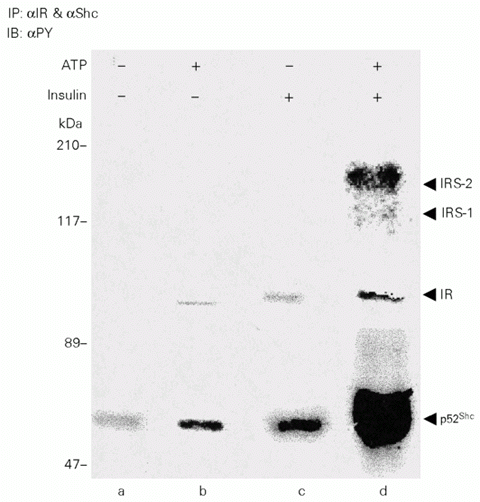
Insulin induces tyrosine phosphorylation of Shc in cell cultures and in insulin-sensitive tissues of the intact rat. However, the ability of insulin receptor (IR) tyrosine kinase to phosphorylate Shc has not been previously demonstrated. In the present study, we investigated insulin-induced IR tyrosine kinase activity towards Shc. Insulin receptor was immunoprecipitated from liver extracts, before and after a very low dose of insulin into the portal vein, and incubated with immunopurified Shc from liver of untreated rats. The kinase assay was performed in vitro in the presence of exogenous ATP and the phosphorylation level was quantified by immunoblotting with antiphosphotyrosine antibody. The results demonstrate that Shc interacted with insulin receptor after infusion of insulin, and, more important, there was insulin receptor kinase activity towards immunopurified Shc. The description of this pathway in animal tissue may have an important role in insulin receptor tyrosine kinase activity toward mitogenic transduction pathways.
tyrosine kinase activity; insulin receptor; Shc; insulin action