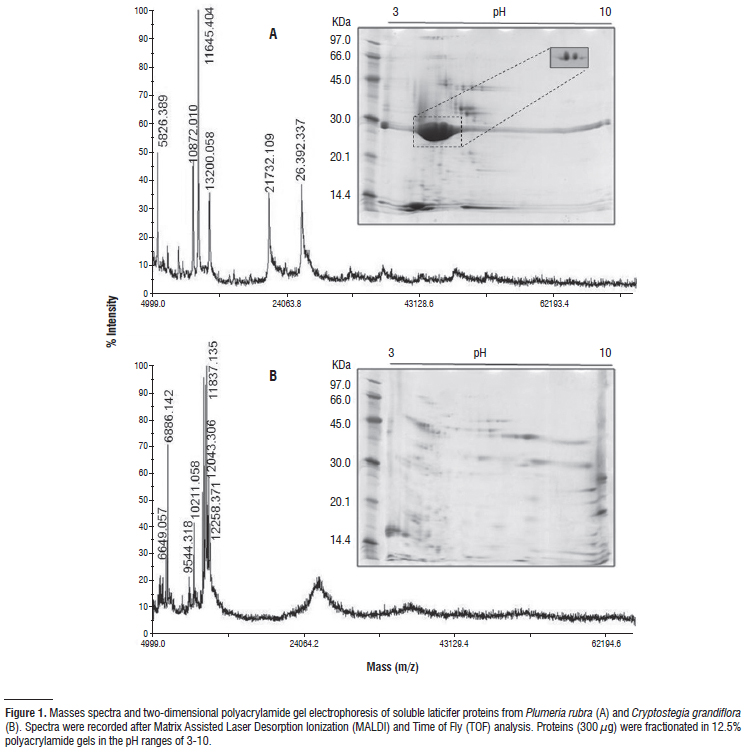
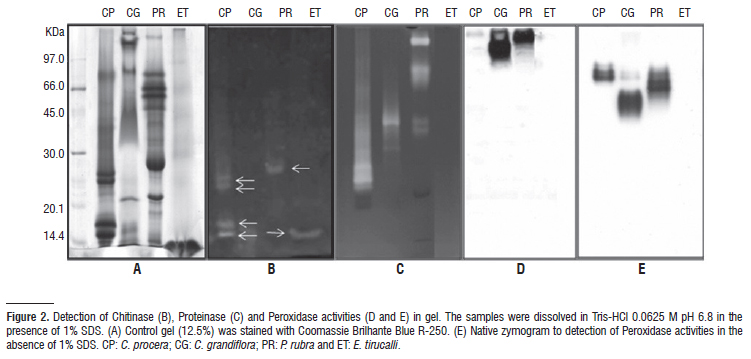
In this study, proteins extracted from laticifer cells of three plants were examined by electrophoresis, mass spectrometry (MALDI-TOF) and characterized in respect of proteolytic, chitinolytic and anti-oxidative activities by means of zymography and colorimetric assays. Acidic proteins with molecular masses between 12.5 and 74.5 kDa predominated in laticifers of P. rubra. This profile was not found in laticifers of C. grandiflora and E. tirucalli. The later was poor in respect of proteins. Strong anti-oxidative activity of superoxide dismutase (E.C. 1.15.1.1) was detected in P. rubra and C. grandiflora latices, and to a lesser extent ascorbate peroxidase (E.C. 1.11.1.1) and isoforms of peroxidase were seen. Catalase (E.C. 1.11.1.6) was detected only in laticifer cells of C. grandiflora. Chitinase (E.C. 3.2.1.14) was the sole activity found in laticifer cells of E. tirucalli, but was also detected in the other latices. The strong proteolytic activity of C. grandiflora was shown to be shared by at least three distinct cysteine proteinases (E.C. 3.4.22.16). Serine, aspartic and metaloproteinases were not detected. In laticifer cells of P. rubra, four proteinases were detected, including cysteine and serine types. This study reports new protein data of laticifers from plants that have been poorly investigated in this respect and contributes to the understanding of biochemical and functional aspects of laticifers in plants.
catalase; chitinase; cysteine proteinase; latex; peroxidase