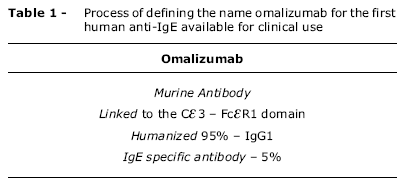
OBJECTIVES: To report on the pharmacology, efficacy and safety of omalizumab , a new option for the treatment of asthma and allergic diseases and the first monoclonal anti-IgE antibody approved for clinical use. SOURCES: MEDLINE, a non-systematic search including reviews and original papers, chosen according to their relevance in the authors' opinion. SUMMARY OF THE FINDINGS: The paper emphasizes the central role IgE plays in allergic diseases and the biological rationale for its use, the evidence upon which the current recommendations for the use of anti-IgE in uncontrolled asthma are based and its possible future applications, in addition to the recommendation that in clinical practice doses must be adjusted for weight and serum IgE levels. Omalizumab was approved in Brazil for patients with severe uncontrolled asthma presenting with a positive skin prick test for one or more relevant aeroallergen, or IgE specific to a relevant allergen detected in serum, having a total IgE level of between 30 and 700 UI/mL. For the time being its use should be restricted to patients aged 12 years or more, but there are prospects that it will be licensed for use with children over 6 years old. CONCLUSIONS: Some severe asthma cases cannot be controlled with the regular treatment options aimed at preventing symptoms and exacerbations, and so require frequent or prolonged use of systemic corticosteroids. These patients may benefit from treatment with anti-IgE, after a meticulous reevaluation of possible reasons for the failure to control asthma.
Omalizumab; anti-IgE; asthma; severe asthma; allergic rhinitis; atopic dermatitis