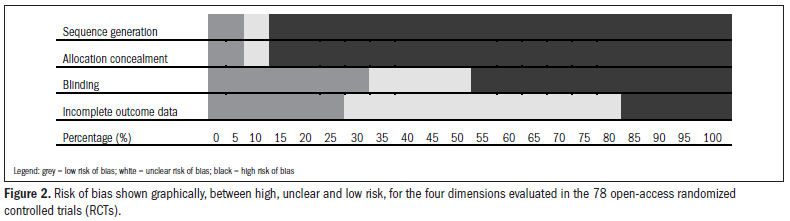
CONTEXT AND OBJECTIVE: Well-conducted randomized controlled trials (RCTs) represent the highest level of evidence when the research question relates to the effect of therapeutic or preventive interventions. However, the degree of control over bias between RCTs presents great variability between studies. For this reason, with the increasing interest in and production of systematic reviews and meta-analyses, it has been necessary to develop methodology supported by empirical evidence, so as to encourage and enhance the production of valid RCTs with low risk of bias. The aim here was to conduct a methodological analysis within the field of dentistry, regarding the risk of bias in open-access RCTs available in the Lilacs (Literatura Latino-Americana e do Caribe em Ciências da Saúde) database. DESIGN AND SETTING: This was a methodology study conducted at Universidade Federal de São Paulo (Unifesp) that assessed the risk of bias in RCTs, using the following dimensions: allocation sequence generation, allocation concealment, blinding, and data on incomplete outcomes. RESULTS: Out of the 4,503 articles classified, only 10 studies (0.22%) were considered to be true RCTs and, of these, only a single study was classified as presenting low risk of bias. The items that the authors of these RCTs most frequently controlled for were blinding and data on incomplete outcomes. CONCLUSION: The effective presence of bias seriously weakened the reliability of the results from the dental studies evaluated, such that they would be of little use for clinicians and administrators as support for decision-making processes.
Randomized controlled trial [publication type]; Selection bias; Double-blind method; Evidence-based dentistry; Empirical research