-period), T-group participants were measured before massaging session.
Treatment
The T-group subjects (in prone position) were treated with 20 minutes of heat pack (40ºC). Heat was applied by placing heat pack on each calf muscle and wrapping with elastic bandage. To place the cushion under the lower leg, the foot was kept risen by 20 cm during treatment.
Measurements
In order to identify DOMS, the criterion measurements consisted of creatine-kinase activity, maximum circumference of calf muscle (CIR), maximum voluntary isometric muscle contraction (MVC), perceived pain (PAIN), one-leg long jumping (JUMP) and dorsiflexed ankle joint angles (D-flex) before (pre-exercise) and immediately after treatment for T-group, immediately before and after the exercise for the C-group. Measurements were taken on each of the three days of the Exercise-period and at 24 hour intervals for the 7 days following Exercise-periods (Recovery-period). Both groups visited the laboratory between 11:00 AM and 1:00 PM for exercise and/or measurements. The subjects recorded and reported the number of walking steps per day using a step counter (Citizen Co. Ltd., Tokyo, Japan).
Creatine Kinase
Plasma CK activity was analyzed using the Reflotron System (Yamanouchi Pharmaceutical Co.Ltd., Tokyo, Japan). 50µL of whole blood was sampled from a fingertip into heparinized glass capillary before and immediately after each exercise period at daily intervals during the three days in the exercise period in the seven days in recovery period. The collected whole blood sample was dropped onto Reflotron CK analysis sheet (Roche Diagnostics K.K., Tokyo, Japan). All samples were analyzed in duplicate (correlation coefficient > 0.9).
Circumference
The investigators measured the subject's largest lower leg circumference using a constant tension tape while the subjects stood with both feet on the table. In the initial measurement, the investigator marked the regions where measurements were made in each leg in order to measure the same region throughout this study. Measurement points were remarked throughout the study to insure precision of measuring sites. The mean value of the best circumference on each leg was calculated and used for further analysis
Maximal voluntary isometric muscle contraction
Maximal Gastrocnemius muscular strength was measured using a Tension meter (Takei Scientific Instruments Co., Ltd, Niigata, Japan) before exercise and immediately after treatment for T-group, and before and after exercise for C-group during the exercise-period and daily during the recovery period. The subject was placed on supine position and firmly attached with a belt to the bed. The attachment cable of Tension meter was placed to the subject's forefoot with 90 degree of the ankle. During measurement, both arms were crossed over the chest. Subjects were asked to sustain maximal effort for 10 seconds. The resting interval between maximal isometric contractions was 1 minute. The best of two maximal isometric plantarflexion contractions of each leg was taken, and the average value of the maximal value for each leg was used as an value of MVC.
Scale of perceived pain
Perceived pain (PAIN) on Gastronomius muscle during resting and walking were evaluated by visual analog scale (VAS) that had a 100-mm line with "no pain" on one end (0 mm) and "extremely painful" on the other end (100 mm). Subjects marked their subjective scale of soreness on the line under the supervision of the examiner. The length of the line from 0 to the marked point provided a numeric measure of soreness. PAIN was evaluated before and immediately after treatment or exercise during the exercise-period and daily during the recovery period.
One-leg long jumping
The average of two long jumps by each leg was used to assess long jump performance. On the floor, the subjects performed the long jump with one leg twice; the rest between measurements was 1 minute. Subjects were encouraged to use their arms during the jump. The subjects needed to land on the floor with both feet. The shortest distance from the starting line was measured. The average distance of the best jump for each leg was used as an indicator of the one-leg long jump performance.
Range of motion
The investigators fully dorsiflexed the subject's foot and measured the angle of ankle using tractograf. The measures of ankle joint angle were taken twice while the subjects fell prone on the table with extended knee. The maximum angle each leg was recorded and the average angle was used for analysis.
Statistical analysis
Data were analyzed using a two-way ANOVA. When ANOVA showed a significant effect, Turkey's post hoc test was used to identify the differences between each time point. Coefficients of correlation were calculated using Person Product Moment Correlation for each data. All analyses were performed using Statistical analysis was using SYSTAT (version 11;SYSTAT software, Inc., Richmond, CA). Statistical significance was set at p < 0.05.
RESULTS
General
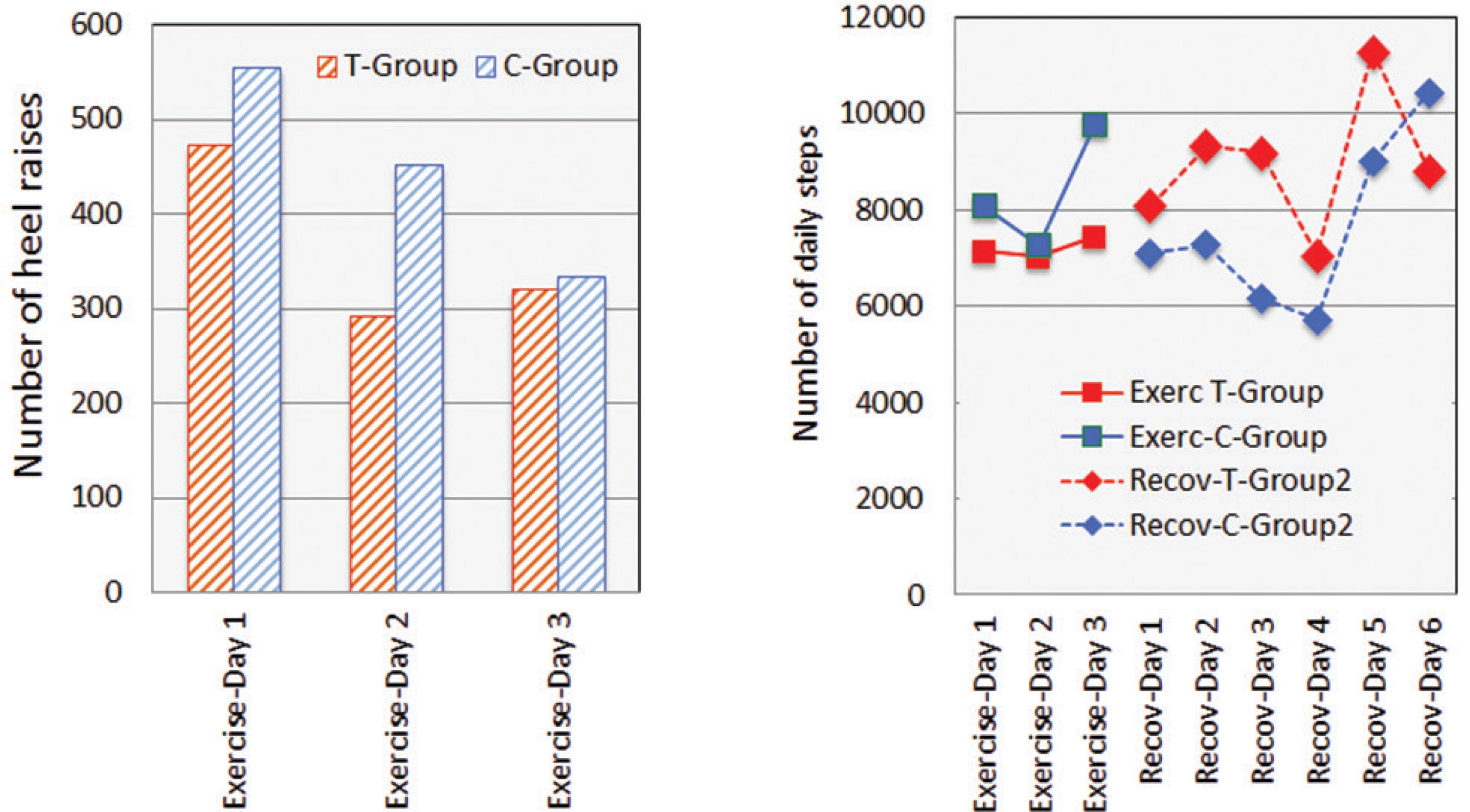
The number of Calf-raise repetitions on Day 2 and Day 3 decreased gradually as compared with the initial exercise. On Day 3, the number of Calf-raise repetitions in C-group was less than T-group as shown in Figires 1 and 2.
Stepping number of Calf raise exercise and changes in creatine kinase(CK) before and after maximum Calf raise exercise for 3 days of exercise period and for 7 days of recovery period. Values are means (± SD). * p < 0.05, ** p < 0.01,*** p < 0.001: significantly different from Day 1; Pre-Ex. # p < 0.05, ##p < 0.01 significantly different between T-group and C-group in each measurement.
Stepping number of Calf raise exercise and stepping number of daily walking after maximum Calf raise exercise for 3 days of exercise period and for 7 days of recovery period. Values are means (± SD).
Creatine-kinase
Figure 1 shows that CK levels in T-group progressively increased in every measurement from Day 3 pre-exercise to day 2 in the Recovery period, and remained significantly higher vs Pre-exercise through Day 5 in the recovery period (p < 0.01-0.05). CK levels in C-group increased moderately in days 1 through 3 in the recovery period. There was no significant relationship between CK and number of exercise both groups. The levels in T-group in each day were consistently higher than in the C-group. There were significant differences between the two groups on Day 3 in Exercise-period to Day 3 in Recovery-period (p < 0.01-0.05). The peak CK levels for both groups occurred on Day 2 in the recovery period.
Number of Calf-raise repetition and daily walking steps
As shown in Figure 2, there were no significant differences on the number of Calf-raise repetition and on the daily walking steps between two groups. There were no significant relationships between the number of Calf-raise repetition and the number of daily walking steps, which were counted from after each exercise to before the next one. T-group subjects walked more than C-group subjects, but the differences were no significant. The daily walking activity did not affect the number of the Calf-raise repetitions in the following day.
Circumference
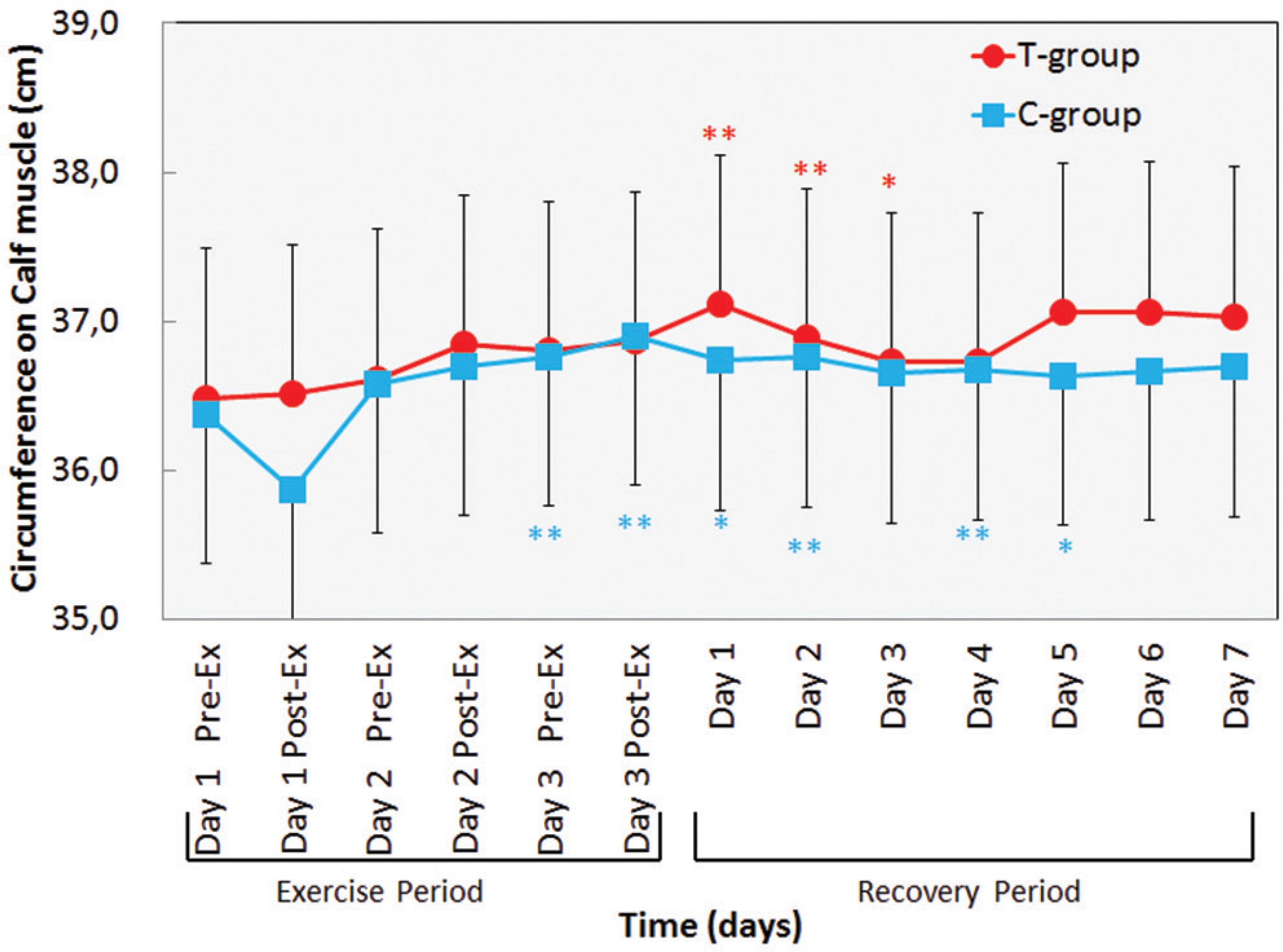
As shown in Figure 3, calf circumference in the T-group and C-group significantly increased on Day 3 in Exercise-period to Day 2 in the recovery period (p < 0.01-0.05). There were no significant differences between C-group and T-group.
Changes in circumference in lower leg before and after daily maximum Calf raise exercise for 3 days of exercise period and for 7 days of recovery period. Values are means(±SD). * p < 0.05; ** p < 0.01: significantly different from Day 1 Pre-Ex.
Perceived pain
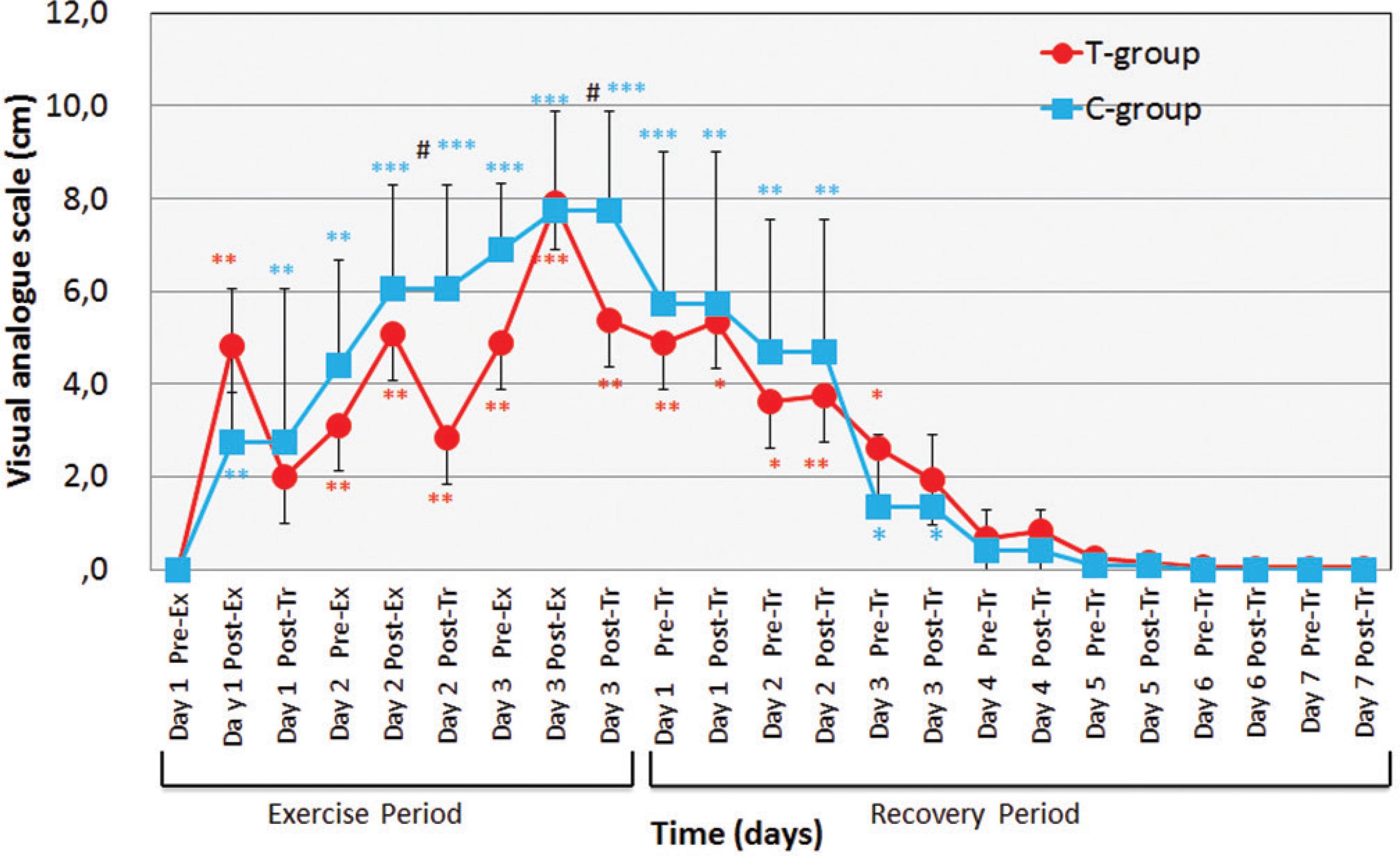
As can be seen in Figure 4, PAIN values in both groups increased significantly during the exercise period, from Day 1 through Day 3. Peak values were reached in Day 3, post-exercise for T-group and for C-group. During the recovery period, PAIN dropped progressively returning to zero by day four of recovery for both groups. However, during this entire phase PAIN was significantly more intense in C-group.
Changes in perceived pain scale for 3 days of exercise period and 7 days of recovery period. Values are means(±SD). * p < 0.05; ** p < 0.01, *** p < 0.001: bsignificantly different from Day 1 Pre-Exercise. # p < 0.05, ## p < 0.01: significantly different between T-group and C-group in each measurement.
PAIN values in T-group significantly increased from Day 1 post-Ex in Exercise-period to Day 4 in Recovery-period (p < 0.01-0.05), and those in C-group significantly and progressively increased from Day 1 post-Ex to Day 3 (p < 0.001-0.01). PAIN values in T-group in each measurement were lower as compared with those in C-group during the present study. T-group after heat treatment decreased PAIN from before treatment during Exercise-period. There were, also, significant differences between C-group and T-group at post-treatment in Exercise-period (p < 0.01).
One-leg jumping
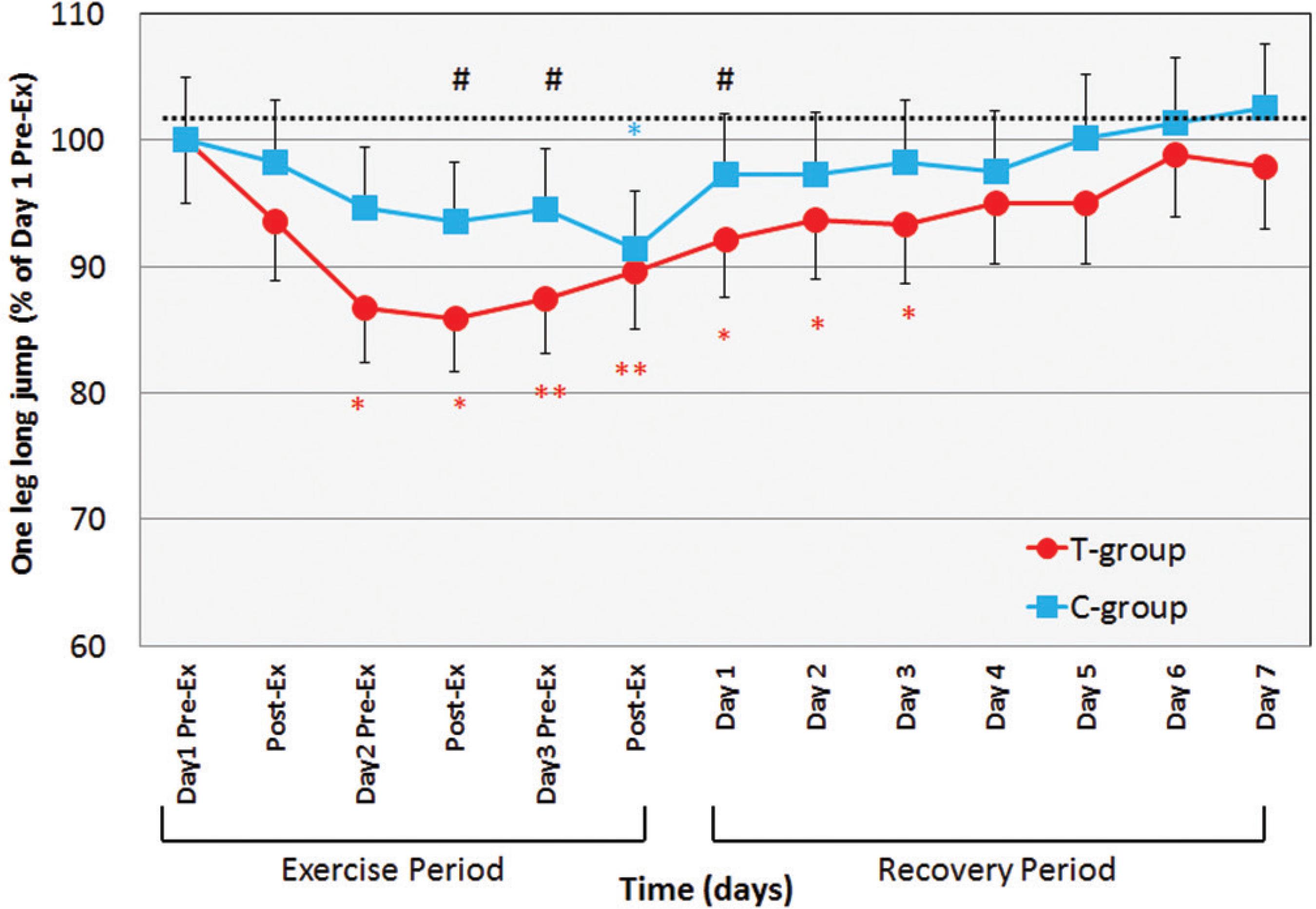
One-leg jumping decreased very slightly during the exercise period, reaching significance versus initial values in the last three measurements for the treated group (Figure 5). During the recovery period, one-leg jumping recovered gradually in both groups, compared to baseline. JUMP values in the T-group decreased significantly from Day 3 pre-Ex to Day 3 and returned on the fourth day of Recovery-period (p < 0.01-0.05). In those days, T-group values were significantly lower than C-group (p < 0.05).
Changes in one-leg long jumping before and after daily maximum Calf raise exercise for 3 days of exercise period and for 7 days of recovery period. Values are means(±SD). * p < 0.05, ** p < 0.01: significantly different from Day 1 Pre-Exercise. #p < 0.05: significantly different between T-group and C-group in each measurement.
Range of motion (ROM) and maximal voluntary isometric muscle contraction (MVC)
Both group slightly decreased ROM (dorsiflexed ankle joint angle) and MVC, but there were no significant differences in MVC and ROM in this study.
DISCUSSION
Heat modality is a common physical therapy treatment which raises the temperature of a given tissue to between 41.5ºC and 45ºC with microwave diathermy and maintains it in this range for a period of time.2929 Guy A. W., Lehmann JF, Stonebrigde JB. Therapeutic application of electromagnetic power. Proc IEEE. 1974;62(1):65-75. In this study, heat pack treatment was at 40ºC for 20 minutes which is the usual form of warm therapy. This treatment is commonly and easily used. Thus, this result is useful information to the general public.
The first finding in this study was to identify the effect of heat pack treatment immediately after strenuous exercise on DOMS. T-group with heat pack treatment increased CK and delayed the recovery from DOMS as compared with C-group. There was no significant difference of circumference between two groups. However, the heat pack treatment immediately after the strenuous exercise decreased PAIN. This result is supported by Petrofsky's study2323 Petrofsky J, Batt J, Bollinger JN, Jensen MC, Maru EH, Al-Nakhli HH. Comparison of different heat modalities for treating delayed-onset muscle soreness in people with diabetes. Diabetes Technol Ther. 2011;13(6):645-55. regarding the attenuation of PAIN. They concluded that muscle soreness following exercise was greatest in people with diabetes, but was reduced by 20 minutes of moist heat.2323 Petrofsky J, Batt J, Bollinger JN, Jensen MC, Maru EH, Al-Nakhli HH. Comparison of different heat modalities for treating delayed-onset muscle soreness in people with diabetes. Diabetes Technol Ther. 2011;13(6):645-55. The most important physiological responses induced by heat therapy are a regional increase in blood flow and the relief of pain. A previous study3030 Trowbridge CA, Hopkins DO, Feland JB, Jutte LS, Eggett DL. Paraspinal musculature and skin temperature changes: comparing the Thermacare Heat Wrap, the Johnson & Johnson Back Plaster, and ABC Warme-Pflaster. J Orthop Sports Phys Ther. 2004;34(9):549-58. concluded that hyperthermia, which transfers heat to deep tissue, caused pain relief through what is know as the gate control theory of pain within the central nervous system and not through an action on the peripherical nervous system. Hyperthermia increases blood perfusion and increases capillary permeability.3131 Giombini A, Giovannini V, Di Cesara A, Pacetti P, Ichinoseki-Sekine N, Shiraishi M, et al. Hyperthermia induced by microwave diathermy in the management of muscle and tendon injury. Br Med Bull. 2007;83(3):379-96. This event could potentially alter delivery and removal of substances essential for energy metabolism and allow the entrance and activation of macrophages and granulocytes to the affected area.3232 Song CW. Effect of local hyperthermia on blood flow and microenvironment: a review. Cancer Res. 1984;44(10 Suppl):4721s-30s.
There are two alternate procedures of heat treatment: (i) hyperthermia modality, through microwave diathermy, shortwave diathermy, and ultrasound, which may be applied to reach therapeutic values from 1 to 4 cm below the skin surface; (ii) moist heat modality in which hot packs increase the temperature of the skin.2929 Guy A. W., Lehmann JF, Stonebrigde JB. Therapeutic application of electromagnetic power. Proc IEEE. 1974;62(1):65-75. Previous studies showed that 15 minutes of shortwave diathermy, which could heat up a larger treatment area and volume of tissue than ultrasound, increased the temperature of soft tissue by 4.58 ± 0,87 ºC at a depth of 3 cm.3333 Draper DO, Knight K, Fujikawa T, Castel CJ. Temperature change in human muscle during and after pulsed short-wave diathermy. J Orthop Sports Phys Ther. 1999;29(1):13-22. Moist heat pack (hot pack) treatment elevated muscle temperature by 1ºC.3434 Minton J. A comparison of thermotherapy and cryotherapy in enhancing supine, extended-leg, hip flexion. J Athl Train. 1993;26(2):174-6. The moist heat modality appears to be advantageous in fast penetration and pain relief during 20 minutes, but the short duration of dry heat application resulted in poor heat transfer to deep tissues.3535 Petrofsky JS, Laymon MS. Heat transfer to deep tissue: the effect of body fat and heating modality. J Med Eng Technol. 2009;33(5):337-48. Petrofsky et al. concluded that individuals with a thick layer of subcutaneous fat exhibited small differences in deep tissue temperature with moist heat modality.3535 Petrofsky JS, Laymon MS. Heat transfer to deep tissue: the effect of body fat and heating modality. J Med Eng Technol. 2009;33(5):337-48. The subjects included in our study, with 26% of body fat, had less subcutaneous fat as compared with subjects in Petrofsky's study. The T-group in our study might transfer heat to exercised muscles because the significantly increased CK values were representative of muscle damage as compared with the C-group.
The last finding in this study was the effective prevention of the decrease in isometric muscle strength and flexibility in the participants that received moist heat to the exercised muscles. A previous study2525 Petrofsky J, Berk L, Bains G, Khowailed IA, Hui T, Granado M, et al. Moist Heat or Dry Heat for Delayed Onset Muscle Soreness. J Clin Med Res. 2013;5(6):416-25. showed that the greatest preservation of strength after two hours of moist heat or eight hours of dry heat on low back after eccentric exercise was related to increased blood flow to deep tissues for a long period of time. However, the two groups in our study exhibited a preventive effect on strength and flexibility of calf muscles, even though the T-group received heat for 20 minutes, while the C-group did not. Both groups maintained elevated blood flow on calf muscles by walking. This walking may allow for better healing of the tissue damage caused by strenuous exercise.
CONCLUSION
The result of this investigation suggests that
(1) heat pack treatment for 20 minutes did not minimize DOMS following the maximum calf-raise exercise.
(2) the muscle soreness immediately after heat pack treatment was decreased as compared with before heating.
AKNOWLEDGEMENT
This project received no external funding. The authors have no relationship with any company or manufacturers. The corresponding author, Eisuke Hiruma, supported this work.
-
Hiruma E, Uchida M, Sasaki H, Umimura M. Heat pack treatment does not attenuate repeated muscle damage in collegiate females. MedicalExpress (São Paulo, online). 2015:2(6):M150607
REFERENCES
-
1Clarkson PM, Teemblay I. Exercise-induced muscle damage, repair, and adaptation in humans. J Appl Physiol 1988;65:1-6.
-
2Newham DJ, McPhail G, Mills KR, Edwards RHT. Ultrastructual changes after concentric and eccentric contractions of human muscle. J Neurol Sci. 1983;61:109-22.
-
3Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fiber injury. Sports Med. 1991;12(3):184-207.
-
4Balnave CD, Thompson MW. Effects of training on eccentric exercise-induced muscle damage. J Appl Physiol. 1993;75(4):1545-51.
-
5Bobbert MF, Hollander AP, Huinjing PA. Factors in delayed onset muscular soreness of man. Med Sci Sports Exerc. 1986;18(1):75-81.
-
6Chen TC, Gsieh SS. The effects of repeated maximal voluntary isokinetic eccentric exercise on recovery from muscle damage. RQES. 2000;71(3):260-6.
-
7Nosaka K, Clarkson PM, McGuiggin ME, Byrne JM. Time course of muscle adaptation after high force eccentric exercise. Eur J Appl Physiol Occup Physiol. 1996;63(1):70-6.
-
8Nosaka K, Newton M. Repeated eccentric exercise bouts do not exacerbate muscle damage and repair. J Strength Cond Res. 2002;16(1):117-22.
-
9Sakamoto A, Maruyama T, Naito H, Sinclair PJ. Effects of exhaustive dumbbell exercise after isokinetic eccentric damage: Recovery of static and dynamic muscle performance. J Strength Cond Res. 2009;23(9):2467-76.
-
10Hiruma E, Umimura M, Naito H, Katamoto S. Effects of massage and compression treatment on performance in three consecutive days. MedicalExpress. 2014;1(6):328-35.
-
11Friden J, Sjostrom M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med. 1983;4(3):170-6.
-
12Jones DA, Newham DJ, Round JM, Tolfree SEJ. Experimental human muscle damage: morphological changes in relation to other indices of damage. J Physiol. 1986;375:435-43.
-
13Nosaka K, Clarkson PM. Muscle damage following repeated bouts of high force eccentric exercise. Med Sci Sports Exerc. 1995;27(9):1263-9.
-
14Al-Nakhli HH, Petrofsky JS, Laymon MS, Arai D, Holland K, Berk LS. The use of thermal infrared imaging to assess the efficacy of a therapeutic exercise program in indivuduals with doabetes. Diabetes Technol Ther. 2012;14(2):159-67.
-
15Allen JD, Mattacola CG, Perrin DH. Effects of microcurrent stimulation on delayed-onset muscle soreness: a double-blind comparison. J Athl Train. 1999;34(4):334-7.
-
16Jakeman JR, Byrne C, Eston RG. Efficacy of lower limb compression and combined treatment of manual massage and lower limb compression on symptoms of exercise-induced muscle damage in women. J Strength Cond Res. 2010;24(11):3157-65.
-
17Mekjavic IB, Exner JS, Tesch PA, Eiken O. Hyperbaric oxygen therapy does affect recovery from delayed onset muscle soreness. Med Sci Sports Exerc. 2000;32(3):558-63.
-
18Best TM HR, Wilcox A, Haq F. Effectiveness of sports massage for recovery of skeletal muscle from strenuous exercise. Clin J Sport Med. 2008;18(5):446-60.
-
19Andersen LL, Jay K, Andersen CH, Jakobsen MD, Sundstrup E, Topp R, et al. Acute effects of massage or active exercise in relieving muscle soreness: randomized controlled trial. J Strength Cond Res. 2013;27(12):3352-9.
-
20Hilbert JE, Sforzo GA, Swensen T. The effects of massage on delayed onset muscle soreness. Br J Sports Med. 2003;37(1):72-5.
-
21Glasgow PD, Ferris R, Bleakley CM. Cold water immersion in the management of delayed-onset muscle soreness: Is dose important? A randomised controlled trial. Phys Ther Sport. 2014;15(4):228-33.
-
22Mayer JM, Mooney V, Matheson LN, Erasala GN, Verna JL, Udermann BE, et al. Continuous low-level heat wrap therapy for the prevention and early phase treatment of delayed-onset muscle soreness of the low back: a randomized controlled trial. Arch Phys Med Rehabil. 2006;87(10):1310-7.
-
23Petrofsky J, Batt J, Bollinger JN, Jensen MC, Maru EH, Al-Nakhli HH. Comparison of different heat modalities for treating delayed-onset muscle soreness in people with diabetes. Diabetes Technol Ther. 2011;13(6):645-55.
-
24Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and clod therapies for musculoskeletal injury. Postgrad Med. 2015;127(1):57-65.
-
25Petrofsky J, Berk L, Bains G, Khowailed IA, Hui T, Granado M, et al. Moist Heat or Dry Heat for Delayed Onset Muscle Soreness. J Clin Med Res. 2013;5(6):416-25.
-
26Nosaka K, Muthalib M, Lavender A, Laursen PB. Attenuation of muscle damage by preconditioning with muscle hyperthermia 1-day prior to eccentric exercise. Eur J Appl Physiol. 2007;99(2):183-92.
-
27Brock ST, Clasey JL, Gater DR, Yates JW. Effects of deep heat as a preventative mechanism on delayed onset muscle soreness. J Strength Cond Res. 2004;18(1):155-61.
-
28Saga N, Katamoto S, Naito H. Effect of heat preconditioning by microwave hyperthermia on human skeletal muscle after eccentric exercise. Journal of Sports Science and Medicine. 2008;7:176-83.
-
29Guy A. W., Lehmann JF, Stonebrigde JB. Therapeutic application of electromagnetic power. Proc IEEE. 1974;62(1):65-75.
-
30Trowbridge CA, Hopkins DO, Feland JB, Jutte LS, Eggett DL. Paraspinal musculature and skin temperature changes: comparing the Thermacare Heat Wrap, the Johnson & Johnson Back Plaster, and ABC Warme-Pflaster. J Orthop Sports Phys Ther. 2004;34(9):549-58.
-
31Giombini A, Giovannini V, Di Cesara A, Pacetti P, Ichinoseki-Sekine N, Shiraishi M, et al. Hyperthermia induced by microwave diathermy in the management of muscle and tendon injury. Br Med Bull. 2007;83(3):379-96.
-
32Song CW. Effect of local hyperthermia on blood flow and microenvironment: a review. Cancer Res. 1984;44(10 Suppl):4721s-30s.
-
33Draper DO, Knight K, Fujikawa T, Castel CJ. Temperature change in human muscle during and after pulsed short-wave diathermy. J Orthop Sports Phys Ther. 1999;29(1):13-22.
-
34Minton J. A comparison of thermotherapy and cryotherapy in enhancing supine, extended-leg, hip flexion. J Athl Train. 1993;26(2):174-6.
-
35Petrofsky JS, Laymon MS. Heat transfer to deep tissue: the effect of body fat and heating modality. J Med Eng Technol. 2009;33(5):337-48.
Publication Dates
-
Publication in this collection
Dec 2015
History
-
Received
27 Aug 2015 -
Reviewed
10 Sept 2015 -
Accepted
20 Oct 2015