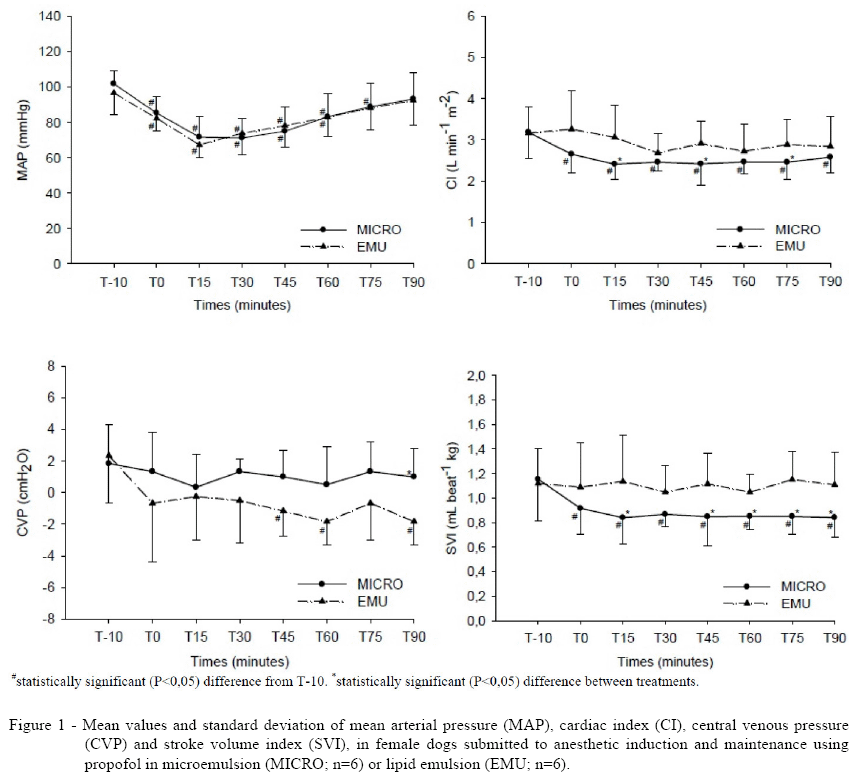
This research aimed to evaluate the clinical and cardiorespiratory effects of a propofol formulation with nanometer droplet diameter in dogs. Six adult healthy female dogs weighing 14.8±1.2kg were used in this study. Each dog received two treatments with a 15-day washout period. A microemulsion (MICRO) or lipid emulsion (EMU) of propofol was administered intravenously (IV) for induction and maintenance of anesthesia. Anesthesia was maintained with a constant rate infusion of propofol (0.4mg kg-1 minute-1). Cardiorespiratory variables were recorded before induction (baseline), immediately after and at 15-minute intervals for 90 minutes after treatment. Arterial blood samples were also taken for blood gas analysis, except at 45 and 75 minutes after induction. The mean arterial pressure decreased significantly during both treatments, while the cardiac index decreased significantly only in MICRO treatment. The time to extubation, sternal recumbency, ambulation and total recovery was similar in both treatments. Opisthotonos was observed in 33% of the animals in each treatment. The propofol microemulsion presented clinical and respiratory parameters similar to those obtained with the lipid emulsion commercially available, but had some significantly different hemodynamic characteristics when used for inducing and maintaining anesthesia. Based only in these results, no advantages are seen in the use of this new microemulsion.
propofol; microemulsion; lipid emulsion; cardiorespiratory; dogs