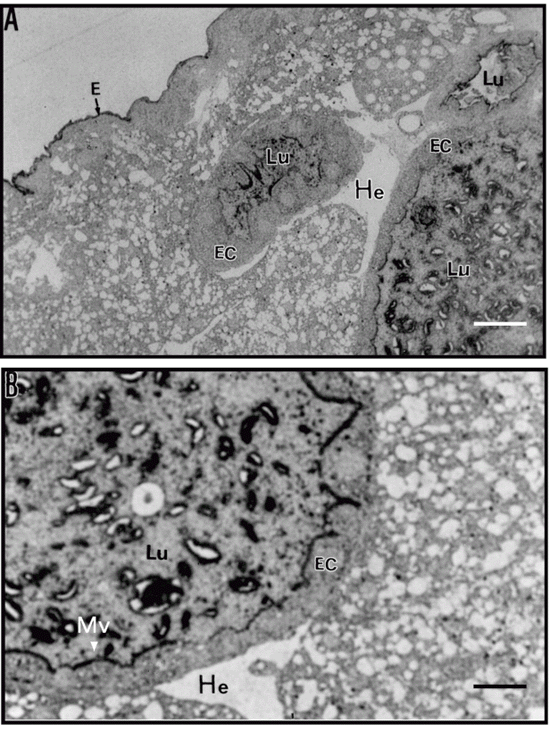
The presence of chitin in midgut structures of Callosobruchus maculatus larvae was shown by chemical and immunocytochemical methods. Detection by Western blotting of cowpea (Vigna unguiculata) seed vicilins (7S storage proteins) bound to these structures suggested that C. maculatus-susceptible vicilins presented less staining when compared to C. maculatus-resistant vicilins. Storage proteins present in the microvilli in the larval midgut of the bruchid were recognized by immunolabeling of vicilins in the appropriate sections with immunogold conjugates. These labeling sites coincided with the sites labeled by an anti-chitin antibody. These results, taken together with those previously published showing that the lower rates of hydrolysis of variant vicilins from C. maculatus-resistant seeds by the insect's midgut proteinases and those showing that vicilins bind to chitin matrices, may explain the detrimental effects of variant vicilins on the development of C. maculatus larvae.
bruchid beetles; Callosobruchus maculatus; cowpea seeds; Vigna unguiculata; vicilins (7S storage proteins); immunolocalization; midgut