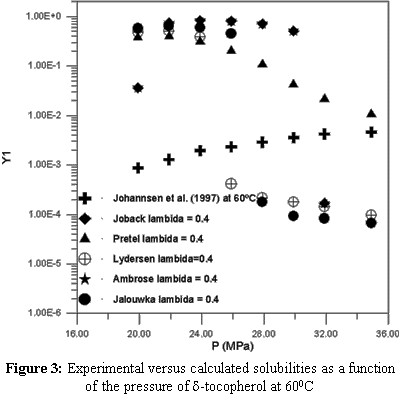
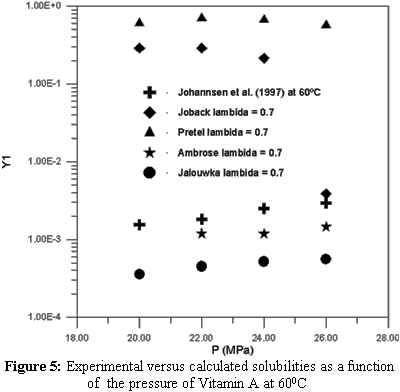
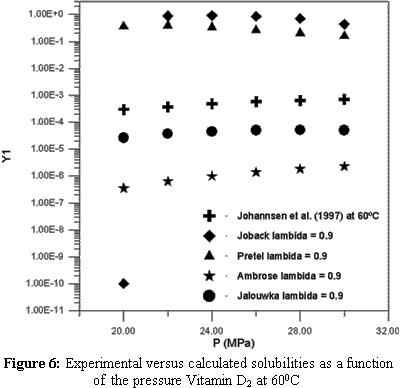
Extraction with supercritical solvents has been used in different areas, such as petroleum desasphaltation, descaffeination of coffee and tea and in the separation of other types of natural products. The supercritical solvent most frequently utilized in the extraction of natural products is carbon dioxide (CO2) due to its several advantages over other solvents such as low cost, atoxicity and volatility. The design, evaluation and optimization of a supercritical extraction that is based on phase equilibrium require phase equilibrium data. This type of data is very scarce for natural compounds like sterols and vitamins. These natural compounds are produced synthetically, but nowadays interest in their extraction from natural sources is increasing. Therefore, the objective of this work is to study the thermodynamic modelling equilibrium of systems containing vitamins A, D, E and K, using the predictive LCVM model. The sensitivity of critical properties in the calculation of the phase behavior was also studied. This study proved that the choice of a group contribution method to calculate thermodynamic properties is very important for obtaining good results in the phase equilibrium calculations.
Phase behavior; Vitamins; Sterols; Solubility data; CO2; LCVM