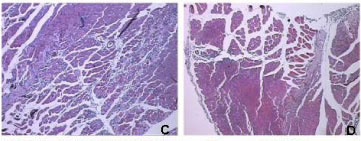
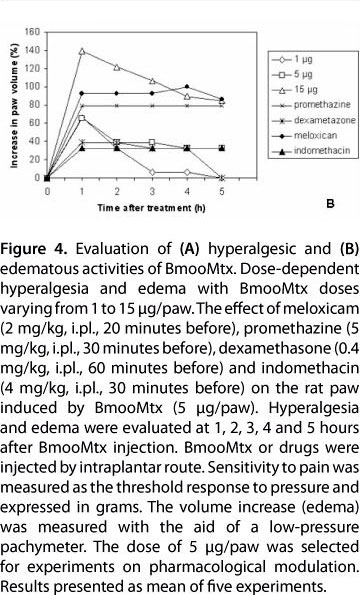
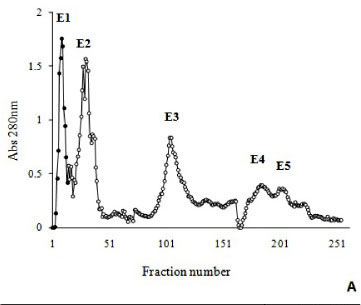
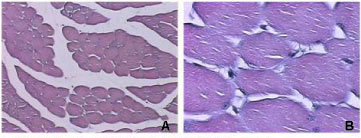
A myotoxin phospholipase A2 homologue, BmooMtx, was isolated from the venom of Bothrops moojeni by a combination of ion-exchange chromatography on DEAE-Sephacel column and gel filtration on Sephadex G-75. SDS-PAGE showed the enzyme to be a monomer with a molecular weight of 16,500. BmooMtx induced release of creatine kinase and morphological analyses indicated that it provoked an intense myonecrosis, with visible leukocyte infiltrate and damaged muscle cells 24 hours after injection. Anti-BmooMTx antibodies partially neutralized the myotoxic activity of BmooMtx and crude B. moojeni venom, as judged by determination of plasma creatine kinase levels and histological evaluation of skeletal muscle in mice. Anti-BmooMTx antibodies were effective in reducing the plasma creatine kinase levels of crude B. alternatus and B. leucurus venoms, evidencing immunological cross-reactivity between BmooMTx and other bothropic venoms. Intraplantar (i.pl.) injection of BmooMtx (1 to 15 μg/animal) caused a dose- and time-dependent hyperalgesia and edematogenic responses. Dexamethasone (0.4 mg/kg), meloxicam (2 mg/kg) and promethazine (5 mg/kg) markedly reduced the hyperalgesia. Our data suggest that these drugs may likely serve as complementary therapies in cases of accidents with Bothrops moojeni, provided that such pharmacological treatments are administered immediately after the incident.
Bothrops moojeni; hyperalgesia; myotoxin; edema