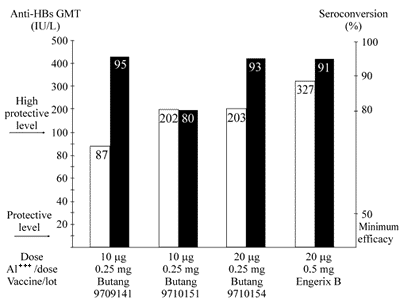
Recombinant yeast-derived hepatitis B vaccine manufactured by Instituto Butantan was administered in two groups of adult volunteers (I, II) following two different schedules of immunization. In the first trial (10 <FONT FACE="Symbol">m</FONT>g doses and 0, 1, 3 months vaccination schedule) 106 individuals completed the full immunization program. The results of seroconversion by age group varied from 70 to 100% and the GMT from 46.5 to 124.9 mIU mL-1. In the second trial with 68 individuals (for dosage comparison and 0, 1, 6 months vaccination schedule) indicated that the vaccine formulated in 20 <FONT FACE="Symbol">m</FONT>g was more effective than in 10 <FONT FACE="Symbol">m</FONT>g. The adverse reactions observed in the vaccinees were less frequent than the ones previously found since the introduction of similar vaccines.
Hepatitis B vaccine; Clinical trials