Abstract
Abstract: Tissue engineering holds as a prominent technique to repair or replace the damaged human parts to recreate its native function. In this research, a novel scaffold based on polyurethane (PU) comprising megni oil was electrospun for tissue engineering applications. The obtained polyurethane blended with megni oil nanofibers were characterized by scanning electron microscopy (SEM), fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), contact angle measurement and atomic force microscopy (AFM). Furthermore, the blood compatibility of the fabricated nanocomposites evaluated through activated prothrombin time (APTT), partial thromboplastin time (PT) and hemolysis assay to determine the anticoagulant nature. The morphological results showed that the fabricated nanocomposites showed reduced fiber size (789 ± 143.106 nm) than the pristine control (890 ± 116.91 nm). The interaction between PU and megni oil was identified by the hydrogen bond formation evident in the FTIR. The incorporation of megni oil in the PU decreased the wettability behavior (113.3° ± 1.528) and improved the surface roughness (646 nm). Preliminary evaluation of blood compatibility assessments was carried out using APTT, PT and hemolysis assay revealed the enhanced antithrombogenicity nature of the fabricated nanocomposites than the PU. Hence, we conclude that the fabricated new nanocomposite membrane with desirable characteristics which might find potential application in the tissue engineering applications.
Key words
polyurethane; megni oil; electrospun scaffold; blood compatibility; tissue engineering
INTRODUCTION
In recent days, remodeling/replacement of damaged tissues had been performed using a tissue engineering technique. It was an emerging and prominent technique to repair the different tissue organs (bone, skin, liver, blood vessel, heart valve, etc.) of the human body to improve the function of human tissues. The main goal in the tissue engineering is fabricating a scaffold which resembles the native ECM structure (MathewMATHEW AP, AUGUSTINE RO, KALARIKKAL NA and THOMAS SA. 2016. Tissue Engineering: Principles, recent trends and the future. Nanomedicine and Tissue engineering-State of the art and recent trends. Nanomed Tissue Eng, p. 31-82. et al. 2016). The structure of ECM is three dimensional which contains proteoglycans and fibrous proteins embedded with fibers in a diameter range of 50 to 150 nm (WangWANG X, DING B and LI B. 2013. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater Today 16(6): 229-241. et al. 2013, CheungCHEUNG HY, LAU KT, LU TP and HUI D. 2007. A critical review on polymer-based bio-engineered materials for scaffold development. Comp Part B: Eng 38(3): 291-300. et al. 2007). The nanofibers prepared from polymers hold great promise in resembling the native function of the extracellular matrix (ECM) (Heydarkhan et al. 2008HEYDARKHAN-HAGVALL S, SCHENKE-LAYLAND K, DHANASOPON AP, ROFAIL F, SMITH H, WU BM, SHEMIN R, BEYGUI RE and MACLELLAN WR. 2008. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomater 29(19): 2907-2914.). Further, the nanofibers revealed better cell adhesion and proliferation which supports the remodeling of the damaged tissue (SawSAW SH, WANG K, YONG T and RAMAKRISHNA S. 2007. Polymeric nanofibers in tissue engineering. Nanotechnol Life Sci 9: 66-134. et al. 2007).
In this, different fabricating techniques like drawing, self-assembly, template-directed synthesis, phase separation and electro-spinning were utilized to build up nanoscale matrices (OkadaOKADA M and MATSUMOTO T. 2016. Fabrication Methods of Hydroxyapatite Nanocomposites. Nano Biomed 8(1): 15-26. and Matsumoto 2016, MazumderMAZUMDER SK. 2002. Composites Manufacturing, Materials, Product and Process Engineering. CRC Taylor & Francis. 2002). Electrospinning, a versatile and least expensive technique is used to pull out ultrafine fibers (drawn from the syringe needle and collected on the drum) from the synthetic and natural polymers by applying a high voltage onto it (SubbiahSUBBIAH T, BHAT GS, TOCK RW, PARAMESWARAN S and RAMKUMAR SS. 2005. Electrospinning of nanofibers. J Appl Polym Sci 96(2): 557-569. et al. 2005, HuangHUANG N, YANG P, LENG YX, CHEN JY, SUN H, WANG J, WANG GJ, DING PD, XI TF and LENG Y. 2003. Hemocompatibility of titanium oxide films. Biomater 24: 2177-2187. et al. 2003). The extracted fibers have high surface area and porosity which facilitates the enhanced adherence, migration and growth of the cell’s response (LohLOH QL and CHOONG C. 2013. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B: Rev 19(6): 485-502. and Choong 2013). The assessment of the blood compatibility in the clinical applications is one of the main parameters which decides the application of the fabricated materials. QiQI J, ZHANG H, WANG Y, MANI MP and JAGANATHAN SK. 2018. Development and blood compatibility assessment of electrospun polyvinyl alcohol blended with metallocene polyethylene and plectranthus amboinicus (PVA/mPE/PA) for bone tissue engineering. Int J Nanomed 13: 2777. et al. 2018 electrospun PVA scaffold added with metallocene polyethylene and plectranthus amboinicus fibers. It was observed that the addition of metallocene polyethylene and plectranthus amboinicus resulted in the improvement of the blood compatibility and suggested a suitable candidate for bone tissue engineering. Del Gaudio et al. 2013DEL GAUDIO C, ERCOLANI E, GALLONI P, SANTILLI F, BAIGUERA S, POLIZZI L and BIANCO A. 2013. Aspirin-loaded electrospun poly (ε-caprolactone) tubular scaffolds: potential small-diameter vascular grafts for thrombosis prevention. J Mater Sci: Mater Med 24(2): 523-532. fabricated electrospun poly (ε-caprolactone) tubular scaffolds loaded with aspirin. It was showed that the prepared poly (ε-caprolactone)/aspirin scaffolds showed improved blood compatibility. JaganathanJAGANATHAN SK and MANI MP. 2018. Electrospun polyurethane nanofibrous composite impregnated with metallic copper for wound-healing application. 3 Biotech 8(8): 327. and Mani 2018 fabricated polyurethane scaffold added with copper particles for wound dressing application. It was observed that the addition of copper particles delayed the coagulation time of the pristine PU. From these literatures, it was clearly evident that the electrospun nanofibers with improved blood compatibility serve as a promising component in tissue engineering applications.
Currently, biodegradable polymers in terms of the scaffold were utilized in tissue engineering and clinical applications like drug delivery equipment’s, surgical devices, tissue structures and implants for bone defects. Polyurethane (PU) finds application in many biomedical devices because of excellent resembling of ECM mainly due to its biodegradable, biocompatibility and good mechanical properties (LambaLAMBA NMK, WOODHOUSE KA and COOPER SL. 1998. Polyurethanes in Biomedical Applications. Florida: CRC Press. et al. 1998, Ma et al. 2011MA ZW, HONG Y, NELSON DM, PICHAMUTHU JE, LEESON CE and WAGNER WR. 2011. Biodegradable polyurethane ureas with variable polyester or polycarbonate soft segments: Effects of crystallinity, molecular weight, and composition on mechanical properties. Biomacromol 12: 3265-3274.). Further significant features such as maintaining a moist environment, high swelling ratio and excellent oxidation stability allow the usage of PU in tissue engineering applications (JaganathanJAGANATHAN SK, MANI MP, AYYAR M and SUPRIYANTO E. 2017a. Engineered electrospun polyurethane and castor oil nanocomposite scaffolds for cardiovascular applications. J Mater Sci 52(18): 10673-10685. et al. 2017a). PU was blended with megni oil to improve the biological properties (blood compatibility and cellular response) of the PU. Megni oil is obtained from Nutmeg seed belongs to Myristicagenus. It was mostly available in Banda Islands of Indonesia, Penang Island in Malaysia and in Grenada, Caribbean. The nutmeg oil mainly contains 60–80% of d-camphene and other constituents like d-pinene, limonene, d-borneol, l-terpineol, geraniol, safrol, and myristicin (MaynardMAYNARD RL. 1997. The Merck Index: 1996. Occupational Env Med 54(4): 288. 1997). The nutmeg oil possesses various medicinal benefits such as treating cough syrups and mixed with almond oil for treating rheumatic pain. Further, along with honey, it utilizes for treating nausea, gastroenteritis, chronic diarrhoea and indigestion (http://www.penmai.com/forums/nature-cure/8384-nutmeg-jathikai-its-benefits.html). The objective is to investigate the physico-chemical characterizations and blood compatibility assays of the newly developed PU-based nanocomposites to evaluate their effectiveness for tissue engineering.
MATERIALS AND METHODS
MATERIALS
Tecoflex EG-80A, a medical polyurethane was obtained from Lubrizol and dissolved in dimethylformamide (DMF) solvent (Sigma Aldrich, UK). Megni oil was supplied from Elpar Herbs Sdn. Bhd, Kedah, Malaysia. The chemical such phosphate buffered saline (PBS) and sodium chloride physiological saline (0.9% w/v) used in the coagulation studies were purchased from Sigma-Aldrich, Kuala Lumpur, Malaysia. The reagents such as rabbit brain activated cephaloplastin, calcium chloride (0.025 M), and thromboplastin (Factor III) used in the APTT and PT studies were obtained from Diagnostic Enterprises, Solan, India.
PREPARATION OF SOLUTION
The homogeneous polyurethane solution with a concentration of 9 wt% was done by mixing 0.450g in 5 ml of DMF for overnight at room temperature. Similarly, the homogeneous solution of megni oil (4 wt%) was done by mixing 120 µl of megni oil in 3 ml of DMF and stirred for 1 hr maximum at room temperature. Finally, the prepared homogeneous PU solution was added into the prepared Megni oil solution at a ratio of 8:1 v/v and stirred for 1 hr maximum to obtain a homogeneous solution.
ELECTROSPINNING PROCESS
The electrospinning setup was used to fabricate the pure PU and composite PU membranes. To fabricate nanofibers, 10 ml syringe is filled with the prepared homogeneous solutions of polyurethane and composite and fitted in the syringe pump. The parameters used in this study as follows: an applied voltage of 10 kV, flow rate 0.5 ml/h and collector distance as 20 cm respectively for both PU and composite membranes. The nanofibers were collected using aluminum foil placed on the collector drum. Finally, the obtained nanofibers were dried under vacuum for 24 hr to remove any residual DMF in the nanofibers.
PHYSICOCHEMICAL CHARACTERIZATION
A detailed investigation of physicochemical characterization was performed. Initially, the morphology of the electrospun membranes were investigated using SEM unit and the fiber size distribution was obtained using Image J. Wettability of the electrospun membranes were calculated manually employing VCA contact angle analysis instrument. The electrospun membranes were inspected over the range of 600-4000 cm-1 using FTIR unit to investigate the chemical peaks present. The thermal properties of the electrospun membranes were carried out using TGA unit at a heating rate of 10 °C/min with the temperature range of 30 °C - 1000 °C. The surface roughness of the electrospun membranes was measured using AFM unit by scanning in size of 20 μm × 20 μm with pixels of 256 * 256.
COAGULATION ASSAYS
APTT and PT assay
Anticoagulant nature of the electrospun membranes was determined using APTT and PT assay. To begin the assay, a small piece of electrospun membrane was cut, washed with PBS and incubated at 37 °C for 30 min. The test was carried out by following the protocol as explained earlier. Lastly, the mean APTT and PT were measured for both electrospun PU and composite membranes (Jaganathan et al. 2017a).
Hemolysis assay
The safety of the blood cells with the electrospun membranes was investigated using a hemolysis assay. To begin the assay, a small piece of electrospun membrane was cut and incubated in physiological saline for 30 min at 37 °C. The assay was carried out by following the protocol as discussed earlier. Lastly, hemolytic index of the electrospun PU and nanocomposites were determined using the procedure explained earlier (JaganathanJAGANATHAN SK, MANI MP, ISMAIL AF and AYYAR M. 2017b. Manufacturing and characterization of novel electrospun composite comprising polyurethane and mustard oil scaffold with enhanced blood compatibility. Polym 9(5): 163. et al. 2017b).
Statistical analysis
All experiments were conducted thrice independently and the statistical significance was determined using Unpaired t-test. The results gained from all experiments are expressed as mean ± SD. In the case of qualitative experiments, a representative of three images is shown.
RESULT AND DISCUSSION
Fig. 1a and b represent the fiber morphology of the electrospun PU and PU/megni oil scaffolds. It was observed from the SEM figures, that both electrospun membranes exhibited randomly oriented fibers without any beads. The fiber diameters of PU and PU/megni oil scaffolds were observed to 890 ± 117 nm and 789 ± 143 nm, respectively. The electrospun PU/megni oil composite showed a reduction in fiber diameter which was due to the decrease in polymer concentration while adding megni oil into the polyurethane matrix (ShiSHI X, ZHOU W, MA D, MA Q, BRIDGES D, MA Y and HU A. 2015. Electrospinning of nanofibers and their applications for energy devices. J Nanomater 16(1): 122. et al. 2015). ChenCHEN R, HUANG C, KE Q, HE C, WANG H and MO X. 2010. Preparation and characterization of coaxial electrospun thermoplastic polyurethane/ collagen compound nanofibers for tissue engineering applications. Colloids Surf B: Biointerface 79: 315-325. et al. 2010 fabricated scaffold utilizing polyurethane incorporated with collagen for tissue engineering application. It was observed that the addition of collagen into the polyurethane matrix resulted in the reduction of fiber diameter than the pristine PU. The reported fiber diameter was in the range of 700–800 nm and exhibited enhanced cell adhesion and proliferation. Similarly, our fabricated PU and PU/megni oil scaffolds showed fiber diameter within these reported values which suggests the candidate for the tissue engineering applications.
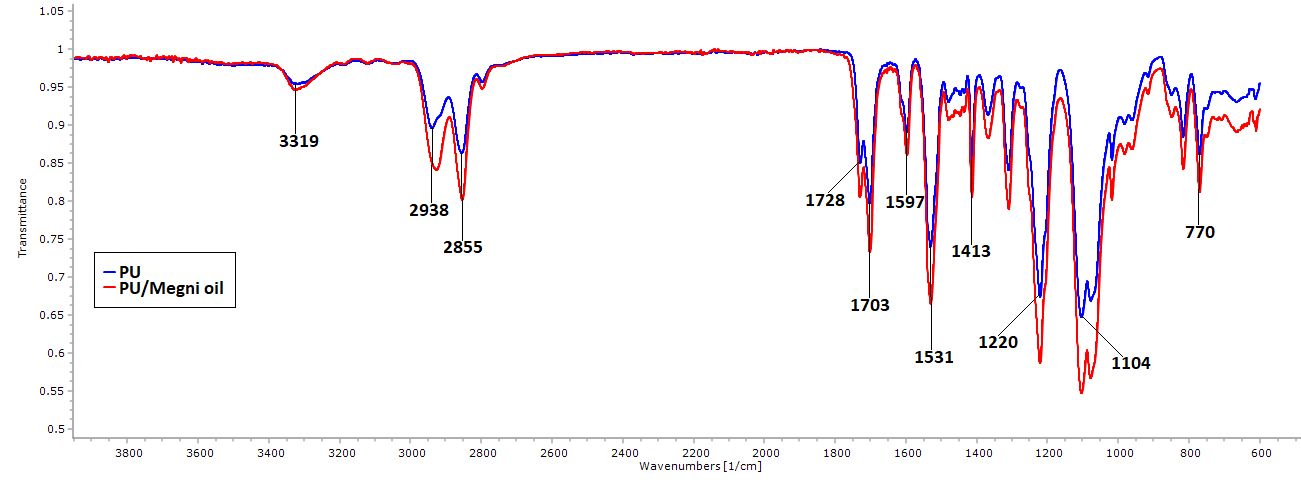
The IR spectrum of the fabricated PU and PU/megni oil scaffolds were indicated in Fig. 2. The spectra of the PU showed three main characteristics peaks observed at 3323 cm-1, 2939 cm-1 and 2854 cm-1 , 1730 cm-1 and 1703 cm-1 were attributes to NH stretching, CH stretching and carbonyl stretching respectively. Further, the other peaks seen at 1597cm-1 and 1531cm-1 denotes the NH vibrations, 1413 cm-1 indicates the CH stretch vibrations and at 1221cm-1 and 1104 cm-1 CO stretch corresponding to the alcohol group (Jaganathan et al. 2017a). In the spectra of the electrospun PU/megni oil scaffold, it was noted no new peak formation but their intensity was increased through hydrogen bond formation (UnnithanUNNITHAN AR, BARAKAT NAM, TIRUPATHI PB, GNANASEKARANE G, NIRMALA R, CHA YS, CHE HJ, EL-NEWEHY M and KIM HY. 2012. Wound-dressing materials with antibacterial activity from electrospun polyurethane–dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr Polym 90: 1786-1793. et al. 2012 ). Further, the CH peak shift was observed in the PU/megni oil scaffold indicates the existence of megni oil in the PU matrix. The CH peak observed at 2939 cm-1 in PU was shifted to 2925 cm-1 in PU/megni oil scaffold which confirms the interaction of PU with megni oil (TijingTIJING LD, RUELO MTG, AMARJARGAL A, PANT HR, PARK CH, KIM DW and KIM CS. 2012. Antibacterial and superhydrophilic electrospun polyurethane nanocomposite fibers containing tourmaline nanoparticles. Chem Eng J 197: 41-48. et al. 2012).
The results of water wettability for the electrospun PU and PU/megni oil nanocomposites were presented in Table I. From the obtained results of water wettability test, it was observed that the PU membrane exhibited hydrophobic with a contact angle of 100° ± 1 and with the addition of megni oil, the wettability was decreased showing contact angle of 113.3° ± 2. Certain studies of CeylanCEYLAN M. 2009. Superhydrophobic behavior of electrospun nanofibers with variable additives. Available at: http://hdl.handle.net/10057/2535.
http://hdl.handle.net/10057/2535...
et al. 2008 and Cui et al. 2009CUI W, LI X, ZHOU S and WENG J. 2008. Degradation patterns and surface wettability of electrospun fibrous mats. Polym Degrad Stab 93(3): 731-738. reported that that the small fiber diameter might favor the increase in the water contact angle. In our study, the addition of megni oil resulted in the reduction of the fiber diameter which might have favored the increase in the contact angle. JansenJANSEN EJ, SLADEK RE, BAHAR H, YAFFE A, GIJBELS MJ, KUIJER R, BULSTRA SK, GULDEMOND NA, BINDERMAN I and KOOLE LH. 2005. Hydrophobicity as a design criterion for polymer scaffolds in bone tissue engineering. Biomater 26(21): 4423-4431. et al. 2005 reported that the hydrophobic surface might facilitate the more proteins absorption which supports the improved healing of bone defects. Hence, the newly fabricated electrospun nanocomposites exhibited hydrophobic nature which might facilitate the enhanced absorption of proteins for the healing of bone defects.
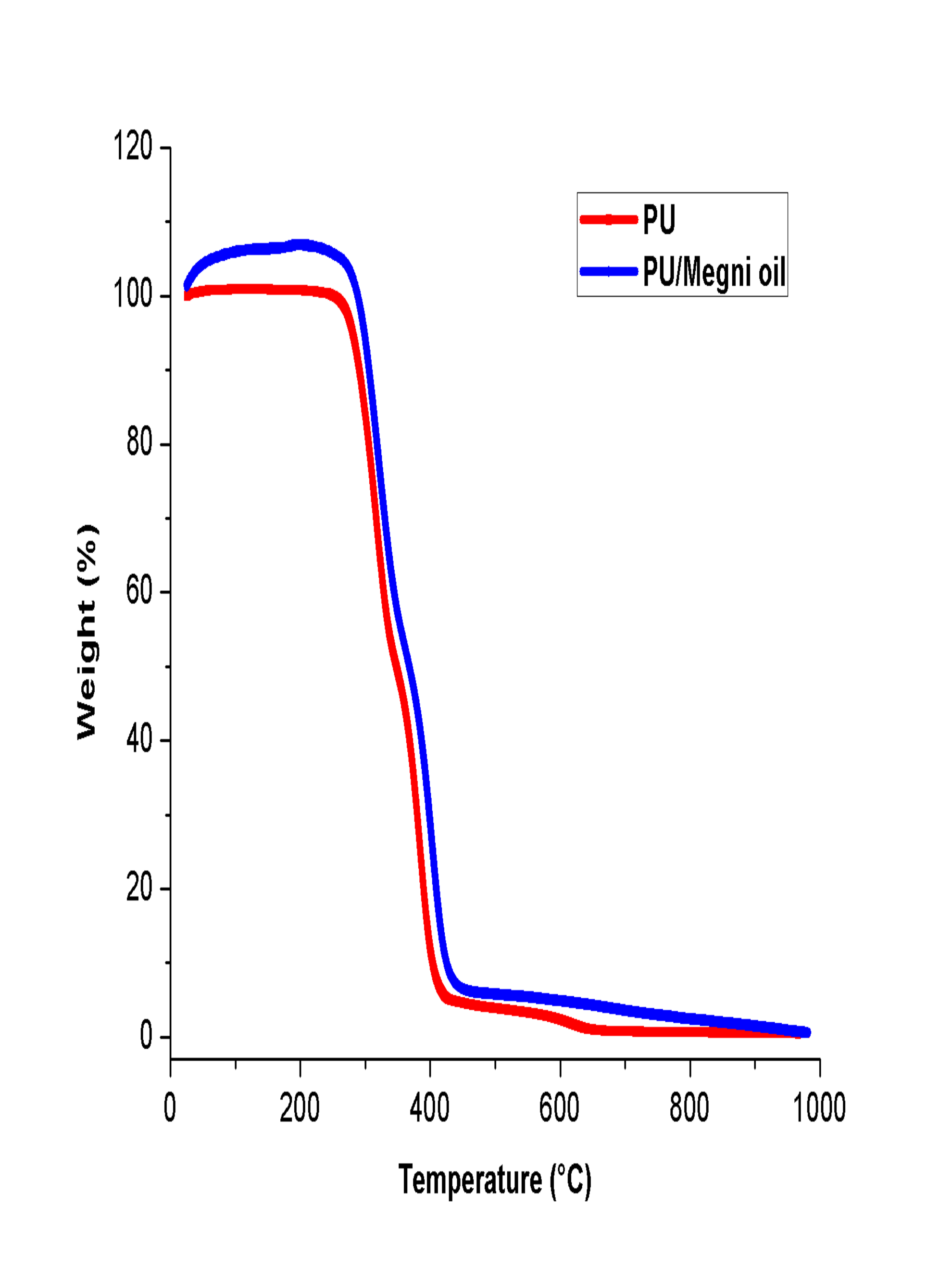
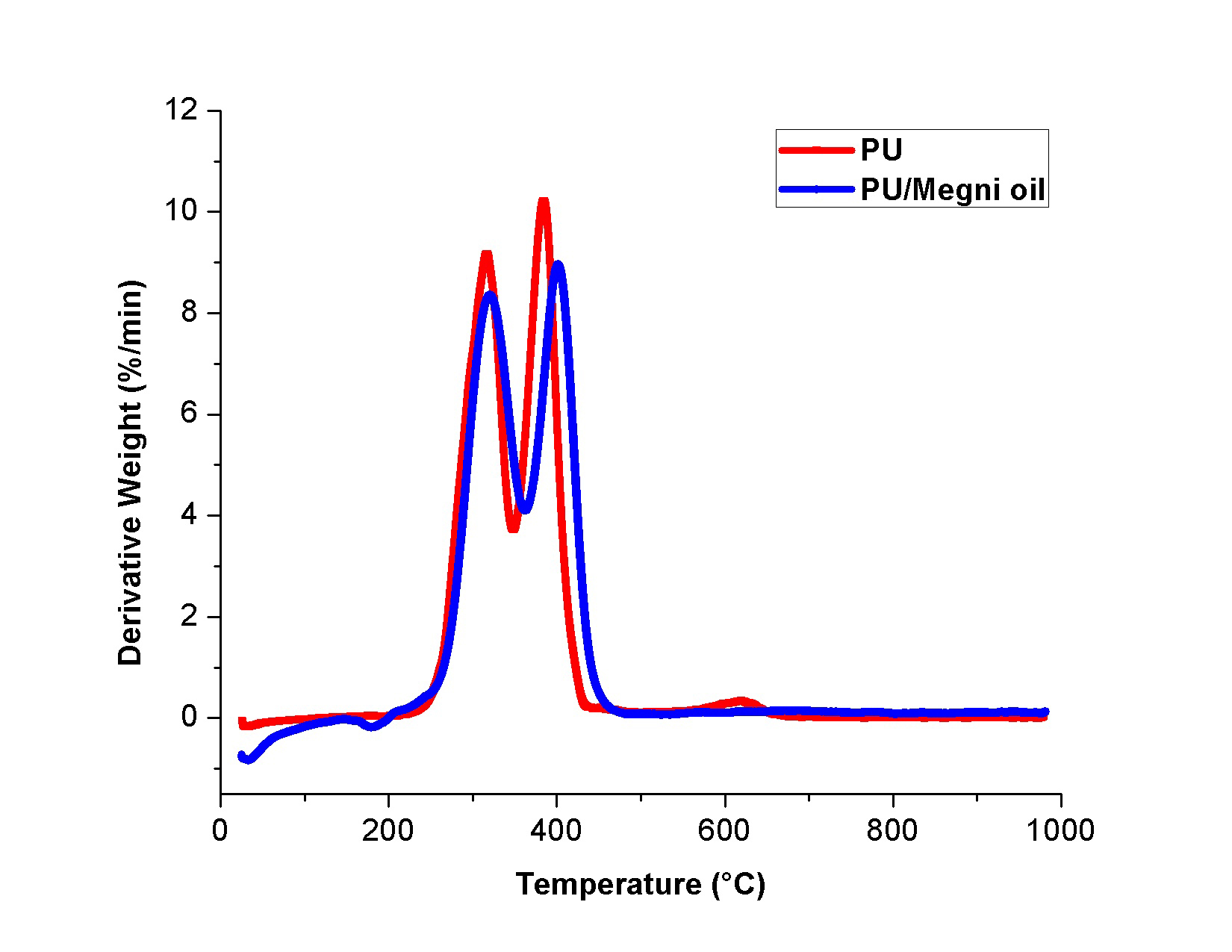
Thermal analysis was carried to investigate the effect of megni oil on the thermal stability of the PU matrix and their curves were showed in Fig. 3. It was revealed from the TGA test that the blending of megni oil improved the thermal stability of the pristine PU. It was noted that the initial onset temperature of PU was observed to 276 °C whereas it was increased to 287 °C in PU/megni oil membrane indicating the enhanced thermal stability. Jaganathan et al. 2017b fabricated polyurethane scaffold incorporated with mustard oil nanofibers. It was observed that the addition of mustard oil into the polyurethane matrix resulted in the enhancement of the thermal stability which correlates with our observations. Further at 1000 °C, the remaining weight residue of the PU membrane was observed to 0.45%, while the electrospun PU/megni oil nanocomposites exhibited weight residue of 0.55% indicating the existence of megni oil in the PU matrix. Moreover, the DTG curves for the electrospun membranes were shown in Fig. 4. It was revealed that the PU/megni oil scaffold showed reduced weight loss compared to the PU. The weight loss in PU occurs in three stages namely first loss begins at 223 °C to 348 °C, the second loss starts at 348 °C to 446 °C and third loss observed at 557 °C to 684 °C respectively. In the electrospun PU/megni oil scaffold, it showed only two loss indicating its reduced weight loss. The first loss was seen at 221 °C to 362 °C and the second loss observed at 362 °C to 481 °C respectively. It was observed that the third weight loss peak in the PU membrane was observed to be disappeared in the fabricated PU/megni oil membrane indicating reduced weight loss. The third weight loss disappearance was due to the addition of megni oil in the polyurethane matrix which had increased the thermal stability by reducing the weight loss of the fabricated PU/megni oil composite. ChaoCHAO CY, MANI MP and JAGANATHAN SK. 2018. Engineering electrospun multicomponent polyurethane scaffolding platform comprising grapeseed oil and honey/propolis for bone tissue regeneration. PLoS ONE 13(10): e0205699. et al. 2018 fabricated polyurethane scaffold added with grape seed oil/honey/propolis. It was shown that the addition of grape seed oil resulted in the disappearance of the third weight loss peak in the pristine PU which resembles our findings.
The surface properties for the electrospun PU and PU/megni oil scaffold were indicated in Fig. 5a and b. The surface property measurement through the AFM analysis revealed that the surface roughness for PU/megni oil scaffold was increased than the pristine PU. The average Ra for the PU membrane was found to 313 nm, while the electrospun PU/megni oil scaffold showed Ra of 646 nm, respectively. The results clearly indicated that the addition of megni oil resulted in the improved surface roughness of the PU. The enhanced surface roughness was due to the megni oil constituents in the polymer matrix. NikpourNIKPOUR MR, RABIEE SM and JAHANSHAHI M. 2012. Synthesis and characterization of hydroxyapatite/ chitosan nanocomposite materials for medical engineering applications. Compos: Part B 43: 1881-1886. et al. 2012 reported the addition of hydroxyapatite into chitosan material favored the increase surface roughness and their findings correlate with our observations. Jaganathan et al. 2017a fabricated polyurethane scaffold added with mustard oil nanofibers. It was reported that the addition of mustard oil improve the surface roughness of the pristine PU and concluded the reason was due to the presence of mustard oil constituents in the polymer matrix. As reported earlier, the megni oil contains numerous active constituents which might have favored the improvement in the surface roughness of the pristine PU.
The blood compatibility assessments of the electrospun PU and PU/megni oil nanocomposites determined through APTT and PT assay. The obtained blood compatibility results revealed that the blood clotting time for the electrospun PU/megni oil scaffold was delayed compared to the pristine PU. The reason for the delayed blood clotting time was due to the presence of the megni oil in the PU matrix. In the measurement of APTT assay, the PU membrane showed blood clotting of 175 ± 4 s, while for electrospun PU/megni oil scaffold, the blood clot formation time was reported to 186 ± 2, respectively. In the measurement of PT assay, the PU membrane showed blood clotting of 76 ± 3 s, while for electrospun PU/megni oil scaffold, the blood clot formation time was reported to 83 ± 2 s, respectively. Further, in order to determine the release of hemoglobin from the electrospun membranes, hemolysis assay was performed. The PU membrane exhibited hemolytic percentage of 2.48% and for the PU/megni oil mats, the measured hemolytic index was observed to be 1.75%. Hence, the electrospun nanocomposite mats exhibited hemolytic percentage of below 2% and it is known as a non-hemolytic materials (Jaganathan et al. 2017a). The blood compatibility is influenced by various surface properties such as fiber diameter, wettability and surface roughness (Huang et al. 2003). Jaganathan et al. 2017b prepared polyurethane/mustard oil composite using electrospinning technique and observed the enhancement in the blood compatibility than the pristine PU because of increase in surface roughness. In another study, Ayyar et al. 2017AYYAR M, MANI MP, JAGANATHAN SK and RATHANASAMY R. 2018. Preparation, characterization and blood compatibility assessment of a novel electrospun nanocomposite comprising polyurethane and ayurvedic-indhulekha oil for tissue engineering applications. Biomed Eng/Biomedizinische Technik 63(3): 245-253. developed electrospun scaffold utilizing polyurethane added with indhulekha oil and investigated its anticoagulant behavior. It was observed that developed PU/indhulekha oil composite with smaller fiber diameter displayed improved blood compatibility than the pristine PU. Further, Jaganathan et al. 2017b prepared a nanocomposite based on polyurethane loaded with castor oil which showed improved blood compatibility. The reason for the improved blood compatibility may be due to their hydrophobic behavior. Hence, the addition of megni oil in PU matrix showed a reduction in fiber diameter, enhanced hydrophobicity and improved surface roughness which might have resulted in the improvement of the blood compatibility.
CONCLUSION
In this research, a novel scaffold comprising PU incorporated with megni oil was fabricated successfully for bone tissue engineering. The results showed that the fabricated nanocomposites showed reduced fiber size than the control indicated in the SEM. The interaction between PU and megni oil was identified by the hydrogen bond formation evident in the FTIR. The incorporation of megni oil in the PU decreased the wettability behavior and improved surface roughness. Preliminary evaluation of blood compatibility assessments was carried out using APTT, PT and hemolysis assay revealed the enhanced antithrombogenicity nature of the fabricated nanocomposites than the PU. Hence, we conclude that the fabricated new nanomaterial which has desirable characteristics which might find potential application in the tissue engineering applications.
ACKNOWLEGMENTS
This work was supported by the Ministry of Higher Education Malaysia with the Grant no. Q.J130000.2545.17H00 and Q.J130000.2545.20H00.
REFERENCES
- AYYAR M, MANI MP, JAGANATHAN SK and RATHANASAMY R. 2018. Preparation, characterization and blood compatibility assessment of a novel electrospun nanocomposite comprising polyurethane and ayurvedic-indhulekha oil for tissue engineering applications. Biomed Eng/Biomedizinische Technik 63(3): 245-253.
- CEYLAN M. 2009. Superhydrophobic behavior of electrospun nanofibers with variable additives. Available at: http://hdl.handle.net/10057/2535
» http://hdl.handle.net/10057/2535 - CHAO CY, MANI MP and JAGANATHAN SK. 2018. Engineering electrospun multicomponent polyurethane scaffolding platform comprising grapeseed oil and honey/propolis for bone tissue regeneration. PLoS ONE 13(10): e0205699.
- CHEN R, HUANG C, KE Q, HE C, WANG H and MO X. 2010. Preparation and characterization of coaxial electrospun thermoplastic polyurethane/ collagen compound nanofibers for tissue engineering applications. Colloids Surf B: Biointerface 79: 315-325.
- CHEUNG HY, LAU KT, LU TP and HUI D. 2007. A critical review on polymer-based bio-engineered materials for scaffold development. Comp Part B: Eng 38(3): 291-300.
- CUI W, LI X, ZHOU S and WENG J. 2008. Degradation patterns and surface wettability of electrospun fibrous mats. Polym Degrad Stab 93(3): 731-738.
- DEL GAUDIO C, ERCOLANI E, GALLONI P, SANTILLI F, BAIGUERA S, POLIZZI L and BIANCO A. 2013. Aspirin-loaded electrospun poly (ε-caprolactone) tubular scaffolds: potential small-diameter vascular grafts for thrombosis prevention. J Mater Sci: Mater Med 24(2): 523-532.
- HEYDARKHAN-HAGVALL S, SCHENKE-LAYLAND K, DHANASOPON AP, ROFAIL F, SMITH H, WU BM, SHEMIN R, BEYGUI RE and MACLELLAN WR. 2008. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomater 29(19): 2907-2914.
- HUANG N, YANG P, LENG YX, CHEN JY, SUN H, WANG J, WANG GJ, DING PD, XI TF and LENG Y. 2003. Hemocompatibility of titanium oxide films. Biomater 24: 2177-2187.
- HUANG ZM, ZHANG YZ, KOTAKI M and RAMAKRISHNA S. 2003. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol 63(15): 2223-2253.
- JAGANATHAN SK and MANI MP. 2018. Electrospun polyurethane nanofibrous composite impregnated with metallic copper for wound-healing application. 3 Biotech 8(8): 327.
- JAGANATHAN SK, MANI MP, AYYAR M and SUPRIYANTO E. 2017a. Engineered electrospun polyurethane and castor oil nanocomposite scaffolds for cardiovascular applications. J Mater Sci 52(18): 10673-10685.
- JAGANATHAN SK, MANI MP, ISMAIL AF and AYYAR M. 2017b. Manufacturing and characterization of novel electrospun composite comprising polyurethane and mustard oil scaffold with enhanced blood compatibility. Polym 9(5): 163.
- JANSEN EJ, SLADEK RE, BAHAR H, YAFFE A, GIJBELS MJ, KUIJER R, BULSTRA SK, GULDEMOND NA, BINDERMAN I and KOOLE LH. 2005. Hydrophobicity as a design criterion for polymer scaffolds in bone tissue engineering. Biomater 26(21): 4423-4431.
- LAMBA NMK, WOODHOUSE KA and COOPER SL. 1998. Polyurethanes in Biomedical Applications. Florida: CRC Press.
- LOH QL and CHOONG C. 2013. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B: Rev 19(6): 485-502.
- MA ZW, HONG Y, NELSON DM, PICHAMUTHU JE, LEESON CE and WAGNER WR. 2011. Biodegradable polyurethane ureas with variable polyester or polycarbonate soft segments: Effects of crystallinity, molecular weight, and composition on mechanical properties. Biomacromol 12: 3265-3274.
- MATHEW AP, AUGUSTINE RO, KALARIKKAL NA and THOMAS SA. 2016. Tissue Engineering: Principles, recent trends and the future. Nanomedicine and Tissue engineering-State of the art and recent trends. Nanomed Tissue Eng, p. 31-82.
- MAYNARD RL. 1997. The Merck Index: 1996. Occupational Env Med 54(4): 288.
- MAZUMDER SK. 2002. Composites Manufacturing, Materials, Product and Process Engineering. CRC Taylor & Francis.
- NIKPOUR MR, RABIEE SM and JAHANSHAHI M. 2012. Synthesis and characterization of hydroxyapatite/ chitosan nanocomposite materials for medical engineering applications. Compos: Part B 43: 1881-1886.
- OKADA M and MATSUMOTO T. 2016. Fabrication Methods of Hydroxyapatite Nanocomposites. Nano Biomed 8(1): 15-26.
- QI J, ZHANG H, WANG Y, MANI MP and JAGANATHAN SK. 2018. Development and blood compatibility assessment of electrospun polyvinyl alcohol blended with metallocene polyethylene and plectranthus amboinicus (PVA/mPE/PA) for bone tissue engineering. Int J Nanomed 13: 2777.
- SAW SH, WANG K, YONG T and RAMAKRISHNA S. 2007. Polymeric nanofibers in tissue engineering. Nanotechnol Life Sci 9: 66-134.
- SHI X, ZHOU W, MA D, MA Q, BRIDGES D, MA Y and HU A. 2015. Electrospinning of nanofibers and their applications for energy devices. J Nanomater 16(1): 122.
- SUBBIAH T, BHAT GS, TOCK RW, PARAMESWARAN S and RAMKUMAR SS. 2005. Electrospinning of nanofibers. J Appl Polym Sci 96(2): 557-569.
- TIJING LD, RUELO MTG, AMARJARGAL A, PANT HR, PARK CH, KIM DW and KIM CS. 2012. Antibacterial and superhydrophilic electrospun polyurethane nanocomposite fibers containing tourmaline nanoparticles. Chem Eng J 197: 41-48.
- UNNITHAN AR, BARAKAT NAM, TIRUPATHI PB, GNANASEKARANE G, NIRMALA R, CHA YS, CHE HJ, EL-NEWEHY M and KIM HY. 2012. Wound-dressing materials with antibacterial activity from electrospun polyurethane–dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr Polym 90: 1786-1793.
- VINCENT M, THOMAS H, HEIKE H, VIOLA V and DANIEL E. 2012. Influence of fiber diameter and surface roughness of electrospun vasculargrafts on blood activation. Acta Biomater 8(12): 4349-4356.
- WANG X, DING B and LI B. 2013. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater Today 16(6): 229-241.
Publication Dates
-
Publication in this collection
19 June 2019 -
Date of issue
2019
History
-
Received
7 Jan 2019 -
Accepted
8 Mar 2019