Abstracts
PURPOSE: To explore the relationship between morphological characteristics and histologic localization of metastasis within sentinel lymph nodes (SLN) and axillary spread in women with breast cancer. METHODS: We selected 119 patients with positive SLN submitted to complete axillary lymph node dissection from July 2002 to March 2007. We retrieved the age of patients and the primary tumor size. In the primary tumor, we evaluated histologic and nuclear grade, and peritumoral vascular invasion (PVI). In SLNs we evaluated the size of metastasis, their localization in the lymph node, number of foci, number of involved lymph nodes, and extranodal extension. RESULTS: Fifty-one (42.8%) patients had confirmed additional metastasis in non-sentinel lymph nodes (NLSN). High histologic grade, PVI, intraparenchymatous metastasis, extranodal neoplastic extension and size of metastasis were associated with positive NLSN. SLN metastasis affecting the capsule were associated to low risk incidence of additional metastasis. After multivariate analysis, PVI and metastasis size in the SLN remained as the most important risk factors for additional metastasis. CONCLUSIONS:The risk of additional involvement of NSLN is higher in patients with PVI and it increases progressively according the histologic localization in the lymph node, from capsule, where the afferent lymphatic channel arrives, to the opposite side of capsule promoting the extranodal extension. Size of metastasis greater than 6.0 mm presents higher risk of additional lymph node metastasis.
Breast neoplasms; Sentinel lymph node biopsy; Lymph nodes; Neoplasm metastasis
OBJETIVO:Explorar a relação entre características morfológicas e localização histológica da metástase dentro dos linfonodos sentinelas (LS) e disseminação axilar em mulheres com câncer de mama. MÉTODOS: Foram selecionados 119 pacientes com LS positivo, submetidas à dissecação completa dos linfonodos axilares entre Julho de 2002 a Março de 2007. Foram recuperados a idade das pacientes e o tamanho do tumor primário. No tumor primário, avaliamos os graus histológico e nuclear e a invasão vascular peritumoral (IVP). Nos LS, avaliamos o tamanho da metástase, sua localização no linfonodo, o número de focos metastáticos, número de linfonodos envolvidos e a extensão extranodal. RESULTADOS: Cinquenta e um (42,8%) pacientes tiveram metástases adicionais confirmadas nos linfonodos não sentinelas (LNS). Alto grau histológico, IVP, metástase intraparenquimatosa, extensão extranodal e tamanho da metástase foram associados com LNS positivos. Metástase afetando a cápsula do LS foi associada com baixo risco de incidência de metástase adicional. Após análise multivariada, IVP e tamanho da metástase no LS foram os fatores de risco mais importantes para metástases adicionais nos LNS. CONCLUSÕES:O risco de envolvimento adicional dos LNS é maior em pacientes com IVP e tal risco aumenta progressivamente de acordo com a localização histológica da metástase no LS, que inicia na cápsula, onde aporta o linfático aferente, e termina no lado oposto, promovendo a extensão extranodal. Tamanho de metástase maior ou igual a 6,0 mm revela maior risco de metástase nos LNS.
Neoplasias da mama; Biopsia de linfonodo sentinela; Linfonodos; Metástase neoplásica
Localization of metastasis within the sentinel lymph node biopsies: a predictor of additional axillary spread of breast cancer?
Localização da metástase no interior dos linfonodos sentinelas: um preditor de disseminação adicional axilar em câncer de mama?
César Augusto AlvarengaI; César Cabello dos SantosII; Marcelo AlvarengaI; Paula Itagyba ParavidinoI; Sirlei Siani MoraisII; Henrique Benedito BrenelliII; Filomena Marino de CarvalhoIII
IInstituto de Patologia de Campinas (Private Laboratory) - Campinas (SP), Brazil
IIDivision of Oncology and Senology, Department of Obstetrics and Gynecology, Faculdade de Ciências Médicas, Universidade Estadual de Campinas - Unicamp - Campinas (SP), Brazil
IIIDepartment of Pathology, Faculdade de Medicina, Universidade de São Paulo - USP - São Paulo (SP), Brazil
Correspondence Correspondence: César Augusto Alvarenga Av. Orozimbo Maia, 165 (pátio da Maternidade de Campinas) Zip code: 13023-910. Campinas (SP), Brazil
ABSTRACT
PURPOSE: To explore the relationship between morphological characteristics and histologic localization of metastasis within sentinel lymph nodes (SLN) and axillary spread in women with breast cancer.
METHODS: We selected 119 patients with positive SLN submitted to complete axillary lymph node dissection from July 2002 to March 2007. We retrieved the age of patients and the primary tumor size. In the primary tumor, we evaluated histologic and nuclear grade, and peritumoral vascular invasion (PVI). In SLNs we evaluated the size of metastasis, their localization in the lymph node, number of foci, number of involved lymph nodes, and extranodal extension.
RESULTS: Fifty-one (42.8%) patients had confirmed additional metastasis in non-sentinel lymph nodes (NLSN). High histologic grade, PVI, intraparenchymatous metastasis, extranodal neoplastic extension and size of metastasis were associated with positive NLSN. SLN metastasis affecting the capsule were associated to low risk incidence of additional metastasis. After multivariate analysis, PVI and metastasis size in the SLN remained as the most important risk factors for additional metastasis.
CONCLUSIONS:The risk of additional involvement of NSLN is higher in patients with PVI and it increases progressively according the histologic localization in the lymph node, from capsule, where the afferent lymphatic channel arrives, to the opposite side of capsule promoting the extranodal extension. Size of metastasis greater than 6.0 mm presents higher risk of additional lymph node metastasis.
Keywords: Breast neoplasms, Sentinel lymph node biopsy, Lymph nodes, Neoplasm metastasis
RESUMO
OBJETIVO:Explorar a relação entre características morfológicas e localização histológica da metástase dentro dos linfonodos sentinelas (LS) e disseminação axilar em mulheres com câncer de mama.
MÉTODOS: Foram selecionados 119 pacientes com LS positivo, submetidas à dissecação completa dos linfonodos axilares entre Julho de 2002 a Março de 2007. Foram recuperados a idade das pacientes e o tamanho do tumor primário. No tumor primário, avaliamos os graus histológico e nuclear e a invasão vascular peritumoral (IVP). Nos LS, avaliamos o tamanho da metástase, sua localização no linfonodo, o número de focos metastáticos, número de linfonodos envolvidos e a extensão extranodal.
RESULTADOS: Cinquenta e um (42,8%) pacientes tiveram metástases adicionais confirmadas nos linfonodos não sentinelas (LNS). Alto grau histológico, IVP, metástase intraparenquimatosa, extensão extranodal e tamanho da metástase foram associados com LNS positivos. Metástase afetando a cápsula do LS foi associada com baixo risco de incidência de metástase adicional. Após análise multivariada, IVP e tamanho da metástase no LS foram os fatores de risco mais importantes para metástases adicionais nos LNS.
CONCLUSÕES:O risco de envolvimento adicional dos LNS é maior em pacientes com IVP e tal risco aumenta progressivamente de acordo com a localização histológica da metástase no LS, que inicia na cápsula, onde aporta o linfático aferente, e termina no lado oposto, promovendo a extensão extranodal. Tamanho de metástase maior ou igual a 6,0 mm revela maior risco de metástase nos LNS.
Palavras-chave: Neoplasias da mama, Biopsia de linfonodo sentinela, Linfonodos, Metástase neoplásica
Introduction
Currently, one of the most important prognostic factors in breast cancer is the regional lymph node status. Lymph node metastasis determines the need for adjuvant therapy and the number of metastases may influence the type of therapy1. For decades, the complete axillary lymph node dissection (CALD) has been the standard of care in patients with invasive breast cancer in order to decide the quality of adjuvant therapy. A significant number of complications was the price to pay to have the knowledge of adequate axillary status2.
The sentinel lymph node (SLN) biopsy proved to be an excellent prognosticator for the outcome of breast cancer patients, without the need for a more morbid procedure as the formal CALD1,3. Therefore, the selective sentinel lymphadenectomy has become the standard approach for assessing axillary status in clinically node-negative breast cancer patients, with less morbidity4-6. On the other hand, CALD is the ideal recommended procedure for patients with SLN metastasis5. Different studies over the last few years have suggested that some patients with positive SLN can be treated without CALD7. The SLN is the only involved axillary node in 38 to 67% patients5,8-13. Some investigators have developed a nomogram to help the identification of subsets of patients who do not need CALD, however, results remain conflicting14.
The presence or absence of metastases in the nonsentinel lymph node (NSLN) seems to be dependent on some morphologic characteristics of the primary breast tumor and characteristics of the SLN metastases15. The purpose of this study was to explore the morphologic characteristics and the histologic localization of metastases in the SLN, together with primary breast cancer features, in order to define subsets of patients that can achieve benefit from CALD.
Methods
The study was retrospective and evaluated 546 consecutive patients with invasive breast carcinomas and clinically negative axillary nodes, from July 2002 to March 2007. All the patients were submitted to SLN biopsy and those with positive SLN underwent CALD. All the patients came from different private clinics from Campinas, the third city of the state of Sao Paulo, Brazil, with a 2010 population of more than one million. All the surgical specimens were processed and analyzed in the Instituto de Patologia de Campinas, a private laboratory in Campinas.
The subjects were selected from the list of women with positive SLN who underwent CALD. We excluded the women with negative SLN and those who refused to be submitted to CALD. SLN were negative in 405 patients (74.2%). One hundred-forty-one (25.8%) patients presented metastases in SLN. Of these cases, 14 patients had isolated tumor cells (2.5%), 24 had micrometastases (4.4%) and 103 had macrometastases (18.9%), defined according the American Joint Committee on Cancer (AJCC) criteria16.
Twenty-two of these patients were not submitted to CALD and were excluded from this study, 13 women with isolated tumor cells, 5 with micrometastases and 4 with small macrometastases in the SLN. One hundred-nineteen invasive breast carcinomas met the inclusion criteria for this study. The SLNs were identified by the blue dye and/or radiolabeled colloid and intraoperative gamma probe techniques. The SLNs were submitted to frozen section and paraffin examination.
All SLN were entirely paraffin embedded after longitudinal or transverse sections at 2 to 3 mm intervals and fixation in 10% buffered formalin. Four-micrometer histological sections were stained by hematoxylin-eosin (H&E). The H&E slides were examined and, if the SLN was negative, six serial sections were taken from the paraffin block at intervals of 50 µm. Immunohistochemical examination was performed in the first histologic section using AE1/AE3 antibody (Dako, Carpinteria, CA, 1:50 dilution), employing the envision-peroxidase technique with antibody retrieval by humid heat. The remaining five sections were stained by H&E and examined microscopically.
We reviewed the hematoxylin-eosin (H&E) slides from the primary tumor, the SLN, and the complementary axillary lymph nodes (NSLN). The breast carcinoma was evaluated for tumor size (recorded by macroscopic description or measured from microscopic sections), histologic type, histological grade according Nottingham criteria17, nuclear grade and peritumoral vascular invasion.
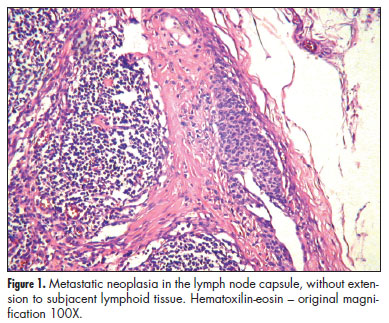
The positive SLNs were evaluated for size of metastatic deposits, localization of the metastasis in the lymph node, number of foci, number of involved lymph nodes, and extranodal extension. The size of metastases was measured by a glass millimeter ruler and classified according to AJCC as isolated tumor cells (measuring less than 0.2 mm at greatest diameter), micrometastases (measuring 0.2 to 2.0 mm) or macrometastases (measuring more than 2.0 mm and subdivided in 2.1 to 4.0 mm, 4.1 to 6.0 mm, 6.1 to 8.0 mm, 8.1 to 10.0 mm and greater than 10.0 mm)16. The localization of metastases was defined as the main component of the neoplasia into the lymph node in the following categories: capsule, with or without subcapsular sinus (without extracapsular or parenchymatous extension); medullary sinus or sinusoids; parenchyma and extranodal extension (Figures 1 to 3). In addition to, the presence/absence of each localizations stated above, parenchymatous and extranodal involvement were analyzed as categorical variables. The number of metastatic foci was counted and the largest focus was considered as the size of the metastasis.
Careful examination of the CALD was carried out and the isolated axillary lymph nodes were dissected, fixed in 10% buffered formalin and entirely embedded in paraffin. After being sliced at 3 to 4 mm intervals in the longitudinal or transverse plane, lymph nodes measuring less than 5 mm were submitted as one piece in toto. A single level of each block was stained with H&E.
All histological analysis were performed by two pathologists (CAA and MA) and presented as a consensus.
Univariate analyses were performed using the χ2 test or Fisher exact test for categorical variables to determine the association between the morphology of the breast tumor and the SLN metastasis with the status of the NSLN. The respective values of crude odds ratios (and confidence intervals) were calculated. Association between histologic localization of metastasis in the SLN and the size of metastasis was analyzed by the Kruskal-Wallis test. A multivariable logistic regression model was constructed to evaluate the association between the statistically significant variables in univariate analyses with the axillary status. Statistical analyses were performed using SAS Institute Incorporation SAS/STAT Software, and a p-value of less than 0.05 was considered significant.
The study was approved by the ethical committee for research of the School of Medical Sciences, from the State University of Campinas (Unicamp), protocol number 0262/2005.
Results
Patients' ages ranged from 29 to 83 years with a mean age of 51 years. A total of 5 to 38 axillary lymph nodes were removed in CALD (mean: 15). Fifty-one out of 119 patients had additional metastases in CALD (42.8%). The size of breast tumors was evaluated according to AJCC16. For purpose of statistical analysis, pT1a cases (4 cases) were grouped with pT1b (30 cases) to form a homogeneous group (total of 33 cases or 27.7%). Sixty-four tumors were pT1c (53.8%), and the pT2, pT3 and pT4 were grouped in 22 cases (18.4%). The histologic grade ranged as follows: grade I (6 cases; 5.0%) that were grouped with grade II (29 cases, 24.2%) for comparison with grade III (85 cases; 70.8%).
The clinico-pathological features of the 119 patients included in the study according to the CALD involvement are summarized in Table 1. High histologic grade and presence of peritumoral vascular invasion (PVI) were associated with neoplastic involvement of CALD. The classification of the metastasis according to their localization within SLN is presented in Table 2.
Pathological characteristics of SLN like parenchymatous metastasis without or with extranodal extension (p=0.004 and respectively OR 4.3; 95%CI 0.91-21.02 and OR 13.0; 95%CI 2.44-69.13) and size of metastasis equal or greater than 4 mm (p=0.01 and OR 2.9; 95%CI 0.69-12.76) and 6 mm also (p=0.01 and OR 7.0; 95%CI 1.60-31.33) were extremely associated with metastases in NSLN by univariate analyses. When the metastasis was associated with capsule involvement in SLN, the risk of additional metastases in NSLN were minimum (p=0.001 and OR 0.05; 95%CI 0.003-0.8).
After multivariable analyses, when histologic grade, PVI, capsular involvement and localization of metastasis were included in the model, only PVI (OR 5.7; 95%CI 2.3-14.0; p=0.0002) and parenchymal involvement with extranodal extension (OR 4.8; 95%CI 1.9-12.4; p=0.001) remained statistically significant. However, when the size of metastasis was included, only metastasis above 6.0 mm (OR 5.8; 95%CI 2.5-13.6; p<0.0001) and PVI (OR 4.7; 95%CI 1.8-11.9; p=0.001) were predictors of additional metastasis.
Discussion
CALD remains the standard of care for SLN positive patients, even in most cases of axillary metastases are limited to the sentinel node9,13,18-21. Moreover, the omission of CALD in patients with limited metastatic disease did not affect overall or disease-free survival of patients with clinical T1-T2 breast cancer as showed in the American College of Surgeons Oncology Group Z0011 randomized trial7. The patients were treated with lumpectomy, adjuvant systemic therapy, and tangential-field whole-breast radiation therapy. Patients were randomly assigned to the CALD group and to the SLN-alone group. In the CALD group, 97 of 355 patients (27.3%) had additional metastasis in lymph nodes removed by CALD7.
The high prevalence of NSLN metastases in our study (42.5%) can be explained because most of our SLN had macrometastases, considered an important risk factor of metastasis in NSLN, as also suggested by Viale et al.22, who reported one of the highest prevalence of NSLN involvement of the literature (50.3%).
The need for CALD is generally highly considered for young patients, but in our study, as well as in previous reports this does not seem to be a risk factor for additional metastasis14,22,23. However, young age is associated with a higher proportion of positive SLN24.
Other factors generally considered in the decision of a completion of axillary dissection are: size of primary tumor, PVI, size of metastasis and number of involved lymph nodes18-23,25-29. In our study, the mean size of breast tumor was 1.6 cm for cases with negative axillary NSLN and 1.8 cm for cases with positive NSLN axillary (variation between 0.1 and 6.0 cm). Two of the four patients with breast tumors less than or equal to 0.5 cm had additional metastases in axillary lymph nodes, a possible reason why the association of primary tumor size with positive NSLN was not significant. One hundred patients with breast carcinomas of this study25 had metastases in 22.6% of SLN, almost the same prevalence of our study. In that study25, as in ours, the size of breast cancer was not associated with positive NSLN. One hundred and eighty patients with SLN metastases of Fleming et al.27 study found a significant association between the size of breast tumor and metastases in SLN, although breast tumor size was not relevant in predicting additional metastases in NSLN27.
However, primary tumor size was significantly associated with risk of NLSN metastasis in various studies19,20,23,26,29,30. All the studies found other characteristics equal or more important than the tumor size. Even in our study, we observed that biggest tumors were associated with additional metastasis. A statistical level of significance was not reached, probably due to the influence of other biological features. For example, in our cases, the high histologic grade of primary breast carcinomas was associated with higher fraction of additional metastases.
The size of metastatic deposits in SLN seems more important than the primary tumor size in predicting NLSN metastasis26. Our results confirm the impact of the size of metastasis as a risk factor to higher lymph node metastatic involvement. Only macrometastases equal or greater than 4 mm had important risk of additional metastases in NSLN, supporting the need of CALD in SLN macrometastases cases9,26. Despite the small number of cases with isolated tumor cells and micrometastases in the SLN (21 cases or 17.5%), we did not find a significant association with positive axilla, as in other studies22,31,32.
PVI was observed in 34 (28.3%) patients of our study and it was significantly associated with additional metastases in the NSLN (p=0.0005). As discussed above regarding primary tumor size, the same controversies exist about the significance of PVI as a risk factor to additional lymph node metastasis. Several studies presented results similar to ours19,22,23,25,33,34, while others did not28,30,35.
The neoplastic emboli reach the SLN throughout the different lymphatic channels, migrate to the capsule of SLN first, then to the subcapsular sinus, after that, infiltrating the sinusoids and then, the lymph node parenchyma, diffusely. Finally the tumor cells migrate beyond the opposite side of capsule promoting the extranodal extension. Our results, in a certain way, reflect this pathway as they show higher aggressiveness from capsule to extranodal extension. The histologic localization of metastases within the SLN was barely explored in literature. Diaz et al. observed that metastatic tumor had a higher probability of being present in the region of the inflow junction of the afferent lymphatic vessel and they suggested an optimization of the method for evaluating axillary sentinel lymph node specimens36. Although the data from this study do suggest that tumor cells appear to localize preferentially to either the afferent lymphatic inflow junction or the area of the axillary SLN with the highest radioactive counts, a more practical method of processing the SLN would need to be developed to achieve a more focused evaluation of a single tissue block. Paish et al.37 developed an elegant and economic three-dimensional reconstruction (3DR) method to evaluate the spatial distribution of metastases in SLN. In this study, the afferent lymphatic pole was involved in 17/19 cases, but confined to the afferent pole only in 7 cases. Metastases were present at the efferent pole in 12/19 cases, and confined to the efferent pole only in two cases. Although the initial objectives of the study were to develop the method of 3DR and to understand of the early metastatic disease inside the lymph node, the authors incidentally found distinct metastatic growth patterns, classified as sinusoidal, nodular and diffuse. Although the number of cases was too small, their results suggested a poor prognosis associated to sinusoidal metastatic pattern and a good prognosis in the group of diffuse pattern37. Although a comparison does not seem appropriate, since the methods and objectives of our study were totally different, we found no significant risk of additional metastasis in the cases with the sinusoidal localization of metastasis, a pattern similar to that described by Paish et al.37 as having a poor prognosis.
We found fifteen cases of metastases located predominantly in the SLN capsule and/or subcapsular sinus, and 13 (81%) of them did not have additional metastases in NSLN. For metastasis with a component in the capsule, even associated with more extensive involvement, the risk of disease in NSLN is lower. Therefore, we can conclude that SLN metastasis restricted to capsule is a good predictor of no additional metastases in axillary lymph nodes.
On the other hand, metastasis in the SLN parenchyma (68%), was 5-times greater associated to the risk of additional NSLN involvement in univariate analyses and 1.5-times greater after multivariate analyses. Extranodal extension of the disease in the SLN was associated to a 4.2-time risk in univariate analysis. Consequently, it may be inferred that the metastasis situated in the capsule represent an initial stage of disease within the SLN, while the parenchymal and extranodal metastases represent an advanced stage of the disease, with bigger metastatic deposits and greater risk of additional involvement of NSLN. Metastasis above 6.0 mm together with the presence of PVI were the most powerful predictors of additional metastasis. There are some doubts about histological localization of metastasis within the SLN, as a tentative of identification of more aggressive cases and as an explanation about neoplastic involvement of SLN restricted to the capsule could be considered a real metastasis or it could correspond to mechanical transport of cells from the primary tumor.
At this moment, we did not analyze the pathological features impact on patients survival or the risk of regional recurrence. Further studies could investigate a possible role of the histological localization of metastasis, particularly the extranodal involvement, on the recurrence.
Our results show that the localization of metastatic cells within SLN is an important predictor of risk of additional metastasis and the findings are consistent with the pattern of metastatic dissemination through afferent vessels to extranodal spread.
Contributions of authors
César Augusto Alvarenga: Conceptualized the study and participated in the design of the manuscript and in the draft of the manuscript, and carried out the histopathological study; César Cabello dos Santos: Participated in the design of the manuscript and coordinated the study; Marcelo Alvarenga: Contributed to the writing of the manuscript and carried out the histopathological study; Paula Itagyba Paravidino: Participated in the writing of the manuscript and collaborated in the histopathological study; Sirlei Siani Morais: Carried out the statistical study; Henrique Benedito Brenelli: Provided critical revisions of the manuscript; Filomena Marino de Carvalho: Participated in the conceptualization and design of the manuscript, contributed with the statistical analysis.
All authors reviewed and approved the final manuscript.
Received: 08/06/2013
Accepted with modifications: 10/23/2013
Conflict of interests: none.
Study carried out at Instituto de Patologia de Campinas - Campinas (SP), Brazil.
- 1. Giuliano AE. Mapping a pathway for axillary staging: a personal perspective on the current status of sentinel lymph node dissection for breast cancer. Arch Surg. 1999;134(2):195-9.
- 2. Ernst MF, Voogd AC, Balder W, Klinkenbijl JH, Roukema JA. Early and late morbidity associated with axillary levels I-III dissection in breast cancer. J Surg Oncol. 2002;79(3):151-5.
- 3. Edge SB, Niland JC, Bookman MA, Theriault RL, Ottesen R, Lepisto E, et al. Emergence of sentinel node biopsy in breast cancer as standard-of-care in academic comprehensive cancer centers. J Natl Cancer Inst. 2003;95(20):1514-21.
- 4. Noguchi M. Sentinel lymph node biopsy as an alternative to routine axillary lymph node dissection in breast cancer patients. J Surg Oncol. 2001;76(2):144-56.
- 5. Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349(6):546-53.
- 6. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233-41.
- 7. Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569-75.
- 8. Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220(3):391-401.
- 9. Czerniecki BJ, Scheff AM, Callans LS, Spitz FR, Bedrosian I, Conant EF, et al. Immunohistochemistry with pancytokeratins improves the sensitivity of sentinel lymph node biopsy in patients with breast carcinoma. Cancer. 1999;85(5):1098-103.
- 10. Albertini JJ, Lyman GH, Cox C, Yeatman T, Balducci L, Ku N, et al. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA. 1996;276(22):1818-22.
- 11. Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, Bedoni M, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349(9069):1864-7.
- 12. Kamath VJ, Giuliano R, Dauway EL, Cantor A, Berman C, Ku NN, et al. Characteristics of the sentinel lymph node in breast cancer predict further involvement of higher-echelon nodes in the axilla: a study to evaluate the need for complete axillary lymph node dissection. Arch Surg. 2001;136(6):688-92.
- 13. Veronesi U, Paganelli G, Viale G, Galimberti V, Luini A, Zurrida S, et al. Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. J Natl Cancer Inst. 1999;91(4):368-73.
- 14. Van Zee KJ, Manasseh DM, Bevilacqua JL, Boolbol SK, Fey JV, Tan LK, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10(10):1140-51.
- 15. Cserni G, Boross G, Maráz R, Leidenius MH, Meretoja TJ, Heikkila PS, et al. Multicentre validation of different predictive tools of non-sentinel lymph node involvement in breast cancer. Surg Oncol. 2012;21(2):59-65.
- 16. American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th ed. New York: Springer Verlag; 2010.
- 17. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403-10.
- 18. Carcoforo P, Maestroni U, Querzoli P, Lanzara S, Maravegias K, Feggi L, et al. Primary breast cancer features can predict additional lymph node involvement in patients with sentinel node micrometastases. World J Surg. 2006;30(9):1653-7.
- 19. Fougo JL, Afonso M, Senhorães Senra F, Dias T, Leal C, Araújo C, et al. Predictive factors for non-sentinel lymph node involvement in breast cancer patients with a positive sentinel node: should we consider sentinel node-related factors? Clin Transl Oncol. 2009;11(3):165-71.
- 20. Hwang RF, Krishnamurthy S, Hunt KK, Mirza N, Ames FC, Feig B, et al. Clinicopathologic factors predicting involvement of nonsentinel axillary nodes in women with breast cancer. Ann Surg Oncol. 2003;10(3):248-54.
- 21. Mustać E, Matusan-Ilijas K, Marijić B, Smokvina M, Jonjić N. Predicting the likelihood of additional nodal metastases in breast carcinoma patients with positive sentinel node biopsy. Int J Surg Pathol. 2010;18(1):36-41.
- 22. Viale G, Maiorano E, Pruneri G, Mastropasqua MG, Valentini S, Galimberti V, et al. Predicting the risk for additional axillary metastases in patients with breast carcinoma and positive sentinel lymph node biopsy. Ann Surg. 2005;241(2):319-25.
- 23. Bolster MJ, Peer PG, Bult P, Thunnissen FB, Schapers RF, Meijer JW, et al. Risk factors for non-sentinel lymph node metastases in patients with breast cancer. The outcome of a multi-institutional study. Ann Surg Oncol. 2007;14(1):181-9.
- 24. Bernardi S, Bertozzi S, Londero AP, Giacomuzzi F, Angione V, Dri C, et al. Nine years of experience with the sentinel lymph node biopsy in a single Italian center: a retrospective analysis of 1,050 cases. World J Surg. 2012;36(4):714-22.
- 25. Abdessalam SF, Zervos EE, Prasad M, Farrar WB, Yee LD, Walker MJ, et al. Predictors of positive axillary lymph nodes after sentinel lymph node biopsy in breast cancer. Am J Surg. 2001;182(4):316-20.
- 26. Chu KU, Turner RR, Hansen NM, Brennan MB, Bilchik A, Giuliano AE. Do all patients with sentinel node metastasis from breast carcinoma need complete axillary node dissection? Ann Surg. 1999;229(4):536-41.
- 27. Fleming FJ, Kavanagh D, Crotty TB, Quinn CM, McDermott EW, O'Higgins N, et al. Factors affecting metastases to non-sentinel lymph nodes in breast cancer. J Clin Pathol. 2004;57(1):73-6.
- 28. Joseph KA, El-Tamer M, Komenaka I, Troxel A, Ditkoff BA, Schnabel F. Predictors of nonsentinel node metastasis in patients with breast cancer after sentinel node metastasis. Arch Surg. 2004;139(6):648-51.
- 29. Wong SL, Edwards MJ, Chao C, Tuttle TM, Noyes RD, Woo C, et al. Predicting the status of the nonsentinel axillary nodes: a multicenter study. Arch Surg. 2001;136(5):563-8.
- 30. Zavagno G, De Salvo GL, Bozza F, Scalco G, Marconato R, Valletta S, et al. Number of metastatic sentinel nodes as predictor of axillary involvement in patients with breast cancer. Breast Cancer Res Treat. 2004;86(2):171-9.
- 31. Meretoja TJ, Vironen JH, Heikkilä PS, Leidenius MH. Outcome of selected breast cancer patients with micrometastasis or isolated tumor cells in sentinel node biopsy and no completion axillary lymph node dissection. J Surg Oncol. 2010;102(3):215-9.
- 32. Ozcinar B, Muslumanoglu M, Igci A, Gurdal SO, Yavuz E, Kecer M, et al. Clinical importance of micrometastasis in sentinel lymph nodes. Breast. 2011;20(1):31-3.
- 33. Turner RR, Chu KU, Qi K, Botnick LE, Hansen NM, Glass EC, et al. Pathologic features associated with nonsentinel lymph node metastases in patients with metastatic breast carcinoma in a sentinel lymph node. Cancer. 2000;89(3):574-81.
- 34. Sachdev U, Murphy K, Derzie A, Jaffer S, Bleiweiss IJ, Brower S. Predictors of nonsentinel lymph node metastasis in breast cancer patients. Am J Surg. 2002;183(3):213-7.
- 35. Reynolds C, Mick R, Donohue JH, Grant CS, Farley DR, Callans LS, et al. Sentinel lymph node biopsy with metastasis: can axillary dissection be avoided in some patients with breast cancer? J Clin Oncol. 1999;17(6):1720-6.
- 36. Diaz LK, Hunt K, Ames F, Meric F, Kuerer H, Babiera G, et al. Histologic localization of sentinel lymph node metastases in breast cancer. Am J Surg Pathol. 2003;27(3):385-9.
- 37. Paish EC, Green AR, Rakha EA, Macmillan RD, Maddison JR, Ellis IO. Three-dimensional reconstruction of sentinel lymph nodes with metastatic breast cancer indicates three distinct patterns of tumour growth. J Clin Pathol. 2009;62(7):617-23.
Correspondence:
Publication Dates
-
Publication in this collection
10 Jan 2014 -
Date of issue
Nov 2013
History
-
Received
08 June 2013 -
Accepted
23 Oct 2013