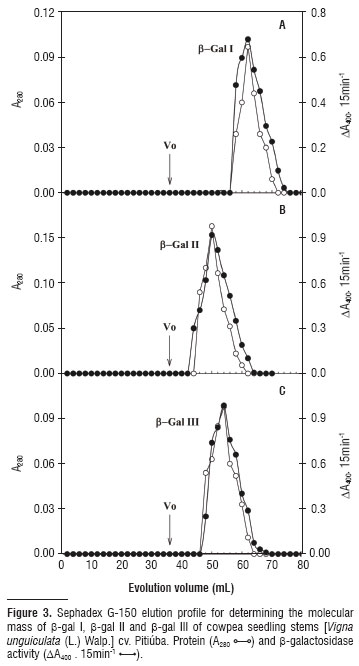
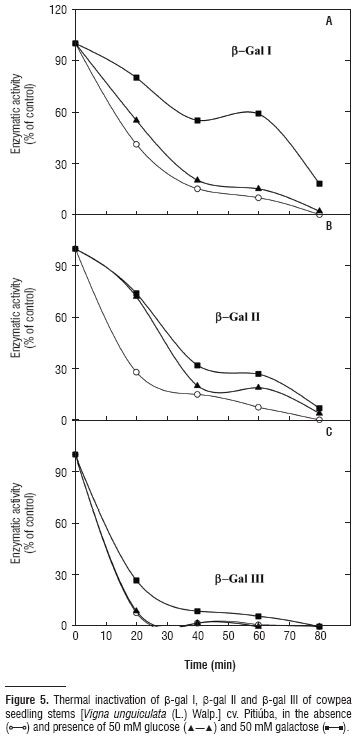
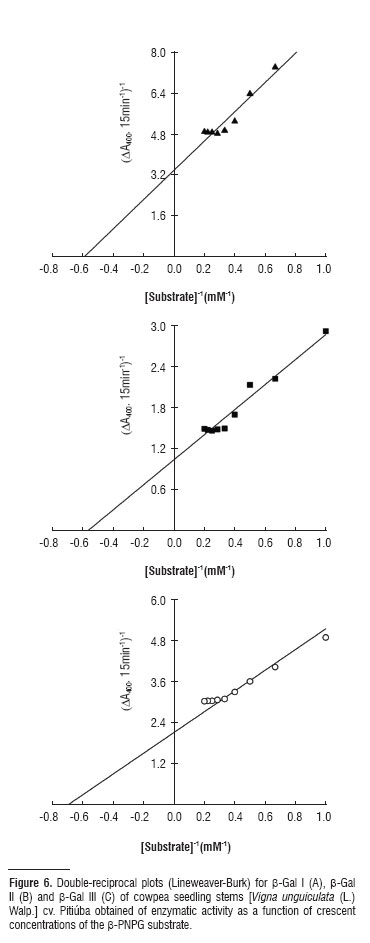
Three β-galactosidase isoforms, β-gal I and β-gal II (cytosolic) and β-gal III (cell wall-associated), were isolated from stems of Vigna unguiculata (L.) Walp. cv. Pitiúba seedlings. Purification consisted of aμMonia sulfate fractionation followed by chromatography in DEAE-Sephadex and Lactosyl-Sepharose columns. The two cytosolic isoforms showed the same chromatography pattern, which differed from that of β-gal III. Electrophoresis revealed a single band of protein for β-gal II and β-gal III which also expressed β-galactosidase activity in gel. The apparent molecular mass of the β-gal I, II and III was 89, 146 and 124 kDa, respectively. The three isoforms revealed the same optimal pH (4.0) and the same optimal assay temperature (55ºC) for enzyme activity. The three isoforms were stable at temperatures up to 50ºC, and incubation with glucose and galactose expanded their thermal stability as well as inhibited their activities. Galactose was the most effective in promoting these effects and β-gal I and II were competitively inhibited by this sugar. Kinetic analysis using β-PNPG as substrate, revealed K M of 1.69, 1.76 and 1.43 for β-gal I, β-gal II and β-gal III, respectively. The β-gal I was able to hydrolyze all synthetic substrates tested, whereas β-gal II exhibited only β-fucosidase and α-arabinosidase activities, and β-gal III was limited to the α-galactosidase, β-fucosidase and α-arabinosidase activities. These results are consistent with three distinct β-galactosidases exhibiting quite similar kinetic features, but endowed with different functional specificities probably related to their specific roles in the plant cell physiology.
enzymatic kinetics; cowpea; optimal pH; enzyme purification; thermal stability; thermal inactivation