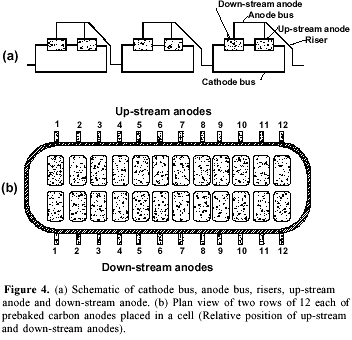
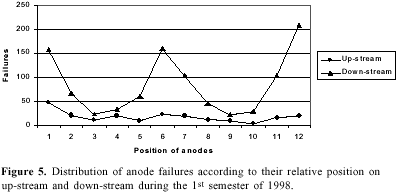
Studies on the electrolysis of cryolite-alumina melt showed that even the best equipped smelter, functioning at 960 ºC, has only 33% energy efficiency. For stable functioning of the smelter at 3% alumina the minimum wt% ratio NaF/AlF3 was found to be 1.11. The anodes located on down-stream row were found to face more turbulence; raising their level by 4 cm resulted in decreasing the number of burnoffs. A careful selection of potlining material improved the pot life. The stability of the aluminum-bath interface is one of the major factors affecting the current efficiency. An improved cell design has been proposed to achieve the ultimate aluminum-bath interface stability. The proposed cell design should allow a reduction of the cathode-to-anode distance producing a lower voltage and improving the power efficiency.
aluminum electrowinning; Hall-Heroult process; electroreduction