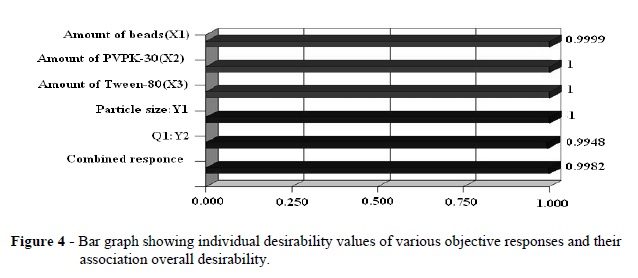
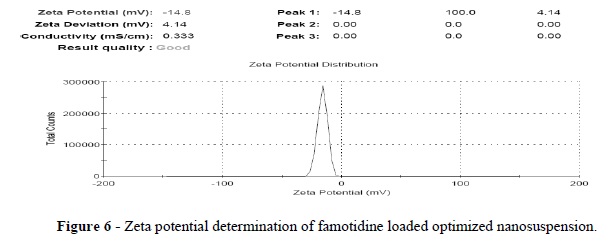
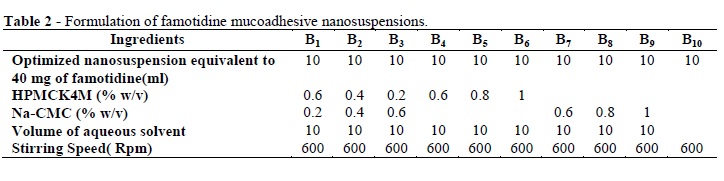
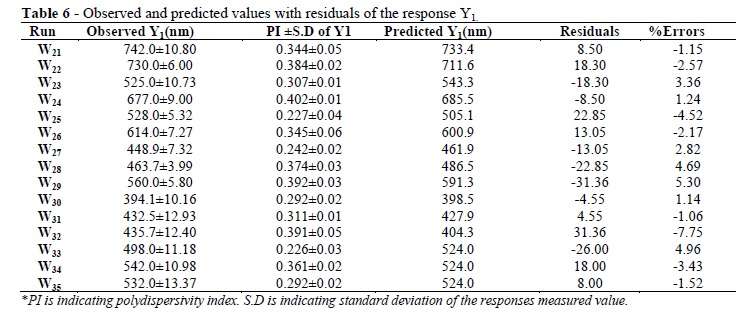
The present study was performed to design and evaluate the famotidine loaded mucoadhesive nanosuspension for aspirin induced ulcer. A 3-factor, 3-level Box-Behnken design was applied to study the effects of amount of the beads (X1), PVPK-30(X2) and Tween-80 (X3) on the particle size (Y1), and cumulative percentage drug released after 1h (Y2). The optimization was performed using the desirability function and contour plots. The scanning electron microscopy (SEM) showed the nanoparticles as spherical in shape. The differential scanning calorimetry (DSC) analysis indicated that there was substantial crystallinity change in the nanoparticle compared with the pure drug. Ex-vivo mucoadhesion study showed that famotidine mucoadhesive nanoparticles possessed higher mucoadhesion than the famotidine nanoparticles. The in vivo studies on aspirin-induced rats indicated the lowering in ulcer index for famotidine mucoadhesive nanoparticles was 0.46+0.011, which was significantly better than the effect of traditional famotidine suspension (0.66+0.035). Famotidine mucoadhesive nanosuspension could be prepared using the media milling technique and allowing significant reduction in ulcer index compared to famotidine suspension.
Famotidine; Nanosuspension; Box-Behnken design; Mucoadhesion; peptic ulcer