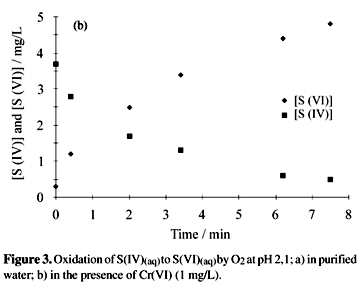
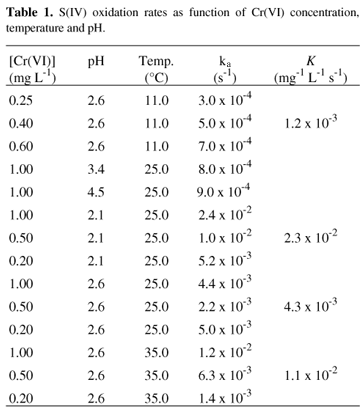
Catalytic effect of metal ions: Cr(VI), Cr(III), Cd(II), V(V) and chloride anion, on the oxidation of S(IV) in aqueous solution, at concentrations of metal ions and S(IV) usually found in urban atmospheres, were studied under controlled experimental conditions (pH (2.1 - 4,5), T (25.0 - 35.0 °C), air flow rate, concentration of reactants, etc...). The kinetic constant determined at 25.0 °C and pH range (2.1 - 4.5), using ultra pure water was 8.0 ± 0.5 x 10-4 s-1. This value was considered as a reference for the oxidation reaction rate. The kinetic constants determined in the presence of Cr(VI) revealed that the oxidation reaction of S(IV) is quite influenced by the acidity. At pH = 2.1 (K = 2.3 x 10-2 mg-1 L s-1) the reaction is carried out with a rate five times greater when compared to pH = 2.6 (K = 4.3 x 10-3 mg-1 L s-1) and thirty times greater when compared to pH = 3.4 (K= 8.0 x 10 -4 mg-1 L s-1). The following rate expression was obtained at pH = 2.6: -r(S(IV) =K [Cr(VI)] [S(IV)] and the activation energy found was: Ea =70.3KJ/mol. No catalytic effects were observed for Cd(II) or chloride ion, while inhibitory effects were observed for Cr(III) and V(V) ions.
catalysis; aqueous S(IV) oxidation; transition metals