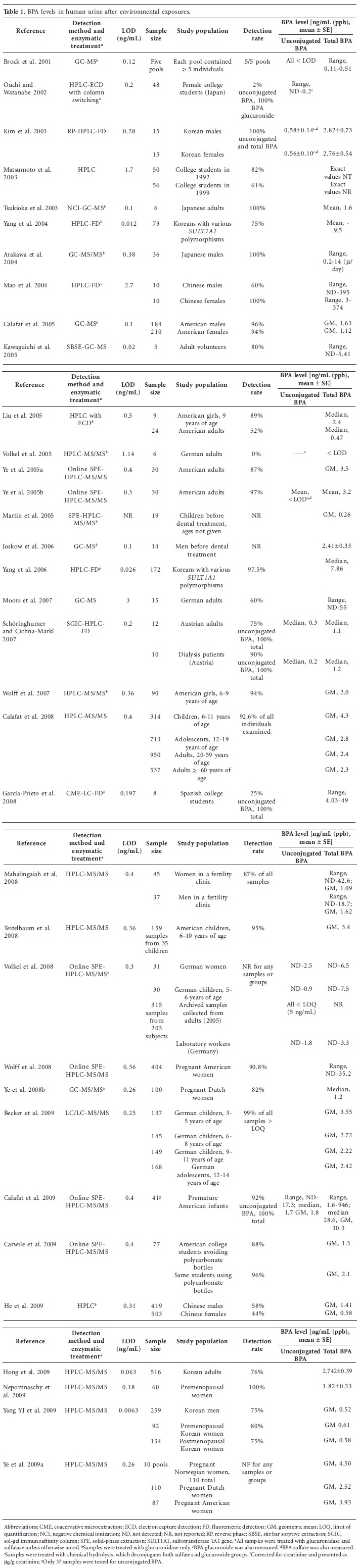
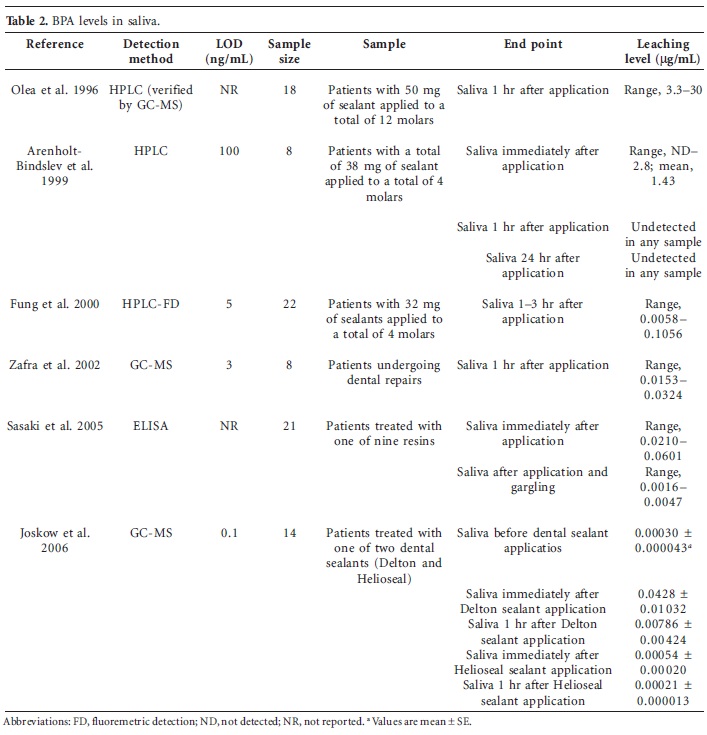
Bisphenol A (BPA) is one of the highest-volume chemicals produced worldwide, and human exposure to BPA is thought to be ubiquitous. Thus, there are concerns that the amount of BPA to which humans are exposed may cause adverse health effects. We examined many possibilities for why biomonitoring and toxicokinetic studies could come to seemingly conflicting conclusions. More than 80 published human biomonitoring studies that measured BPA concentrations in human tissues, urine, blood, and other fluids, along with two toxicokinetic studies of human BPA metabolism were examined. Unconjugated BPA was routinely detected in blood (in the nanograms per milliliter range), and conjugated BPA was routinely detected in the vast majority of urine samples (also in the nanograms per milliliter range). In stark contrast, toxicokinetic studies proposed that humans are not internally exposed to BPA. Available data from biomonitoring studies clearly indicate that the general population is exposed to BPA and is at risk from internal exposure to unconjugated BPA. The two toxicokinetic studies that suggested human BPA exposure is negligible have significant deficiencies, are directly contradicted by hypothesis-driven studies, and are therefore not reliable for risk assessment purposes.
Endocrine disruptor; Human exposure; PBPK/PBTK model; Pregnancy; Risk assessment; Toxicokinetics