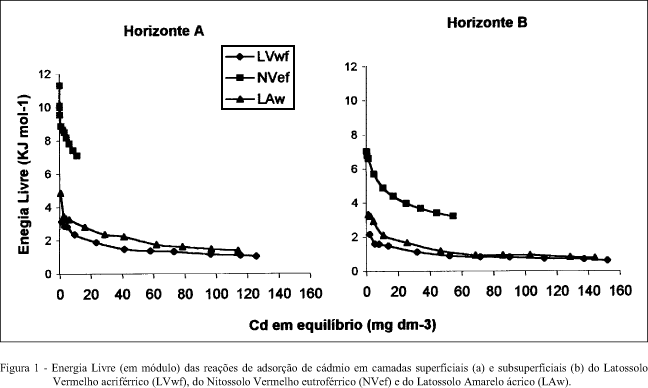
O cádmio é um metal pesado que pode ser adicionado ao solo por meio do lixo urbano ou industrial, lodo de esgoto e fertilizantes fosfatados. É facilmente absorvido e translocado pelas plantas, tendo potencial de entrar na cadeia alimentar humana. A persistência e a mobilidade do cádmio no solo é determinada pela intensidade da adsorção pelos colóides do solo. A avaliação da energia livre de adsorção de um elemento ao solo pode servir como medida da força da reação. Estudou-se a energia livre da reação de adsorção de cádmio em amostras superficiais (0-0,2m) e subsuperficiais (na maior expressão do horizonte B) de um Nitossolo Vermelho eutroférrico, um Latossolo Vermelho acriférrico e um Latossolo Amarelo ácrico, após a adição de 5, 10, 15, 25, 50, 75, 100, 125, 150, 175 e 200mg L-1 de cádmio. A adsorção de cádmio pelos solos foi espontânea, pois a energia livre apresentou valores negativos em todas as concentrações estudadas. Os valores de energia livre diminuíram com o aumento da dose de cádmio adicionada. O Nitossolo apresentou maior energia livre do que os Latossolos, sobretudo na camada superficial. Os horizontes superficiais apresentaram maior energia livre para as reações de adsorção de cádmio dos que os subsuperficiais, provavelmente devido ao efeito da matéria orgânica, que apresenta alta afinidade pelo elemento.
material orgânica; óxido de ferro; ponto de efeito salino nulo