Abstracts
Thyroxine (T4) withdrawal or recombinant TSH is used for the stimulation of thyroglobulin (Tg), whole-body scanning (WBS) and iodine-131 treatment in patients with thyroid carcinoma. This study evaluated the T4 dose reduction protocol as an alternative for patients' preparation. Fifty-one patients were submitted to total T4 withdrawal for WBS and Tg measurement. T4 treatment was then resumed and maintained until TSH reached levels < 0.3 mIU/l. The T4 dose was then decreased to 0.8 µg/kg/day and TSH was measured weekly. Tg was assayed when TSH was > 30 mIU/l. Patients diagnosed with the disease upon initial evaluation were treated. We also evaluated the clinical and laboratory changes observed for both preparations. Using the reduced dose protocol, TSH levels > 30 mIU/l were reached within 6 and 8 weeks in 84.6 and 100% of the patients, respectively. T4 withdrawal was associated with more common symptoms of hypothyroidism and elevation of creatine kinase (CK) and LDL cholesterol. The T4 dose reduction protocol proved to be useful for Tg stimulation and ablative therapy, without the complication of severe hypothyroidism or the cost of recombinant TSH.
Thyroid cancer; T4 dose reduction
A suspensão da tiroxina (T4) ou o TSH recombinante são usados para a estimulação da tireoglobulina (Tg), para o mapeamento de corpo inteiro (MCI) e para o tratamento com 131Iodo em pacientes com carcinoma tireoideano. Esse estudo avaliou um protocolo de redução de dose do T4 como alternativa para o preparo desses pacientes. Cinquenta e um pacientes submeteram-se à suspensão total de T4 para o MCI e a medida de Tg. Tratamento com T4 foi então reinstituído e mantido até que o TSH atingisse níveis < 0.3 mUI/l. A dose de T4 foi então dominuída para 0,8 µg/kg/dia e o TSH medido semanalmente. A Tg foi analisada quando o TSH estava > 30 mUI/l. Pacientes diagnosticados com a doença na fase inicial da avaliação foram tratados. Nós também avaliamos as alterações clínicas e laboratoriais observadas para ambos os preparos. Usando o protocolo de redução de dose, níveis de TSH > 30 mUI/l foram atingidos em 6 e 8 semanas em 84,6 and 100% dos pacientes, respectivamente. A suspensão do T4 esteve associada com sintomas mais comuns de hipotireoidismo e com elevação da creatino- quinase (CK) e LDL-colesterol. O protocolo de redução da dose de T4 mostrou-se útil para a estimulação da Tg e terapia ablativa, sem apresentar as complicações do hipotireoidismo severo ou chegar ao custo do TSH recombinante.
Câncer de tireóide; Redução da dose de T4
ARTIGO ORIGINAL
Managing thyroid cancer without thyroxine withdrawal
Controlando o câncer de tireóide sem suspender a tiroxina
Pedro Weslley S. do Rosário; Flavio P.J. Vasconcelos; Ludmilla D. Cardoso; Márcio W. Lauria; Leonardo L. Rezende; Eduardo L. Padrão; Álvaro L. Barroso; Valéria C. Guimarães; Saulo Purisch
Department of Thyroid, Endocrinology Service, Santa Casa de Belo Horizonte, MG
Endereço para correspondência Endereço para correspondência: Pedro Weslley Souza do Rosário Centro de Estudos e Pesquisa Clínica de Endocrinologia e Metabologia (CEPCEM) Av. Francisco Sales 1111, 5 o andar, Ala D 30150-221 Belo Horizonte, MG Fax: (31) 321-0836 E-mail: pedrorosario@globo.com
ABSTRACT
Thyroxine (T4) withdrawal or recombinant TSH is used for the stimulation of thyroglobulin (Tg), whole-body scanning (WBS) and iodine-131 treatment in patients with thyroid carcinoma. This study evaluated the T4 dose reduction protocol as an alternative for patients' preparation. Fifty-one patients were submitted to total T4 withdrawal for WBS and Tg measurement. T4 treatment was then resumed and maintained until TSH reached levels < 0.3 mIU/l. The T4 dose was then decreased to 0.8 µg/kg/day and TSH was measured weekly. Tg was assayed when TSH was > 30 mIU/l. Patients diagnosed with the disease upon initial evaluation were treated. We also evaluated the clinical and laboratory changes observed for both preparations. Using the reduced dose protocol, TSH levels > 30 mIU/l were reached within 6 and 8 weeks in 84.6 and 100% of the patients, respectively. T4 withdrawal was associated with more common symptoms of hypothyroidism and elevation of creatine kinase (CK) and LDL cholesterol. The T4 dose reduction protocol proved to be useful for Tg stimulation and ablative therapy, without the complication of severe hypothyroidism or the cost of recombinant TSH.
Keywords: Thyroid cancer; T4 dose reduction
RESUMO
A suspensão da tiroxina (T4) ou o TSH recombinante são usados para a estimulação da tireoglobulina (Tg), para o mapeamento de corpo inteiro (MCI) e para o tratamento com 131Iodo em pacientes com carcinoma tireoideano. Esse estudo avaliou um protocolo de redução de dose do T4 como alternativa para o preparo desses pacientes. Cinquenta e um pacientes submeteram-se à suspensão total de T4 para o MCI e a medida de Tg. Tratamento com T4 foi então reinstituído e mantido até que o TSH atingisse níveis < 0.3 mUI/l. A dose de T4 foi então dominuída para 0,8 µg/kg/dia e o TSH medido semanalmente. A Tg foi analisada quando o TSH estava > 30 mUI/l. Pacientes diagnosticados com a doença na fase inicial da avaliação foram tratados. Nós também avaliamos as alterações clínicas e laboratoriais observadas para ambos os preparos. Usando o protocolo de redução de dose, níveis de TSH > 30 mUI/l foram atingidos em 6 e 8 semanas em 84,6 and 100% dos pacientes, respectivamente. A suspensão do T4 esteve associada com sintomas mais comuns de hipotireoidismo e com elevação da creatino- quinase (CK) e LDL-colesterol. O protocolo de redução da dose de T4 mostrou-se útil para a estimulação da Tg e terapia ablativa, sem apresentar as complicações do hipotireoidismo severo ou chegar ao custo do TSH recombinante.
Descritores: Câncer de tireóide; Redução da dose de T4
AFTER INITIAL THERAPY of differentiated thyroid carcinoma with thyroidectomy and ablation of remnants with iodine-131 (1-3), follow-up is generally carried out by serum thyroglobulin (Tg) measurement alone in low risk patients (4) or combined with imaging methods in high-risk cases (5,6). Despite its limitations, iodine whole-body scanning (WBS) is widely used to evaluate these patients (7,8). Tg sensitivity is increased by TSH stimulation (9-11), as is the uptake of iodine-131 for diagnostic scanning or ablative therapy (12-15). In order to obtain satisfactory TSH levels, usually higher than 30 mU/l, T4 therapy may be discontinued, rapidly inducing hypothyroidism (12-15), which can compromise the patients' quality of life and result, among other changes, in hypercholesterolemia and myopathy accompanied by a rise in creatine kinase (CK) (16-18). Recombinant TSH effectively stimulates Tg production and iodine uptake by tumor cells for WBS (11,19-21) without causing hypothyroidism. However, Pacini et al. (22) showed that hypothyroidism is more effective than recombinant TSH for ablative treatment. In addition to this lower efficacy in ablative therapy, the high cost of recombinant TSH is a restrictive factor for its routine use. Therefore, an alternative that does not present the symptoms caused by T4 withdrawal is required for follow-up tests in patients for whom recombinant TSH is not available. Guimarães & DeGroot (23) have suggested an alternative protocol to increase TSH in which the thyroxine dose was reduced by half, reporting good results in terms of efficacy and reduction of morbidity. However, this method, which has always appealed a lot to us, has received some criticism (24). In the present study we reevaluated the dose reduction protocol compared to total T4 withdrawal, taking into account aspects such as efficiency in raising TSH, symptoms, serum CK and LDL cholesterol levels, capacity to stimulate Tg production, and efficacy of ablative therapy.
PATIENTS AND METHODS
Patient characteristics and study design
Patients with differentiated thyroid carcinoma who had undergone total thyroidectomy followed by remnant ablation and who did not present distant metastases were evaluated 6 to 12 months after initial therapy. The patients were first prepared with T4 withdrawal for 5 weeks, receiving fixed doses of 50 µg/day triiodothyronine (T3) for the first 2 weeks. The patients were then submitted to iodine WBS and Tg measurement during hypothyroidism. Antithyroglobulin antibodies (TgAb) were not detected in any case. After evaluation, T4 treatment was resumed up to the point when TSH reached levels < 0.3 mIU/l, but still detectable, and maintained for 4 weeks. The T4 dose was then changed to 0.8 µg/kg/day (the difference between the calculated and administered dose varied from -6 to +5 µg, average of +2.3 µg) and serial measurements (weekly) of TSH were obtained. Patients were maintained on this preparation for a minimum of 6 and a maximum of 8 weeks. If during these evaluations (6 or 8 weeks) patients showed levels > 30 mIU/l, Tg was measured and patients whose initial evaluation indicated recurrence of the disease received a new dose of iodine-131 (100 mCi). In addition, we determined morbidity, preference for one of the two types of preparation, heart rate, CK, LDL cholesterol and free T4 during hypothyroidism, and the efficacy of ablative treatment in those patients submitted to the dose reduction protocol. Fifty-one patients, 43 women and 8 men, were studied. Age ranged from 19 to 56 years (mean of 38.6 years). Forty patients had papillary carcinoma and 11 had follicular carcinoma. Thirty-six patients were in stage I (tumor within the thyroid) and 15 were in stage II (metastases to the lymph nodes). A control group consisting of 24 patients who also had cervical disease after initial treatment and who had received the same 100-mCi dose but were prepared with the classical protocol was retrospectively selected for comparison. All patients who were again treated with radioiodine showed cervical uptake < 3% (control WBS) and Tg < 20 ng/ml (TSH > 30 mIU/l).
The protocol was approved by the Research Ethics Committee of the institution and the patients gave informed consent to participate.
Tg and TgAb measurements
Tg was measured by an immunoradiometric assay (ELSA-hTG, CIS Bio International, France) with a functional sensitivity of 0.8 ng/ml, with the reference value established by the laboratory ranging from 3 to 42 ng/ml. TgAb were determined by a chemiluminescent assay (Chemiluminescent ICMA, Nichols Institute Diagnostics, San Juan Capistrano, CA) with a detection limit of 1 IU/ml and with a reference value < 2 IU/ml. No TgAb were detected in these cases.
CK, LDL cholesterol, TSH and free T4 measurements
CK was measured by an enzyme assay, with a reference value of up to 165 U/l for women and up to 190 U/l for men. LDL cholesterol was determined by an enzyme colorimetric method, with a reference value of up to 160 mg/dl. TSH was measured by an immunoradiometric assay (TSH MAIAclone, BioChem ImmunoSystems, Bologna, Italy), with a sensitivity of 0.03 mIU/l and the reference value established by the laboratory ranging from 0.3 to 4 mIU/l. Free T4 was measured by radioimmunoassay (GammaCoat Free T4, Diasorin, Stillwater, Minnesota, USA), with a sensitivity of 0.08 ng/dl and a reference value ranging from 0.73 to 2.1 ng/dl.
Imaging methods
Diagnostic WBS was performed with a tracer dose of 5 mCi iodine-131 during hypothyroidism and administration of a low iodine diet during the 2 weeks preceding the exam. Anterior and posterior images of the whole body were obtained 72 h after iodine administration. Post-therapy WBS was performed 7 days after the administration of the ablative dose. Other imaging methods used for the definition of disease status were cervical ultrasound and contrast-free chest and mediastinum-computed tomography.
Statistical methods
Significance was determined by c2 analysis and p values < 0.05 were considered to be significant.
RESULTS
Raising TSH
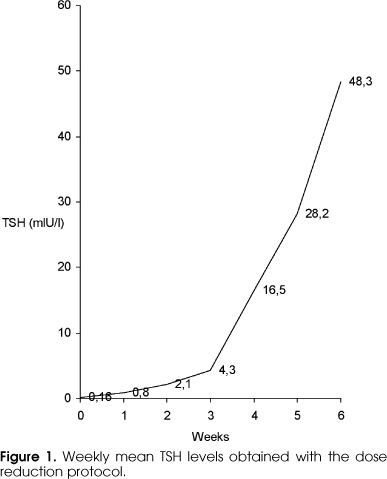
Using the classical protocol, TSH levels > 30 mIU/l were reached in 78.4 and 100% of patients in the absence of thyroid hormone within 2 and 3 weeks, respectively. When TSH was > 30 mIU/l, free T4 levels ranged from undetectable to 0.48 ng/dl (mean ± SD: 0.21 ± 0.23 ng/dl), and TSH ranged from 53 to 186 mIU/l (83 ± 23 mIU/l). Using the reduced dose protocol, TSH levels > 30 mIU/l were reached within 6 weeks in 84.6% of patients and within 8 weeks in the remaining patients. A TSH value > 15 mIU/l was predictive of levels > 25 mIU/l after 1 week. Basal TSH did not change among patients who successfully reached the required level within 6 and 8 weeks. When TSH was > 30 mIU/l (51.64 ± 11.1 mIU/l), free T4 ranged from 0.53 to 1.19 ng/dl (0.69 ± 0.15 ng/dl) (table 1). Figure 1 shows the weekly mean TSH values obtained with the T4 reduced dose protocol.
Adverse effects
Symptoms
Using the classical protocol, symptoms attributable to hypothyroidism (table 2) were observed in 70.6% of patients and 29.4% remained free of new complaints. With the reduced dose protocol, hypothyroidism symptoms were present in only 23.5% of cases, with most patients (76.5%) being symptom-free (table 1). When questioned about their preference, 35.3% of the patients reported no difference between the two types of preparation and 64.7% chose the reduced dose protocol. None of the patients preferred total T4 withdrawal.
CK
Basal CK levels were normal in all patients and the same concentrations were observed before application of the classical protocol and the reduced dose preparation (90 ± 19 versus 94 ± 17 U/l, p= ns). After T4 withdrawal, 70.5% of the patients presented an increase in CK values (from 236 to 1,015 U/l), an average of 364 ± 168 U/l among the 51 patients and significantly higher than the 178 ± 71 U/l found when the T4 dose was simply reduced. In this case, 80.3% of the patients showed normal levels of this enzyme (table 1).
LDL cholesterol
Basal LDL cholesterol levels were < 160 mg% in all patients, with no difference between values obtained before the classical protocol and the reduced dose preparation (102 ± 21U/l versus 108 ± 18U/l, p= ns). After T4 withdrawal, 62.7% of the patients showed values > 160 mg% (162 to 265 mg/dl), with an average of 172 ± 22 mg/dl, a value significantly higher than the 138 ± 18 mg/dl found when the T4 dose was simply reduced. In this case, 70.6% of the patients showed normal levels (< 160 mg/dl) (table 1).
Efficacy of ablative treatment with the reduced dose protocol
Twenty patients showed recurrence of cervical remnants after initial therapy, all with < 3% uptake in the cervical bed (control WBS) and without distant metastases. These patients received a 100-mCi dose after preparation with the reduced protocol and the success of the new therapy (negative WBS and Tg < 2 ng/ml, 6 months to 1 year after treatment) was 75%. A group of 24 subjects prepared by total T4 withdrawal and showing the same characteristics (cervical recurrence, second treatment, cervical uptake < 3%, dose of 100 mCi) was retrospectively selected for comparison among the patients seen at our institution. The efficacy of ablative therapy in this situation was 79.1%, with no difference compared to the reduced dose preparation (p= ns).
Efficacy of thyroglobulin generation
No treatment was performed after Tg off T4 measurement until a new evaluation was done with the reduced dose protocol. Therefore, no change in the status of the disease was observed. Initial stimulated Tg was undetectable (< 1 ng/ml) in 31 cases that were free of the disease and the same was observed for the T4 reduced dose preparation. Detectable Tg off T4 ranged from 2.4 to 20 ng/ml (8.87 ± 6.05 ng/ml) in patients with disease recurrence and from 1.8 to 18.3 ng/ml (6.94 ± 5.14 ng/ml, p= ns) in reduced dose preparation.
DISCUSSION AND CONCLUSIONS
We considered a minimum TSH value of 30 mIU/l as ideal based on previous studies demonstrating an increase in iodine-131 uptake with these values (12-15). Furthermore, exaggerated TSH rises have no additional benefits, as demonstrated by Goldman et al. (13), who did not detect any difference in uptake when employing median TSH levels of 68 mIU/l (2 week T3-off) versus 96 mIU/l (4 week T3-off). Using the reduced dose protocol, 84.3% of the patients reached these values within 6 weeks, demonstrating good efficacy. Basal TSH (> 0.03 and < 0.3 mIU/l) was reduced in the present patients and no difference was observed between patients who reached the required TSH level within 6 or 8 weeks. Nevertheless, it is possible that the efficacy of this preparation is higher when TSH is > 0.3 mIU/l and lower if basal levels are undetectable. Values > 15 mIU/l were predictive of TSH levels > 25 mIU/l after 1 week. These factors had already been defined by Guimarães & DeGroot (23). In the present study we only evaluated patients who had undergone total thyroidectomy and ablative therapy, and therefore without functioning thyroid remnants which might have altered the efficacy of the protocol to raise TSH. In addition, none of the patients presented functioning metastases. We recognize that in the presence of large remnants or metastases this protocol may not be as successful, requiring longer preparation times.
Analysis of the symptoms observed in the present study clearly shows the advantage and patients' preference of treatment without T4 withdrawal, as also demonstrated by Guimarães & DeGroot (23). In addition, laboratory parameters such as free T4, CK and LDL cholesterol significantly differed between the two protocols, in favor of the reduced dose protocol. Therefore, it is unquestionable that this preparation resulted in benefits in terms of quality of life and reduction of morbidity, in agreement with a previous study (23).
Concerning iodine-131 uptake, Greenspan (24) has indicated the possibility that iodine-127, present in the thyroxine molecule, may compete with the tracer if T4 therapy is continued. In view of the small quantity of iodine-127 and considering the use of a reduced dose only and the prescribed low iodine diet, we do not believe that this fact may interfere. In addition, if there were interference, the use of recombinant TSH in patients on full T4 therapy would not be efficient to stimulate iodine uptake when performing WBS (11,19-21). Despite the similar efficacy of the two preparations in remnant treatment with high iodine-131 doses, the use of the reduced dose protocol for ablative therapy still requires caution and further studies because of the small size of the present series.
Additionally, we compared Tg measurement between the two preparations and observed that the reduced dose protocol is effective for Tg stimulation.
Another important aspect refers to the prolonged period of time under increased TSH, which could stimulate tumor growth more intensively than the classical protocol that results in fast TSH rises over a short period of time. Using the preparation without T4 withdrawal, increased TSH values were only observed in the last three weeks, showing that the delay is longer when starting from decreased TSH. In addition, the smaller concentrations compensate for the longer TSH exposure. Although we did not evaluate the TSH decrease after T4 treatment had been resumed, it is possible that the classical protocol delays normalization of TSH since the values obtained were higher.
After conclusion of this study, T3 was withdrawn from the Brazilian market due to some problems involving the company responsible for its manufacturing and distribution. Considering the high risk of an incorrect dosage during laboratory handling, we believe that the T4 dose reduction protocol is of greater importance in our country.
Weekly monitoring of TSH after complete T4 withdrawal can also be used, reducing the duration of hypothyroidism since the desired elevation of TSH can be rapidly achieved (average of 17 days) (25).
In conclusion, the dose reduction protocol is an especially effective alternative after total thyroidectomy and ablation of remnants and stimulates TSH for Tg measurement and possibly for WBS without the complication of severe hypothyroidism and the high cost of recombinant TSH. Concerning ablative therapy, we recommend caution and further studies including a larger number of patients.
ACKNOWLEDGMENTS
We thank Prof. Leslie J. DeGroot (University of Chicago) for his valuable contribution to the revision of this article.
Recebido em 14/04/04
Revisado em 22/09/04 e 15/10/04
Aceito em 15/12/05
- 1. DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab 1990;71:414-24.
- 2. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 1994;97:418-28.
- 3. Schlumberger MJ. Medical progress papillary and follicular thyroid carcinoma. N Engl J Med 1998;338:297-306.
- 4. Mazzaferri EL, Robbins RJ, Spencer CA, Braverman LE, Pacini F, Wartofsky L, et al. A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma. J Clin Endocrinol Metab 2003;88:1433-41.
- 5. Robbins RJ, Chon JT, Fleisher M, Larson SM, Tuttle RM. Is the serum thyroglobulin response to recombinant human thyrotropin sufficient, by self, to monitor for residual thyroid carcinoma? J Clin Endocrinol Metab 2002;87:3242-7.
- 6. Rosario PW, Maia FF, Cardoso LD, Fagundes TA, Reis JS, Purisch S. Usefulness of radioiodine scanning in patients with moderate/high risk differentiated thyroid carcinoma in whom thyroglobulin (without thyroxine) is undetectable after initial treatment. Arq Bras Endocrinol Metabol 2004;48:384-8.
- 7. Grigsby P. Cost minimization analysis and utility of pretreatment and posttreatment total body iodine-131 scans in patients with thyroid carcinoma. Cancer 1998;82:931-5.
- 8. Lind P. 131-I whole body scintigraphy in thyroid cancer patients. Q J Nucl Med 1999;43:188-94.
- 9. Ozata M, Suzuki S, Miyamoto T, Liu RT, Fierro-Renoy F, DeGroot LJ. Serum thyroglobulin in the follow-up of patients with treated differentiated thyroid cancer. J Clin Endocrinol Metab 1994;79:98-105.
- 10. Schlumberger M, Baudin E. Serum thyroglobulin determination in the follow-up of patients with differentiated thyroid carcinoma. Eur J Endocrinol 1998;138:249-52.
- 11. Haugen BR, Pacini F, Reiners C, Schlumberger M, Ladenson PW, Sherman SI, et al. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab 1999;84:3877-85.
- 12. Maxon HR, Smith HS. Radioiodine-131 in the diagnostic and treatment of metastatic well differentiated thyroid cancer. Endocrinol Metab Clin North Am 1990;19:685-717.
- 13. Goldman JM, Line BR, Aamodt RL, Robbins J. Influence of triiodothyronine withdrawal time on 131I uptake post-thyroidectomy for thyroid cancer. J Clin Endocrinol Metab 1980;50:734-9.
- 14. Edmonds CJ, Hayes S, Kermode JC, Thompson BD. Measurement of serum TSH and thyroid hormones in the management and treatment of thyroid carcinoma with radioiodine. Br J Radiol 1977;50:799.
- 15. Hilts SV, Hellman D, Anderson J, Woolfenden J, Van Antwerp J, Patton D. Serial TSH determination after T3 withdrawal or thyroidectomy in the therapy of thyroid carcinoma. J Nucl Med 1979;20:928.
- 16. Lien EA, Nedrebo BG, Varhaug JE, Nygard O, Aakvaag A, Ueland PM. Plasma total homocysteine levels during short-term iatrogenic hypothyroidism. J Clin Endocrinol Metab 2001;85:1049-53.
- 17. Weissel M, Kainz H, Hofer R. Changes in biochemical parameters during complete thyroid hormone deficiency of short duration in athyreotic patients. J Nucl Med 1986;27:1528-32.
- 18. Dubois A, Goldman JM. Gastric secretion and emptying in hypothyroidism. Dig Dis Sci 1984;29:407-10.
- 19. Giovanni V, Arianna LG, Antonio C, Francesco F, Michele K, Giovanni S, et al. The use of recombinant human TSH in the follow-up of differentiated thyroid cancer: experience from a large patient cohort in a single centre. Clin Endocrinol (Oxf) 2002;56:247-52.
- 20. Robbins RJ, Tuttle RM, Sharaf RN, Larson SM, Robbins HK, Ghossein RA, et al. Preparation by recombinant human thyrotropin or thyroid hormone withdrawal are comparable for the detection of residual differentiated thyroid carcinoma. J Clin Endocrinol Metab 2001;86:619-25.
- 21. Durski JM, Weigel RJ, McDougall IR. Recombinant human thyrotropin (rhTSH) in the management of differentiated thyroid cancer. Nucl Med Commun 2001;21:521-8.
- 22. Pacini F, Molinaro E, Grazia Castagna M, Lippi F, Ceccarelli C, Agate L, et al. Ablation of thyroid residues with 30 mCi 131I: a comparison in thyroid cancer patients prepared with recombinant human TSH or thyroid hormone withdrawal. J Clin Endocrinol Metab 2002;87:4063-8.
- 23. Guimarães V, DeGroot LJ. Moderate hypothyroidism in preparation for whole body 131 I scintiscans and thyroglobulin testing. Thyroid 1996;6:69-73.
- 24. Greespan FS. Moderate hypothyroidism in preparation for whole body 131 I scintiscans and thyroglobulin testing. Thyroid 1996;6:493.
- 25. Liel Y. Preparation for radioactive iodine administration in differentiated thyroid cancer patients. Clin Endocrinol (Oxf) 2002;57:523-7.
Publication Dates
-
Publication in this collection
17 Apr 2006 -
Date of issue
Feb 2006
History
-
Accepted
15 Dec 2005 -
Reviewed
15 Oct 2004 -
Received
14 Apr 2004