Abstract
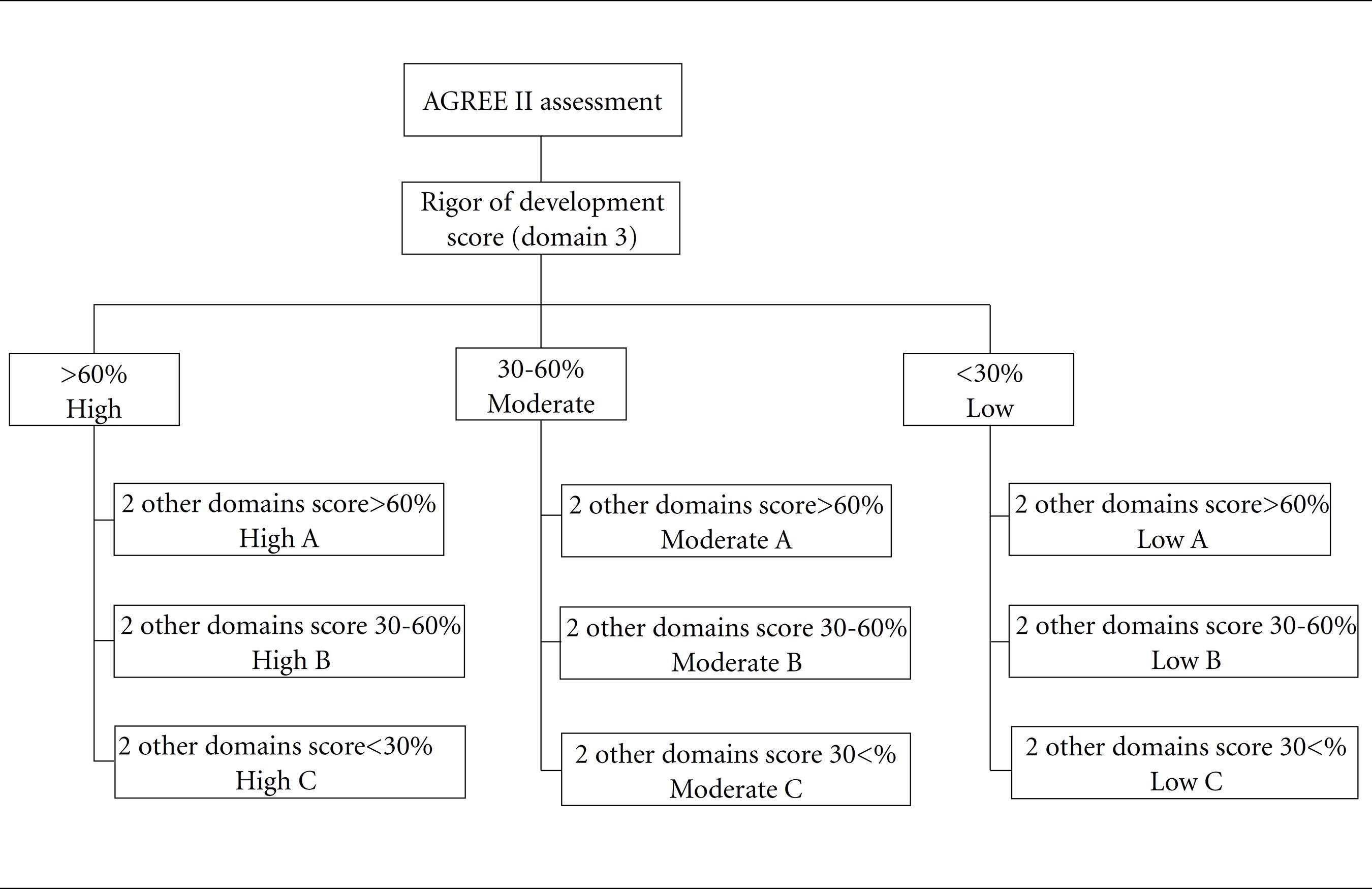
This study aims to compare the differences between clinical practice guidelines (CPGs) of the Ministry of Health (MoH) and those of other Brazilian health institutions. A systematic review of Brazilian CPGs was carried out. CPGs with recommendations for the pharmacological treatment of non-communicable disease (NCDs) were included. CPG methodological quality and transparency was independently assessed by 2 reviewers using the AGREE II. CPGs were rated as high, moderate, and low quality (ranging from A to C). Twenty-six CPGs were assessed for quality. MoH CPGs were published more recently, and were of better quality than the others: 6/6 (100%) were rated as Moderate-A. Although CPGs presented a wide range of methodological quality and transparency, MoH CPGs presented better consistency in the preparation method. To avoid confusion and to improve the quality of care within finite resources in Brazil, and to avoid potential bias, conflicts of interest, national CPGs used within SUS should be developed by Conitec with partners who have no conflict of interest.
Key words
Practice guidelines; Chronic disease; Primary health care; Technology assessment biomedical; Delivery of health care