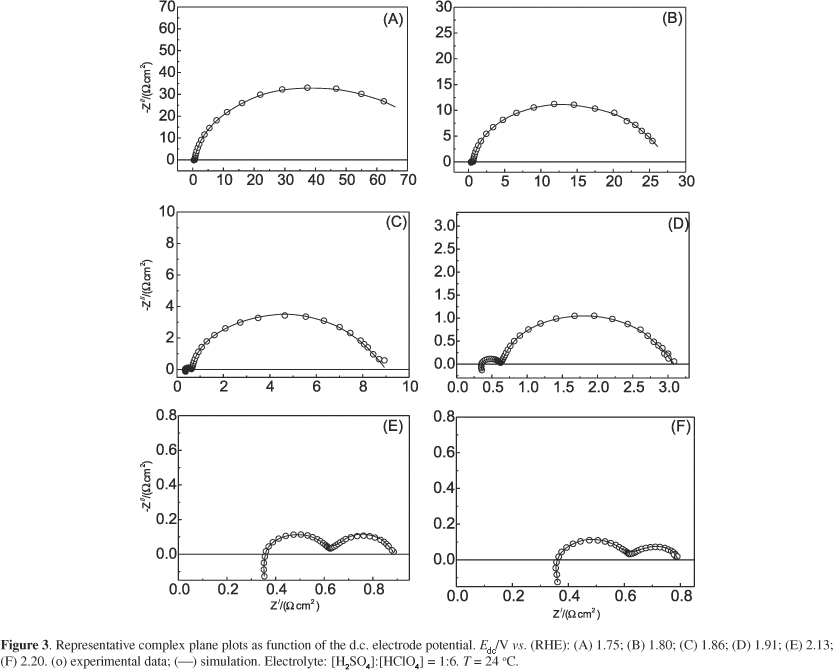
The influence of electrolyte composition (pure sulphuric or perchloric acid and their mixtures) on the kinetics of the oxygen evolution reaction (OER) and the electrochemical ozone production (EOP) on beta-PbO2 electrodes was investigated by electrochemical impedance spectroscopy and quasi-stationary polarisation curves. Analysis of the impedance spectra revealed a dependency of the electric double layer capacity and the adsorption pseudocapacitance, due to reaction intermediates, on electrolyte composition. The pseudocapacitance is a function of electrode potential and electrolyte composition, indicating the surface coverage by the reaction intermediates depends on these parameters. Tafel slope data support primary water discharge as rate determining step of the OER and EOP electrode processes. EOP current efficiency data (phiEOP) show pure electrolytes lead to maximum O3 production when compared to mixed electrolytes. According to the theoretical analysis of the electrode mechanism, this behaviour is a consequence of the reduction in surface concentration of the active centres leading to EOP caused by anion adsorption.
impedance spectroscopy; ozone production; oxygen evolution; kinetics; mixed electrolytes