Abstract
Store-operated Ca2+ entry plays an important role in Ca2+ homeostasis in cells but the mechanisms of control of these channels are not completely understood. We describe an investigation of the role of the CD38-cyclic-ADP-ribose (cADPR)-ryanodine-channel (RyR) signaling pathway in store-operated Ca2+ entry in human smooth muscle. We observed that human myometrial cells have a functional store-operated Ca2+ entry mechanism. Furthermore, we observed the presence of transient receptor potential 1, 3, 4, 5, and 6 ion channels in human myometrial cells. Store-operated Ca2+ transient was inhibited by at least 50-70% by several inhibitors of the RyR, including ryanodine (10 µM), dantrolene (10 µM), and ruthenium red (10 µM). Furthermore, the cell permeable inhibitor of the cADPR-system, 8-Br-cADPR (100 µM), is a potent inhibitor of the store-operated entry, decreasing the store operated entry by 80%. Pre-incubation of cells with 100 µM cADPR and the hydrolysis-resistant cADPR analog 3-deaza-cADPR (50 µM), but not with ADP-ribose (ADPR) leads to a 1.6-fold increase in the store-operated Ca2+ transient. In addition, we observed that nicotinamide (1-10 mM), an inhibitor of cADPR synthesis, also leads to inhibition of the store-operated Ca2+ transient by 50-80%. Finally, we observed that the transient receptor potential channels, RyR, and CD38 can be co-immunoprecipitated, indicating that they interact in vivo. Our observations clearly implicate the CD38-cADPR-ryanodine signaling pathway in the regulation of store-operated Ca2+ entry in human smooth muscle cells.
Cyclic-ADP-ribose; IP3; Endoplasmic reticulum; Ryanodine channel; Calcium entry; Human smooth muscle
Braz J Med Biol Res, June 2006, Volume 39(6) 739-748
Modulation of store-operated Ca2+ entry by cyclic-ADP-ribose
M. Thompson1, T. White2 and  Correspondence and Footnotes
Correspondence and Footnotes
 E.N. Chini1
E.N. Chini1
1Signal Transduction Laboratory, Departments of Anesthesiology and Internal Medicine, 2Department of Physiology, Mayo Clinic and Foundation, Rochester, MN, USA
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
Store-operated Ca2+ entry plays an important role in Ca2+ homeostasis in cells but the mechanisms of control of these channels are not completely understood. We describe an investigation of the role of the CD38-cyclic-ADP-ribose (cADPR)-ryanodine-channel (RyR) signaling pathway in store-operated Ca2+ entry in human smooth muscle. We observed that human myometrial cells have a functional store-operated Ca2+ entry mechanism. Furthermore, we observed the presence of transient receptor potential 1, 3, 4, 5, and 6 ion channels in human myometrial cells. Store-operated Ca2+ transient was inhibited by at least 50-70% by several inhibitors of the RyR, including ryanodine (10 µM), dantrolene (10 µM), and ruthenium red (10 µM). Furthermore, the cell permeable inhibitor of the cADPR-system, 8-Br-cADPR (100 µM), is a potent inhibitor of the store-operated entry, decreasing the store operated entry by 80%. Pre-incubation of cells with 100 µM cADPR and the hydrolysis-resistant cADPR analog 3-deaza-cADPR (50 µM), but not with ADP-ribose (ADPR) leads to a 1.6-fold increase in the store-operated Ca2+ transient. In addition, we observed that nicotinamide (1-10 mM), an inhibitor of cADPR synthesis, also leads to inhibition of the store-operated Ca2+ transient by 50-80%. Finally, we observed that the transient receptor potential channels, RyR, and CD38 can be co-immunoprecipitated, indicating that they interact in vivo. Our observations clearly implicate the CD38-cADPR-ryanodine signaling pathway in the regulation of store-operated Ca2+ entry in human smooth muscle cells.
Key words: Cyclic-ADP-ribose, IP3, Endoplasmic reticulum, Ryanodine channel, Calcium entry, Human smooth muscle
Introduction
The agonist-induced increase in intracellular free Ca2+ concentration is an important component of several signaling pathways (1). Two major mechanisms can increase cytosolic free Ca2+ in cells, namely: a) release of Ca2+ from intracellular stores, and b) influx of Ca2+ from the extracellular media into the cell. It has been shown that agonist-stimulated intracellular Ca2+ transients are biphasic, with the initial, transient rise in Ca2+ representing sarcoplasmic Ca2+ release (1-4), and the later sustained Ca2+ response representing predominantly Ca2+ influx (1-4). One of the mechanisms of extracellular Ca2+ influx is the so-called store-operated Ca2+ entry (2-5). The store-operated Ca2+ entry (also called capacitative Ca2+ entry) appears to be mediated by a large family of channels called the transient receptor potential ion channels (TRPC; 2-5). In this process depletion of intracellular Ca2+ stores leads to activation of channels located in the plasma membrane that are permeable to Ca2+ (2-5). The mechanisms of activation of TRPCs by store depletion are not completely understood (2-5). Several intracellular signaling pathways have been implicated as regulators of the TRPCs, including the IP3, cAMP, and cGMP systems of second messengers (2-7). Cyclic-ADP-ribose (cADPR) is a calcium-mobilizing metabolite of ß-NAD. cADPR was initially described as a potent activator of the ryanodine receptor channel (RyR) and as an endogenous modulator of Ca2+ release from intracellular stores (8-12). Guse et al. (13) were the first to implicate cADPR as a potential activator of Ca2+ entry in T-lymphocytes. More recently, it has been demonstrated that cADPR can also regulate the influx of extracellular Ca2+ into neutrophils, suggesting a mechanism involving activation of store-operated Ca2+ entry (14,15). However, whether cADPR is a modulator of store-operated Ca2+ entry has not been completely elucidated. Recently, Kiselyov et al. (16,17) described a direct interaction of the RyR with TRPC3. Furthermore, they reported that activation of the RyR leads to gating of the capacitative Ca2+ entry (16,17). Because of the emerging importance of cADPR as a cellular modulator of Ca2+ homeostasis, we explored the role of the CD38-cADPR-RyR signaling pathway in the activation of the store-operated Ca2+ transient. We found that cADPR is an endogenous regulator of the store-operated Ca2+ transient in human smooth muscle cells, and that TRPCs, RyR, and CD38 can be co-immunoprecipitated, suggesting that these proteins can interact in vivo.
Material and Methods
Cell preparation
Following Institutional Research Board approval, human myometrium was obtained from patients undergoing elective hysterectomy. Human myometrial cells were isolated using techniques previously described (18). Briefly, the tissue was minced in Hanks' balanced salt solution (HBSS) containing 10 mM glucose and 10 mM HEPES, pH 7.4. The tissue was then suspended in fresh HBSS, aerated with 95% O2/5% CO2, and incubated in a 37ºC water bath with gentle shaking for 2 h in the presence of 20 U/mL papain and 2,000 U/mL DNase. Subsequently, the tissue was incubated for an additional 2 h at 37ºC, with the addition of 1 mg/mL type IV collagenase. Human myometrial cells were released by triturating and then centrifuged and suspended in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, 100 U/L penicillin, 100 µg/L streptomycin, and 2.5 µg/mL amphotericin B. Cultures were grown and maintained in 75-cm2 plastic flasks in a humidified incubator supplied with 5% CO2/95% air at 37ºC. Subcultures were obtained as needed by detaching the cells with a Ca2+/Mg2+-free HBSS solution containing 0.25% trypsin and 5 mM EDTA. Only cultures between passages 2 and 10 were used. Cells isolated by this procedure stain positive for smooth muscle actin and negative for keratin. For the experiments, cells were made quiescent by replacing the growth medium with DMEM without serum. Cell medium was again replaced with DMEM containing testing agents solubilized in 0.1% DMSO or water at the appropriate final concentrations.
Fura 2 loading and [Ca2+]i measurements
Human myometrial smooth muscle cells were plated onto 8-chambered Lab-Teks plates (Nalge Nunc International Corp., Naperville, IL, USA) at a density of 25,000 cells/well and grown until approximately 80% confluence in DMEM. Cells were then washed in HBSS and medium was replaced with DMEM lacking serum for 24 h. Cells were then incubated with 5 µM Fura 2/AM and test agents for 60 min at 37ºC and washed 3X with HBSS containing 1 mM MgSO4/not containing CaCl2. The Lab-Teks plate was then transferred to an inverted Nikon Diaphot microscope equipped with a Princeton Instrument RTE/CCD 12-bit digital camera (Trenton, NJ, USA) controlled through a PC workstation and feed inlets and vacuum outlets for solution changes. A Nikon 20X/0.75 objective lens was used and image size was set to 720 x 540 pixels. A fluorescent light excited the cells alternatively at 340 and 380 nm with a Lambda 10 filter wheel (Sutter Instruments, Novato, CA, USA). Emitted fluorescence (510 nm) was acquired for 100 ms at each excitation wavelength. Cells were perfused for 2 min with HBSS containing 2 mM calcium followed by 4 min of perfusion with HBSS not containing calcium. Subsequently, the cells were perfused with HBSS not containing calcium, 1 µM nifedipine and HBSS not containing calcium, 1 µM nifedipine, and 10 µM cyclopiazonic acid (CPA) for 4 min each. Finally, cells were perfused with HBSS containing 2 mM calcium, 1 µM nifedipine, and 10 µM CPA. Influx of calcium was determined using Metamorph software (Jandel Scientific, Santa Barbara, CA, USA) and averaging changes in fluorescent ratio of 50 individual cells by the following equation: [Ca2+] = Kd*(Fmin/Fmax)*((R-visc*Rmin)/(visc*Rmax-R)), where Kd is the apparent dissociation constant (224 nM at room temperature), F stands for fluorescence at 380 nm, and R for the ratio at 340/389 nm. Fmin and Rmin are the measurements in the absence of Ca2+, and Fmax and Rmax are the fluorescent values at 380 nm and 340/380 nm ratios in saturating Ca2+, R is equivalent to the ratio of fluorescent intensity at 340/380 nm minus the background, and visc is the viscosity value of the cytoplasm (visc = 1). Alternatively, cells were seeded onto 9 x 22-mm coverslips and grown as previously described. When cells were approximately 80% confluent, coverslips were loaded with Fura 2/AM, rinsed in HBSS not containing CaCl2 and placed in the thermostated cuvette (25ºC) of a Hitachi F-2000 fluorescence spectrophotometer. Cells were then monitored as previously described and results determined by comparing the 340/380-nm wavelength ratio.
Immunoprecipitation and Western blot
Human myometrial cells were rinsed twice with HBSS, harvested and subjected to sonication (3 x 5 s) in radio-immunoprecipitation assay (RIPA) homogenizing buffer containing 1 µmol/L PMSF. The homogenates were centrifuged at 10,000 g for 10 min and the resultant supernatant was assayed for protein using the DC protein assay (BioRad, Hercules, CA, USA). The lysates (200 µL) were adjusted to contain 200 µg protein and 2 µg mouse monoclonal anti-CD38 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti-RyR (Santa Cruz Biotechnology), or rabbit polyclonal anti-TRPC antibody (Alomone Labs, Jerusalem, Israel) added overnight at 4oC with gentle rocking. The antibody-protein complex was then immunoprecipitated for 2 h using Protein A/G Plus-Agarose (Santa Cruz Biotechnology), the pellet was washed twice in RIPA buffer, and resuspended in 30 µL RIPA buffer and 30 µL Laemmli buffer. The supernatants were denatured at 100ºC for 3-5 min and 40 µL of the sample subjected to SDS-PAGE using the Criterion Gel System (BioRad) and a 4-15% gradient gel. The gels were run at a constant current of 200 V for 70 min followed by transfer to PVDF membranes (BioRad). The membranes were blocked for 1 h in 5% nonfat dry milk in Tris-buffered saline (TBS) containing 0.1% Tween 20 followed by incubation with appropriate primary antibodies and horseradish-peroxidase-conjugated secondary antibody or horseradish-peroxidase-linked Protein A. Blots were then visualized by exposing them to BioMax MR film (Eastman Kodak Co., Rochester, NY, USA) using LumiGLO (Cell Signaling Technology, Beverly, MA, USA). The specificity of the immunoprecipitation was determined by the use of non-specific IgG (Santa Cruz Biotechnology) in control experiments.
Immunocytochemistry of transient receptor potential channels
Cells were grown on 8-chambered Lab-Teks plates as described, fixed in 2% paraformaldehyde, rinsed three times in TBS and blocked in 10% donkey serum for 1 h at room temperature with gentle shaking. The blocking solution was aspirated and rabbit polyclonal anti-TRPC antibody diluted 1:200 in 5% donkey serum added overnight at 4ºC with gentle rocking. Following the incubation period, chambers were aspirated, rinsed three times in TBS and donkey anti-rabbit CY3-linked secondary antibody (Sigma, St. Louis, MO, USA) diluted 1:200 in TBS was added for 3 h at room temperature with gentle shaking. Cells were rinsed three times in TBS, dried and visualized with an Olympus confocal microscope using Fluoview version 2.0 software.
Material
Fura 2/AM was purchased from Calbiochem (San Diego, CA, USA). 3-Deaza-cADPR was purchased from Molecular Probes (Eugene, OR, USA). All other reagents, of the highest purity grade available, were supplied by Sigma.
The experiments were repeated 3-6 times and data are reported as mean ± SEM. The unpaired t-test was used to establish statistical significance, with P values <0.05 considered to be significant.
Results
Role of cADPR in agonist-stimulated Ca2+ transients
We have demonstrated that human smooth muscle cells utilize the CD38/cADPR pathway to modulate [Ca2+]i transients during agonist stimulation (18). The components of this pathway are present and functional in myometrial, vascular and airway smooth muscle cells (18-24). One important observation is the fact that the intracellular Ca2+ transients induced by oxytocin are dependent on both the CD38/cADPR signaling pathway and extracellular Ca2+ in human myometrial cells. Stimulation of myometrial cells with oxytocin, vasopressin and endothelin-1 leads to [Ca2+]i responses that are largely dependent on influx of extracellular Ca2+, whereas responses to bradykinin or histamine are not (Figure 1). An interesting observation is that agonist responses that are dependent on extracellular Ca2+, i.e., oxytocin, vasopressin and endothelin-1, are also significantly inhibited by 8-Br-cADPR, a cell permeant antagonist of the cADPR signaling pathway, and the level of inhibition is similar to levels observed in the absence of extracellular Ca2+ (Figure 1). An equally intriguing observation is that the responses to bradykinin or histamine, which are largely independent on Ca2+ influx, are attenuated by 8-Br-cADPR to a much lesser extent and again the attenuation mimics the effects of zero extracellular Ca2+ (Figure 1). These results clearly implicate the CD38/cADPR signaling pathway in agonist-induced Ca2+ responses involving Ca2+ influx, as 8-Br-cADPR significantly attenuates those responses primarily dependent on Ca2+ influx.
Store-operated Ca2+ transients in human smooth muscle cells
A significant amount of Ca2+ influx in smooth muscle cells is mediated by store-operated Ca2+ entry (25-27). First, we examined whether myometrial cells contain a functional store-operated Ca2+ entry pathway. Store-operated Ca2+ entry can be evoked in cells by depletion of intracellular Ca2+ stores, namely the sarcoplasmic reticulum (SR) in smooth muscle cells. Using CPA or thapsigargin (Tg), inhibitors of the SR Ca2+ ATPase, to deplete the stores we found that human smooth muscle cells have a functional store-operated Ca2+ transient pathway (Figure 2A and data not shown). The Ca2+ influx stimulated by depletion of intracellular stores was not inhibited by 1 µM nifedipine (an inhibitor of the L-type Ca2+ channels). Furthermore, CPA- and Tg-induced Ca2+ transients were completely blocked by the store-operated Ca2+ entry inhibitors 100 µM 2-APB and 1 mM Gd3+ (Figure 2B) and the relatively non-specific cation channel blockers 1 mM La3+, 1 mM Ni2+, and 10 µM SKF-96365 (data not shown). These data indicate that human smooth muscle cells exhibit functional store-operated Ca2+ entry. Next, we determined the expression of TRPCs on human smooth muscle cells. In agreement with previously published data we observed that several isoforms of TRPCs are expressed in human smooth muscle cells (26,27). We observed expression of TRPC1, 3, 4, 5, and 6 protein in myometrial cells by immunohistochemistry (Figure 3). In summary, these data demonstrate that transient receptor potential channels are expressed and that a Ca2+ influx pathway typical of store-operated Ca2+ entry is present in human myometrial cells.
Role of the RyR in the store-operated Ca2+ transient pathway in human smooth muscle cells
The mechanisms regulating the activity of the store-operated Ca2+ entry have not been completely elucidated. One possibility is that intracellular Ca2+ channels and signaling pathways are coupled to the activation of TRPCs resulting in store-operated Ca2+ entry. In fact, both IP3 and the RyR have been implicated as modulators of the gating properties of TRPCs (5,16,17). It has been previously shown that the RyR co-immunoprecipitates with TRPC3 (16,17), and that activation of the RyR leads to gating of the TRPC (16,17). In fact, it has been proposed previously that coupling between the RyR and store-operated Ca2+ entry is important for the modulation of this Ca2+ influx pathway (16,17).
We have shown that human smooth muscle cells have functional RyR (18). Here we explored the role of the RyR in CPA-stimulated store-operated Ca2+ transients. We found that inhibition of the activity of RyRs using three different structurally unrelated RyR inhibitors, namely ruthenium red, dantrolene, and ryanodine, leads to a significant decrease in CPA-induced store-operated Ca2+ transients (Figure 4B). These data indicate that modulation of the activity of RyRs mediates store-operated Ca2+ entry in human myometrial cells. Inhibition of store-operated Ca2+ transients by RyR inhibition is likely due to gating of TRPCs as opposed to decreased SR depletion, as we also observed that CPA- and Tg-induced intracellular Ca2+ store depletion was not altered by the RyR inhibitors used in this study. Next we examined whether coupling of RyRs and store-operated Ca2+ transients may involve a direct association of RyRs with TRPCs. Immunoprecipitation of TRPCs and the subsequent immunoblotting for the RyR revealed co-immunoprecipitation of the RyR with TRPC3 and TRPC5 (Figure 4A). Combined, these data imply a coupling of RyRs with the store-operated Ca2+ entry pathway possibly via direct interaction with TRPCs.
Role of cADPR in store-operated Ca2+ entry
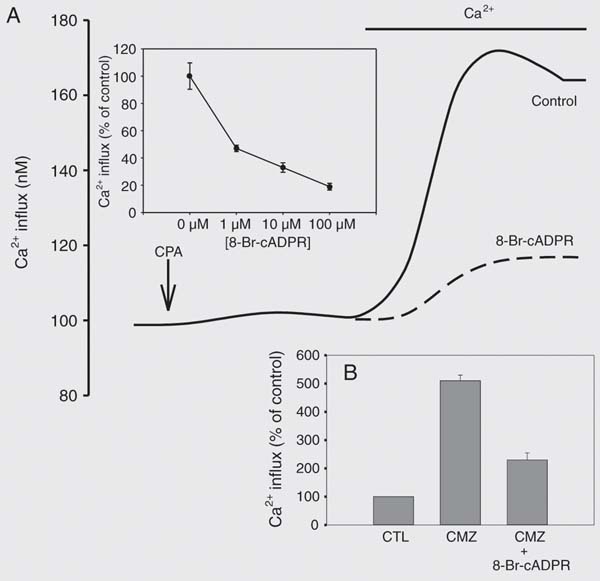
cADPR has recently emerged as a second messenger mediating Ca2+ homeostasis in a variety of cell types by acting as an endogenous modulator of the RyR (8-12). We have shown that cADPR is important for agonist-stimulated Ca2+ transients in smooth muscle cells (18-21). Since RyR activity appears to be important for the gating of the store-operated Ca2+ entry channels and cADPR is an endogenous regulator of RyR activity, we explored the role of this nucleotide on the store operated Ca2+ entry pathway. Using a cell-permeable cADPR antagonist, 8-Br-cADPR, we determined that cADPR modulates both CPA- and Tg-stimulated store-operated Ca2+ transients (Figure 5A). We observed that 8-Br-cADPR dose-dependently inhibits the store-operated Ca2+ transient (Figure 5A, inset). Recently, Bolotina and colleagues (27) have demonstrated that calmidazolium (CMZ) activates the store-operated Ca2+ influx pathway through a mechanism involving phospholipase A2. This mechanism leads to the generation of lysophospholipids which then triggers store-operated Ca2+ channels. We examined whether CMZ activates store-operated Ca2+ transients in human myometrial cells. CMZ treatment enhanced store-operated Ca2+ transients and this enhancement was significantly inhibited by 8-Br-cADPR (Figure 5B). This would suggest that cADPR modulates store-operated Ca2+ via a mechanism upstream of phospholipase A2 (27). In addition, we observed that pre-incubation of human myometrial cells with cADPR or the hydrolysis-resistant cADPR agonist, 3-deaza-cADPR, leads to more than a 50% increase in CPA-induced store-operated Ca2+ transients (Figure 6B). In contrast, the cADPR metabolite ADPR had no effect on store-operated Ca2+ transients (Figure 6B). Next, we used an inhibitor of cADPR synthesis, nicotinamide, to determine the role of endogenous synthesis of this nucleotide in the activity of store-operated Ca2+ transients. We found that pre-incubation of cells with nicotinamide leads to a significant dose-dependent inhibition of CPA-stimulated store-operated Ca2+ transients (Figure 6A). Finally, we explored the possibility of a direct association of CD38, the enzyme responsible for cADPR synthesis, with TRPCs by co-immunoprecipitation studies. Immunoprecipitation of TRPC1, 3, 4, 5, and 6 with the subsequent Western blotting for CD38 revealed that CD38 was co-immunoprecipitated with all of the TRPC family members examined (Figure 7). In agreement with this observation, immunoprecipitation of CD38 followed by probing for the TRPC isoforms also revealed tight association of CD38 with the various TRPC isoforms (Figure 8). These data demonstrate a role for the CD38/cADPR signaling pathway in modulating store-operated Ca2+ entry in human myometrial cells and suggest a mechanism involving the coupling of TRPCs to all of the players in the CD38/cADPR signaling pathway.
Role of cADPR on agonist stimulated Ca2+ transients. Human myometrial smooth muscle cells were grown as described in the Material and Methods section on 9 x 22-mm coverslips. Cells were then incubated with 8-Br-cADPR (100 µM) or vehicle (controls and 0 mM Ca2+) for 60 min, the coverslips were rinsed with HBSS containing 2 or 0 mM Ca2+ and the calcium transients measured with the stated agonists (final concentrations = 1 µM for oxytocin (Oxy), vasopressin (AVP), and endothelin-1 (ET-1); 10 nM for bradykinin (BK), and 10 µM for histamine (Hist)). CTL = control. Data are reported as percentage of control and are the means ± SEM of four independent experiments. The treatment of cells with 8-Br-cADPR or not containing Ca2+ (0 mM Ca2+) led to a significant decrease of the oxytocin-, vasopressin- and endothelin-induced Ca2+ transients. These treatments had no significant effect on the Ca2+ release induced by bradykinin or histamine.
Characterization of store-operated calcium transients in human myometrial smooth muscle cells. Influx of calcium was measured as described in the Material and Methods section. A, Human myometrial cells were perfused with HBSS containing 0 mM Ca2+ for 4 min with or without CPA (10 µM) followed by perfusion with HBSS containing 2 mM Ca2+. Influx of calcium in cells not perfused with CPA was 95% less than cells perfused with CPA (6 vs 122 nM Ca2+, respectively). The figure is representative of three different experiments. B, Cells were grown on 9 x 22-mm coverslips, loaded with 5 µM Fura 2-AM, rinsed briefly in HBSS containing 0 mM Ca2+ and the coverslips placed in a cuvette with calcium-free HBSS. Cells were then treated with or without CPA (10 µM), 2-APB (100 µM), Gd3+ (1 mM), or nifedipine (1 µM final concentrations). Results are reported as percentage of control and are representative of three to five independent experiments. CTL = control; CPA = cyclopiazonic acid; 2-APB = 2-aminoethoxydiphenyl borate; Gd3+ = gadolinium.
Immunocytochemistry of transient receptor protein channel (TRCP) 1, 3, 4, 5, and 6. Cells were grown as described in Material and Methods on 8-chambered Lab-Teks plates until approximately 80% confluence. Cells were then fixed with 2% paraformaldehyde, rinsed in TBS, and blocked for 1 h in 10% donkey serum, and rabbit polyclonal anti-human TRPC antibodies were added overnight. Cells were rinsed in TBS, anti-rabbit CY3-linked secondary antibody added for 1 h and the TRPC visualized using an Olympus confocal microscope. Controls consisted of adding secondary antibody only. Figures are representative of two separate experiments.
Immunoprecipitation of ryanodine receptor (RyR) with transient receptor protein channel (TRPC) antibodies and the effect of ryanodine receptor antagonists on calcium transients. A, Cell extracts were prepared in RIPA buffer, proteins adjusted to 200 µg/vial and 2 µg rabbit polyclonal anti-TRPC antibodies added overnight. The antibody-protein complex was then immunoprecipitated using Protein A/G-agarose, the pellet washed, resuspended in Laemmli buffer, and the sample separated by SDS-PAGE (4-15% gel) using standard protocols. The proteins were transferred to a PVDF membrane and the RyR detected using a rabbit polyclonal antibody. Results indicate bands >250 kDa associated with TRPC3 and 5 and weakly with TRPC4 immunoprecipitates. The arrow indicates the molecular mass of RyR. The figure is representative of three different experiments. B, Human myometrial smooth muscle cells were grown in 8-chambered Lab-Teks plates, incubated for 60 min with antagonists (10 µM each) or vehicle (controls), loaded with Fura 2-AM and store-operated calcium transient was measured. All test agents inhibited store-operated Ca2+ transients (ruthenium red = -70%, dantrolene = -48%, ryanodine = -77%) compared to controls. Data are reported as means ± SEM for at least three independent experiments. *P < 0.05 compared to control (unpaired t-test).
Effect of 8-Br-cADPR on store-operated Ca2+ transients. In A we show a representative tracing of the treatment of human myometrial cells with cyclopiazonic acid (CPA) and Ca2+ as described in Figure 2, in the absence or presence of pre-incubation with 100 µM 8-Br-cADPR. Inset, Human myometrial smooth muscle cells were grown in 8-chambered Lab-Teks plates and incubated for 60 min with different concentration(s) of 8-Br-cADPR prior to measuring the calcium transients. In comparison to controls, 8-Br-cADPR produced significant inhibition at all tested concentrations (1 µM = -53%, 10 µM = -68%, 100 µM = -82%). Data are reported as the mean of three independent experiments. *P < 0.05 compared to controls (unpaired t-test). B, Smooth muscle cells were grown on 9 x 22-mm coverslips, loaded with 5 µM Fura 2-AM, incubated with 8-Br-cADPR (100 µM) for 60 min and rinsed briefly in HBSS containing 0 mM Ca2+. Coverslips were placed in a cuvette containing calcium-free HBSS for 4 min and treated with 1 µM calmidazolium (CMZ) for 4 min; 2 mM Ca2+ was added and store-operated Ca2+ transients were determined. Cells treated with calmidazolium demonstrated store-operated Ca2+ transients which were significantly (P £ 0.05, unpaired t-test) inhibited by 8-Br-cADPR (-60%). Data are reported as percentage of control and are the means ± SEM of three independent experiments. 8-Br-cADPR = 8-Br-cyclic-ADP-ribose; CTL = control.
Effect of nicotinamide and cADPR on store-operated Ca2+. A, Nicotinamide was incubated for 60 min with human myometrial smooth muscle cells grown in 8-chambered Lab-Teks plates prior to measuring the store-operated calcium transient, as described in the Material and Methods section. Concentrations of 5 mM (-27%) and 10 mM (-57%) nicotinamide produced significant inhibition in comparison to controls (P < 0.05, paired t-test). B, ADPR, cADPR, and 3-deaza-cADPR were incubated for 60 min with cells followed by measurement of store-operated Ca2+ transient. cADPR (100 µM) and 3-deaza-cADPR (50 µM) produced a significant (P < 0.05, compared to control, unpaired t-test) stimulation of store-operated Ca2+ transients compared to control. ADPR had no significant effect. Data are reported as means ± SEM of three independent experiments and are reported as percentage of controls. cADPR = cyclic-ADP-ribose.
Co-immunoprecipitation of CD38 with the transient receptor protein channels (TRPC). Cell extracts were prepared in RIPA buffer and immunoprecipitated with a rabbit polyclonal anti-TRPC antibody as previously described. The resultant antibody-protein complex was electrophoresed and transferred to a PVDF membrane as described in the Material and Methods section. The blot was then probed with a mouse monoclonal anti-CD38 antibody and the film developed following incubation with a horse radish peroxidase-linked secondary donkey anti-mouse antibody. A ~50-kDa band corresponding to CD38 was associated with TRPC1, 3, 4, 5, and 6 immunoprecipitates. Control experiments were performed as above using a non-specific (NS) rabbit polyclonal IgG antibody which produced no corresponding 50-kDa band. Data are representative of 3-5 experiments.
Co-immunoprecipitation of the transient receptor protein channels (TRPC) with CD38. Cell extracts were prepared as described previously and immunoprecipitated with a mouse monoclonal anti-CD38 antibody. The resultant antibody-protein complex was electrophoresed and transferred to a PVDF membrane as described in the Material and Methods section. The blot was then probed with rabbit polyclonal anti-TRPC1, 3, 4, 5, and 6 antibodies. Following incubation with horse radish peroxidase-linked Protein A, the film was developed, showing bands corresponding to the ~100-kDa marker. Control experiments were performed similarly using a non-specific mouse monoclonal IgG antibody. Data are representative of 3-5 experiments. IP = immunoprecipitation.
Discussion
The mechanisms governing the activation of store-operated Ca2+ entry are not completely understood. In the present study, we demonstrate that store-operated Ca2+ entry exists in human smooth muscle cells and that key players in the CD38-cADPR-RyR signaling pathway are involved in modulating this Ca2+ entry. This is the first study which implicates cADPR as an endogenous modulator of store-operated Ca2+ entry in human smooth muscle cells. We also demonstrate that modulation of the RyR leads to modulation of store-operated Ca2+ entry, as inhibitors of the RyR also inhibited store-operated Ca2+ entry in human myometrial cells. We also show that CD38, RyR and members of the all TRPC family can be co-immunoprecipitated, suggesting a direct interaction of the CD38/cADPR/RyR and store-operated Ca2+ entry signaling pathways. A role for cADPR in mediating Ca2+ influx was first suggested in T-lymphocytes (13). In that study, microinjection of cADPR into Jurkat T-lymphocytes induced Ca2+ spikes which were almost completely dependent on the presence of extracellular Ca2+. Further evidence was provided in studies of neutrophils from CD38-deficient mice (14). In those studies stimulation of neutrophils from wild-type mice showed a biphasic Ca2+ response, with an initial transient rise followed by a second transient rise.
The second rise in [Ca2+]i was dependent on the presence of extracellular Ca2+ and was absent in neutrophils from CD38-deficient mice. The second rise was also inhibited by the cADPR antagonist 8-Br-cADPR. Interestingly, in the present study, agonists that depend largely on Ca2+ influx, namely oxytocin, endothelin-1 and vasopressin, in human myometrial cells were also largely dependent on cADPR-induced Ca2+ mobilization and the presence of extracellular Ca2+. Antagonism of the cADPR signaling pathway using 8-Br-cADPR resulted in almost complete attenuation of the Ca2+ responses and this attenuation was mimicked by the omission of extracellular Ca2+. An attractive hypothesis, and one that is supported by the data presented here, is that signaling pathways activated during agonist stimulation are coupled with the gating of the TRPCs (5,16,17). We suggest a model in which agonist stimulation leads to the generation
of cADPR and that the cADPR produced leads to activation of RyR which are in turn coupled to TRPCs in the plasma membrane resulting in store-operated Ca2+ influx.
We also provide evidence that cADPR may directly modulate gating of the TRPCs since CPA-induced activation of store-operated Ca2+ entry can also be inhibited by 8-Br-cADPR although we cannot rule out the possibility that 8-Br-cADPR is again acting via inhibition of the RyR. The co-immunoprecipitation of CD38 and RyR with TRPCs suggests a novel multi-protein signaling complex which may modulate communication between intracellular Ca2+ stores and plasma membrane Ca2+ channels.
This is the first evidence for the coupling of Ca2+ channels at both the SR and plasma membrane and a second messenger-generating enzyme. The conformational coupling of SR Ca2+ channels and plasma membrane Ca2+ channels was first described in skeletal muscle cells. In these studies, conformational coupling was demonstrated between RyR in the SR and dihydropyridine receptors in the plasma membrane (28). Kiselyov et al. (16,17) have recently provided evidence for the conformational coupling of IP3Rs and RyRs to TRPCs. Couplings of IP3Rs with TRPC3 or RyRs with TRPC3 were found to be mutually exclusive and involved segregation of these complexes in microdomains (16,17).
We hypothesize that in human smooth muscle cells, microdomains exist that allow for the conformational coupling of RyR and CD38 to TRPCs. Although most of the data presented here were obtained with human myometrial cells, our observation that cADPR can modulate store-operated Ca2+ entry appears to apply to other cell types, since 8-Br-cADPR can also inhibit the store-operated Ca2+ in HL-60 cells by 82%.
We conclude that cADPR is a regulator not only of Ca2+ release from intracellular stores mediated by the RyR, but also of the mechanisms of extracellular Ca2+ influx mediated by the TRPCs. This regulation appears to be mediated by a direct interaction between the components of the cADPR-CD38-RyR pathway and the TRPCs. A similar interaction between the inositol 1,4,5-triphosphate system and the store-operated Ca2+ entry pathway has been proposed (5). It is possible that the crosstalk between intracellular channels and the plasma membrane may play an important role in the mechanism of replacement of intracellular Ca2+ stores.
References
1. Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 2000; 1: 11-21.
2. Putney JW Jr, Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J Cell Sci 2001; 114: 2223-2229.
3. Putney JW Jr The enigmatic TRPCs: multifunctional cation channels. Trends Cell Biol 2004; 14: 282-286.
4. Putney JW Jr, Trebak M, Vazquez G, Wedel B, Bird GS. Signalling mechanisms for TRPC3 channels. Novartis Found Symp 2004; 258: 123-133.
5. van Rossum DB, Patterson RL, Kiselyov K, Boehning D, Barrow RK, Gill DL, et al. Agonist-induced Ca2+ entry determined by inositol 1,4,5-trisphosphate recognition. Proc Natl Acad Sci USA 2004; 101: 2323-2327.
6. Wedel BJ, Vazquez G, McKay RR, St JB, Putney JW Jr. A calmodulin/inositol 1,4,5-trisphosphate (IP3) receptor-binding region targets TRPC3 to the plasma membrane in a calmodulin/IP3 receptor-independent process. J Biol Chem 2003; 278: 25758-25765.
7. Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2004; 286: L909-L917.
8. Lee HC, Walseth TF, Bratt GT, Hayes RN, Clapper DL. Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J Biol Chem 1989; 264: 1608-1615.
9. Dousa TP, Chini EN, Beers KW. Adenine nucleotide diphosphates: emerging second messengers acting via intracellular Ca2+ release. Am J Physiol 1996; 271: C1007-C1024.
10. Galione A, White A. Ca2+ release induced by cyclic ADP-ribose. Trends Cell Biol 1994; 4: 431-436.
11. Galione A, Lee HC, Busa WB. Ca(2+)-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-ribose. Science 1991; 253: 1143-1146.
12. Guse AH. Cyclic ADP-ribose: a novel Ca2+-mobilising second messenger. Cell Signal 1999; 11: 309-316.
13. Guse AH, Berg I, da Silva CP, Potter BV, Mayr GW. Ca2+ entry induced by cyclic ADP-ribose in intact T-lymphocytes. J Biol Chem 1997; 272: 8546-8550.
14. Partida-Sanchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat Med 2001; 7: 1209-1216.
15. Partida-Sanchez S, Iribarren P, Moreno-Garcia ME, Gao JL, Murphy PM, Oppenheimer N, et al. Chemotaxis and calcium responses of phagocytes to formyl peptide receptor ligands is differentially regulated by cyclic ADP ribose. J Immunol 2004; 172: 1896-1906.
16. Kiselyov KI, Shin DM, Wang Y, Pessah IN, Allen PD, Muallem S. Gating of store-operated channels by conformational coupling to ryanodine receptors. Mol Cell 2000; 6: 421-431.
17. Kiselyov K, Shin DM, Shcheynikov N, Kurosaki T, Muallem S. Regulation of Ca2+-release-activated Ca2+ current (Icrac) by ryanodine receptors in inositol 1,4,5-trisphosphate-receptor-deficient DT40 cells. Biochem J 2001; 360: 17-22.
18. Barata H, Thompson M, Zielinska W, Han YS, Mantilla CB, Prakash YS, et al. The role of cyclic-ADP-ribose-signaling pathway in oxytocin-induced Ca2+ transients in human myometrium cells. Endocrinology 2004; 145: 881-889.
19. White TA, Kannan MS, Walseth TF. Intracellular calcium signaling through the cADPR pathway is agonist specific in porcine airway smooth muscle. FASEB J 2003; 17: 482-484.
20. Yusufi AN, Cheng J, Thompson MA, Burnett JC, Grande JP. Differential mechanisms of Ca(2+) release from vascular smooth muscle cell microsomes. Exp Biol Med 2002; 227: 36-44.
21. de Toledo FG, Cheng J, Liang M, Chini EN, Dousa TP. ADP-Ribosyl cyclase in rat vascular smooth muscle cells: properties and regulation. Circ Res 2000; 86: 1153-1159.
22. Li P, Zou AP, Campbell WB. Metabolism and actions of ADP-riboses in coronary arterial smooth muscle. Adv Exp Med Biol 1997; 419: 437-441.
23. Chini EN, Klener P Jr, Beers KW, Chini CC, Grande JP, Dousa TP. Cyclic ADP-ribose metabolism in rat kidney: high capacity for synthesis in glomeruli. Kidney Int 1997; 51: 1500-1506.
24. Kannan MS, Fenton AM, Prakash YS, Sieck GC. Cyclic ADP-ribose stimulates sarcoplasmic reticulum calcium release in porcine coronary artery smooth muscle. Am J Physiol 1996; 270: H801-H806.
25. Shlykov SG, Yang M, Alcorn JL, Sanborn BM. Capacitative cation entry in human myometrial cells and augmentation by hTrpC3 overexpression. Biol Reprod 2003; 69: 647-655.
26. Yang M, Gupta A, Shlykov SG, Corrigan R, Tsujimoto S, Sanborn BM. Multiple Trp isoforms implicated in capacitative calcium entry are expressed in human pregnant myometrium and myometrial cells. Biol Reprod 2002; 67: 988-994.
27. Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol 2004; 6: 113-120.
28. Beam KG, Franzini-Armstrong C. Functional and structural approaches to the study of excitation-contraction coupling. Methods Cell Biol 1997; 52: 283-306.
 Address for correspondence: E.N. Chini, Department of Anesthesiology, Mayo Clinic and Foundation, Rochester, MN 55905, USA. Fax: +1-507-284-8566. E-mail: chini.eduardo@mayo.edu
Address for correspondence: E.N. Chini, Department of Anesthesiology, Mayo Clinic and Foundation, Rochester, MN 55905, USA. Fax: +1-507-284-8566. E-mail: chini.eduardo@mayo.edu
Research supported by Mayo Foundation. Received May 31, 2005. Accepted January 18, 2006.
- 1. Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 2000; 1: 11-21.
- 2. Putney JW Jr, Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J Cell Sci 2001; 114: 2223-2229.
- 3. Putney JW Jr The enigmatic TRPCs: multifunctional cation channels. Trends Cell Biol 2004; 14: 282-286.
- 4. Putney JW Jr, Trebak M, Vazquez G, Wedel B, Bird GS. Signalling mechanisms for TRPC3 channels. Novartis Found Symp 2004; 258: 123-133.
- 5. van Rossum DB, Patterson RL, Kiselyov K, Boehning D, Barrow RK, Gill DL, et al. Agonist-induced Ca2+ entry determined by inositol 1,4,5-trisphosphate recognition. Proc Natl Acad Sci USA 2004; 101: 2323-2327.
- 6. Wedel BJ, Vazquez G, McKay RR, St JB, Putney JW Jr. A calmodulin/inositol 1,4,5-trisphosphate (IP3) receptor-binding region targets TRPC3 to the plasma membrane in a calmodulin/IP3 receptor-independent process. J Biol Chem 2003; 278: 25758-25765.
- 7. Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2004; 286: L909-L917.
- 8. Lee HC, Walseth TF, Bratt GT, Hayes RN, Clapper DL. Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J Biol Chem 1989; 264: 1608-1615.
- 9. Dousa TP, Chini EN, Beers KW. Adenine nucleotide diphosphates: emerging second messengers acting via intracellular Ca2+ release. Am J Physiol 1996; 271: C1007-C1024.
- 10. Galione A, White A. Ca2+ release induced by cyclic ADP-ribose. Trends Cell Biol 1994; 4: 431-436.
- 11. Galione A, Lee HC, Busa WB. Ca(2+)-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-ribose. Science 1991; 253: 1143-1146.
- 12. Guse AH. Cyclic ADP-ribose: a novel Ca2+-mobilising second messenger. Cell Signal 1999; 11: 309-316.
- 13. Guse AH, Berg I, da Silva CP, Potter BV, Mayr GW. Ca2+ entry induced by cyclic ADP-ribose in intact T-lymphocytes. J Biol Chem 1997; 272: 8546-8550.
- 14. Partida-Sanchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat Med 2001; 7: 1209-1216.
- 15. Partida-Sanchez S, Iribarren P, Moreno-Garcia ME, Gao JL, Murphy PM, Oppenheimer N, et al. Chemotaxis and calcium responses of phagocytes to formyl peptide receptor ligands is differentially regulated by cyclic ADP ribose. J Immunol 2004; 172: 1896-1906.
- 16. Kiselyov KI, Shin DM, Wang Y, Pessah IN, Allen PD, Muallem S. Gating of store-operated channels by conformational coupling to ryanodine receptors. Mol Cell 2000; 6: 421-431.
- 17. Kiselyov K, Shin DM, Shcheynikov N, Kurosaki T, Muallem S. Regulation of Ca2+-release-activated Ca2+ current (Icrac) by ryanodine receptors in inositol 1,4,5-trisphosphate-receptor-deficient DT40 cells. Biochem J 2001; 360: 17-22.
- 18. Barata H, Thompson M, Zielinska W, Han YS, Mantilla CB, Prakash YS, et al. The role of cyclic-ADP-ribose-signaling pathway in oxytocin-induced Ca2+ transients in human myometrium cells. Endocrinology 2004; 145: 881-889.
- 19. White TA, Kannan MS, Walseth TF. Intracellular calcium signaling through the cADPR pathway is agonist specific in porcine airway smooth muscle. FASEB J 2003; 17: 482-484.
- 20. Yusufi AN, Cheng J, Thompson MA, Burnett JC, Grande JP. Differential mechanisms of Ca(2+) release from vascular smooth muscle cell microsomes. Exp Biol Med 2002; 227: 36-44.
- 21. de Toledo FG, Cheng J, Liang M, Chini EN, Dousa TP. ADP-Ribosyl cyclase in rat vascular smooth muscle cells: properties and regulation. Circ Res 2000; 86: 1153-1159.
- 22. Li P, Zou AP, Campbell WB. Metabolism and actions of ADP-riboses in coronary arterial smooth muscle. Adv Exp Med Biol 1997; 419: 437-441.
- 23. Chini EN, Klener P Jr, Beers KW, Chini CC, Grande JP, Dousa TP. Cyclic ADP-ribose metabolism in rat kidney: high capacity for synthesis in glomeruli. Kidney Int 1997; 51: 1500-1506.
- 24. Kannan MS, Fenton AM, Prakash YS, Sieck GC. Cyclic ADP-ribose stimulates sarcoplasmic reticulum calcium release in porcine coronary artery smooth muscle. Am J Physiol 1996; 270: H801-H806.
- 25. Shlykov SG, Yang M, Alcorn JL, Sanborn BM. Capacitative cation entry in human myometrial cells and augmentation by hTrpC3 overexpression. Biol Reprod 2003; 69: 647-655.
- 26. Yang M, Gupta A, Shlykov SG, Corrigan R, Tsujimoto S, Sanborn BM. Multiple Trp isoforms implicated in capacitative calcium entry are expressed in human pregnant myometrium and myometrial cells. Biol Reprod 2002; 67: 988-994.
- 27. Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol 2004; 6: 113-120.
- 28. Beam KG, Franzini-Armstrong C. Functional and structural approaches to the study of excitation-contraction coupling. Methods Cell Biol 1997; 52: 283-306.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
29 May 2006 -
Date of issue
June 2006
History
-
Accepted
18 Jan 2006 -
Received
31 May 2005