Abstract
PURPOSE:To investigate the seminiferous tubule histological morphology after an 8 mmHg pneumoperitoneum in the rat model. METHODS: Fourteen rats were divided into two groups: a Sham group submitted to anesthesia and a pneumoperitoneum (Pp) group submitted to abdominal insufflation at 8 mmHg during three hours, followed by desuflation. All rats were killed after six weeks, testicles were collected and evaluated for the tubule diameter, germinative epithelium height and Johnsen´s score. Means were compared by using the Student's-t-test. RESULTS:The seminiferous tubule diameter was diminished by 11.3% in the group submitted to pneumoperitoneum (p<0.05). No significant difference was found among the groups when analyzing the epithelium height and Johnsen´s score. CONCLUSION:In the rat model, the seminiferous tubules present structural alterations when subjected to pneumoperitoneum of 8 mmHg during three hours.
Pneumoperitoneum; Testis; Histology; Rats
3 ORIGINAL ARTICLE
EXPERIMENTAL UROLOGY
Pneumoperitoneum induces morphological alterations in the rat testicle1 1 Research performed at Urogenital Research Unit, Rio de Janeiro State University (UERJ), Rio de Janeiro, Brazil.
Carina Teixeira RibeiroI; Diogo Benchimol De SouzaII; Jorge Luiz Medeiros Jr.III; Waldemar Silva CostaIV; Marco Aurélio Pereira-SampaioV; Francisco José Barcellos SampaioVI
IFellow Master degree, Postgraduate Program in Physiopathology and Surgical Sciences, Urogenital Research Unit, UERJ, Rio de Janeiro, Brazil. Acquisition and interpretation of data, manuscript preparation.
IIPhD, Postdoctoral Fellow, Postgraduate Program in Physiopathology and Surgical Sciences, Urogenital Research Unit, UERJ, Rio de Janeiro, Brazil. Main author. Conception and design of the study, acquisition and interpretation of data, manuscript writing.
IIIMaster, Fellow PhD degree, Postgraduate Program in Physiopathology and Surgical Sciences, Urogenital Research Unit, UERJ, Rio de Janeiro, Brazil. Acquisition of data, manuscript preparation.
IVPhD, Visiting Professor, Postgraduate Program in Physiopathology and Surgical Sciences, Urogenital Research Unit, UERJ, Rio de Janeiro, Brazil. Conception and design of the study, critical revision.
VPhD, Associate Professor, Department of Morphology, Fluminense Federal University. Postdoctoral Fellow, Postgraduate Program in Physiopathology and Surgical Sciences, Urogenital Research Unit, UERJ, Rio de Janeiro, Brazil. Conception and design of the study, acquisition and interpretation of data, manuscript writing.
VIPhD, Full Professor, Postgraduate Program in Physiopathology and Surgical Sciences, Urogenital Research Unit, UERJ, Rio de Janeiro, Brazil. Conception and design of the study, critical revision.
ABSTRACT
PURPOSE:To investigate the seminiferous tubule histological morphology after an 8 mmHg pneumoperitoneum in the rat model.
METHODS: Fourteen rats were divided into two groups: a Sham group submitted to anesthesia and a pneumoperitoneum (Pp) group submitted to abdominal insufflation at 8 mmHg during three hours, followed by desuflation. All rats were killed after six weeks, testicles were collected and evaluated for the tubule diameter, germinative epithelium height and Johnsen´s score. Means were compared by using the Student's-t-test.
RESULTS:The seminiferous tubule diameter was diminished by 11.3% in the group submitted to pneumoperitoneum (p<0.05). No significant difference was found among the groups when analyzing the epithelium height and Johnsen´s score.
CONCLUSION:In the rat model, the seminiferous tubules present structural alterations when subjected to pneumoperitoneum of 8 mmHg during three hours.
Key words: Pneumoperitoneum. Testis. Histology. Rats.
Introduction
The widespread use of laparoscopic surgical procedures has aroused great interest in experimental studies, especially concerning the organ damage caused by the increasing intra abdominal pressure1-3.
The pneumoperitoneum used in laparoscopy promotes an increased intra-abdominal pressure, which compresses the vessels, compromising the drainage of abdominal organs. Although the normal venous drainage is reestablished after abdominal desuflation in the end of the laparoscopic procedure, deleterious lesions have been shown in different organs4-6.
However, few articles have focused on how the pneumoperitoneum affects the testicles. Despite the extra-abdominal localization of the testicles, its arteries and veins are branches from the abdominal aorta and tributaries of the inferior vena cava. Thereby, the increased intra abdominal pressure could affect the blood supply and the venous drainage of this organ.
It was previously reported that a 20mmHg pneumoperitoneum increased apoptosis of germinal cells in pig testicles7 and a 10 or 20mmHg pneumoperitoneum resulted in a decreased blood flow with augmented oxidative stress and histologic damage in testicles of a rat model8 Thus, these studies demonstrated that the testicles can be affected by pneumoperitoneum. On the other hand, surgeons commonly use pneumoperitoneum pressures of up to 15 mmHg in humans, what may corresponds to an 8 mmHg in rats9. So, the intra abdominal pressure used in the animal studies seems to be higher than those normally used in clinical settings.
Therefore, the aim of this study was to investigate, by objective methods, the seminiferous tubule histological morphology after an 8 mmHg pneumoperitoneum in the rat model.
Methods
Fourteen male Wistar rats, four to six months old and weighing 250 to 360 g were used in this study. The rats were kept in a room with controlled temperature (25 ± 1 C) and with an artificial darklight cycle (lights on from 7:00 am to 7:00 pm). They were fed with standard rat food and water ad libitum.
All experiments were performed in accordance with the Brazilian laws for scientific use of animals, and the project was approved by the institutional ethical committee.
The rats were randomly divided into two groups: a Sham group (n = 7), which was submitted only to anesthesia for 210 minutes, and a pneumoperitoneum (Pp) group (n = 7) which underwent to a 180 minutes of Pp at 8 mmHg followed by 30 minutes of desufflation, under the same anesthetic protocol of the Sham group10. All rats were killed six weeks after the procedure and their testes were used for seminiferous tubule diameter, seminiferous tubule epithelium height analyses and Johnsen`s score. All analyses were blindly performed.
Pneumoperitoneum
The animals were anesthetized by intraperitoneal injection of ketamine (80 mg/kg; Cetamin 10%, Syntec do Brasil, Cotia-SP, Brazil) and xylazine (10 mg/kg; Xilazin 20%, Syntec do Brasil, Cotia-SP, Brazil) and kept with spontaneous breathing during the experiment. In the Pp group, a 21-gauge needle was inserted into the abdominal cavity and a Pp at 8 mmHg was established with CO2 by using a laparoscopic insufflator (Eletronic Endoflator 264305 20, Karl Storz, Tuttlingen, Germany). After 180 minutes of Pp, the abdominal cavity was desufflated, and the animals remained anesthetized for 30 minutes.
Histologic evaluation
The tests were fixed in 10% formaldehyde and then routinely processed for paraffin embedding. Serial 5-µm sections of the entire testicle were obtained and stained with hematoxylin and eosin (HE). Images were randomly captured by a digital camera (DP 70, Olympus, Tokyo, Japan) coupled to the microscope (BX 51, Olympus, Tokyo, Japan) under x100 or x200 magnification for diameter and epithelium height, respectively . The diameter of the seminiferous tubules and seminiferous epithelium height were measured using ImageJ software. For this purpose, five tubules in five fields of five sections in each testis were used, summing 125 measures per animal for each parameter. The epithelium height was measured with the "straight line" plugin and considered as the distance from the tunica propria to the inner germinal cell, excluding the spermatozoids. The tubular diameter was measured in the most circular tubules with the same "straight line" plugin passing in the center of the tubule, from tunica propria to tunica propria11,12.
Johnsen´s score
It was evaluated fifty randomly selected tubules per testis under x400 magnification and the germinal epithelium were scored according to Johnsen`s criteria13. A 1-10 score was given to each tubule according to the maturation of germ cells: ten to the complete spermatogenesis, sperm with many germinal epithelium and arranged with regular thickness leaving an open lumen; nine for many sperm, but the epithelium disorganized, germ scaly or obliteration of the lumen, eight for only a few sperm; seven for no sperm but many spermatids; six for no sperm and only a few spermatids (less than 5 -10), five for no sperm and spermatids, but several spermatocytes; four for only a few spermatocytes (less than 5) and no spermatid and sperm, three for only spermatogonia and germ cells; two for no germ cells but presence of Sertoli cells; one for an absence of cells in the section tubular.
Statistical analysis
The two-tailed Student's t test was used for all mean comparisons. Significance was set at a probability value of less than 0.05. Analyses were performed using GraphPad Prism software.
Results
The results of the seminiferous epithelium height and the Johnsen´s score showed that there was no difference between the seminiferous epithelium of controls and rats submitted to the 8mmHg pneumoperitoneum (p>0.05). The epithelium height of control animals was 28.7±6.1 and 28.7±5.1 in Pp animals. Johnsen´s score was 8.2±0.45 in controls and 8.4±0.43 in Pp animals.
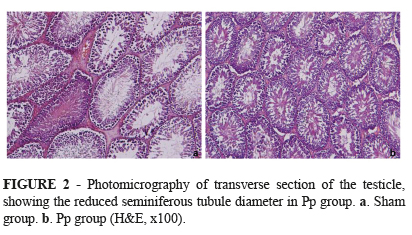
However, regarding the seminiferous tubule diameter, it was found a 11.3% decrease (p<0.05) in the Pp group compared to the sham group (Figure 1). This difference can be observed in Figure 2.
Discussion
This work proposes to study the morphology of the seminiferous tubule of rats subjected to a 8 mmHg pneumoperitoneum during three hours. There are few experimental studies upon this topic, moreover they investigated the effects of intra-abdominal pressures in pigs7,8 and rats7,8 using higher pressures than what corresponds to clinical practice in humans, since the 8mmHg was established for rats as the pressure what corresponds to higher routinely used pressures in humans9. Thus, this is the first study that investigates whether a pneumoperitoneum of 8 mmHg can cause morphological changes in the seminiferous tubules.
Most experiments that investigate morphological alterations after pneumoperitoneum focused on intra-abdominal organs. Intra-abdominal pressures of 15 mmHg for 60 minutes in rats increased the oxidative stress in intestinal tissue14. Another study evaluated the effect of insufflations at 4, 8, or 12 mmHg over the kidney and liver blood flow. It was found a decrease in renal blood flow during inflation with CO2, but this decrease was only significant in the group that was inflated to 8 and 12 mmHg. Also, these pressures did not induce changes in hepatic arterial flow5. Regarding the hepatic portal blood flow, a pneumoperitoneum of 14 mmHg was sufficient to cause a significant decrease15.
Previous studies demonstrated that a 15 mmHg pneumoperitoneum increases the inferior vena cava pressure, while the venous flow is kept constant, as the cardiac output was not affected by this intra-abdominal pressure. However, there is a peripheral blood stasis in vessels of the lower limbs4. Based on this report, the effects of pneumoperitoneum on the testicles could be explained. Just as the lower limbs, the testicles are located outside the abdominal cavity but its vascular irrigation and drainage passes thru the abdominal cavity and thus, may be influenced by modifications in inferior vena cava and abdominal aorta pressures. It has been supposed that, as the pampiniform plexus does not have a valve system and are more compressible than arteries, it might be more compressed by increased abdominal pressure than other venous and arterial systems8.
The testicles are very sensitive to blood supply changes. This aspect have been thoroughly studied in experimental testicular torsion, in which there is impaired blood flow that causes major damage to the germ cells16. Similarly, it was demonstrated that during pneumoperitoneum the increased abdominal pressure caused a decreased testicular blood flow with increased oxidative stress in a rat model8. It is possible that the pressure that decreases testicular blood flow increases reactive oxygen species further leading to seminiferous tubule morphological alterations as seen in the present study.
In a porcine model submitted to a 20 mmHg pneumoperitoneum for four hours, there was an increased cell apoptosis and eNOS and iNOS positive cells in the testicles. However, no difference was observed in relation to the Johnsen's score7, which corroborates to our findings.
Further information about the reproductive function of animals submitted to pneumoperitoneum would be of especial interest to investigate the sperm production after these alterations. Also, long term analysis would help informing if and when normal testicle morphology is restored.
Conclusion
In rat model, the seminiferous tubules present structural alterations when subjected to pneumoperitoneum of 8 mmHg during three hours.
Received: February 14, 2013
Review: April 12, 2013
Accepted: May 10, 2013
Conflict of interest: none
Financial sources: National Council of Scientific and Technological Development (CNPq), Rio de Janeiro Research Foundation (FAPERJ) and Coordination of Improvement of Higher Academic Staff (CAPES), Brazil
- 1. Demyttenaere S, Feldman LS,Fried GM. Effect of pneumoperitoneum on renal perfusion and function: a systematic review. Surg Endosc. 2007;21(2):152-60.
- 2. Guven S, Muci E, Unsal MA, Yulug E, Alver A, Kadioglu Duman M,Mentese A. The effects of carbon dioxide pneumoperitoneum on ovarian blood flow, oxidative stress markers, and morphology during laparoscopy: a rabbit model. Fertil Steril. 2010;93(4):1327-32.
- 3. Avraamidou A, Marinis A, Asonitis S, Perrea D, Polymeneas G, Voros D,Argyra E. The impact of ischemic preconditioning on hemodynamic, biochemical and inflammatory alterations induced by intra-abdominal hypertension: an experimental study in a porcine model. Langenbecks Arch Surg. 2012;397(8):1333-41.
- 4. Lindberg F, Bergqvist D, Rasmussen I,Haglund U. Hemodynamic changes in the inferior caval vein during pneumoperitoneum. An experimental study in pigs. Surg Endosc. 1997;11(5):431-7.
- 5. Brundell SM, Tsopelas C, Chatterton B, Touloumtzoglou J,Hewett PJ. Experimental study of peritoneal blood flow and insufflation pressure during laparoscopy. Br J Surg. 2002;89(5):617-22.
- 6. Lindstrom P, Wadstrom J, Ollerstam A, Johnsson C,Persson AE. Effects of increased intra-abdominal pressure and volume expansion on renal function in the rat. Nephrol Dial Transplant. 2003;18(11):2269-77.
- 7. Istanbulluoglu MO, Piskin M, Zor M, Celik A, Ozgok A, Ates M, Ustun H,Ozgok Y. The acute effects of increased intra-abdominal pressure on testicular tissue: an experimental study in pigs. Urology. 2011;77(2):510 e12-6.
- 8. Imamoglu M, Cay A, Unsal MA, Aydin S, Ozdemir O, Karahan C, Sari A,Sarihan H. The effects of increased intraabdominal pressure on testicular blood flow, oxidative stress markers, and morphology. J Pediatr Surg. 2006;41(6):1118-24.
- 9. Avital S, Itah R, Szomstein S, Rosenthal R, Inbar R, Sckornik Y,Weinbroum A. Correlation of CO2 pneumoperitoneal pressures between rodents and humans. Surg Endosc. 2009;23(1):50-4.
- 10. de Souza DB, Costa WS, Cardoso LE, Benchimol M, Pereira-Sampaio MA,Sampaio FJB. Does prolonged pneumoperitoneum affect the kidney? Oxidative stress, stereological and electron microscopy study in a rat model. Int Braz J Urol. 2013;39(1):30-6.
- 11. Gallo CB, Miranda AF, Felix-Patricio B, Ramos CF, Cardoso LE, Costa WS, Sampaio FJ. Effects of castration and hormone replacement in the urinary bladder of rats: structural, ultrastructural, and biochemical analysis. J Androl. 2012;33(4):684-90.
- 12. Mirhoseini M, Mohamadpour M,Khorsandi L. Toxic effects of Carthamus tinctorius L. (Safflower) extract on mouse spermatogenesis. J Assist Reprod Genet. 2012;29(5):457-61.
- 13. Johnsen SG. Testicular biopsy score count--a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1(1):2-25.
- 14. Bulbuloglu E, Yildiz H, Senoglu N, Coskuner I, Yuzbasioglu MF, Kilinc M, Dogan Z, Deniz C, Oksuz H, Kantarceken B,Atli Y. Protective effects of zinc, pentoxifylline, and N-acetylcysteine in an animal model of laparoscopy-induced ischemia/reperfusion injury of the small intestine. J Laparoendosc Adv Surg Tech A. 2011;21(10):947-51.
- 15. Tunon MJ, Gonzalez P, Jorquera F, Llorente A, Gonzalo-Orden M,Gonzalez-Gallego J. Liver blood flow changes during laparoscopic surgery in pigs. A study of hepatic indocyanine green removal. Surg Endosc. 1999;13(7):668-72.
- 16. Altunoluk B, Soylemez H, Bakan V, Ciralik H,Tolun FI. Protective effects of zofenopril on testicular torsion and detorsion injury in rats. Urol J. 2011;8(4):313-9.
Publication Dates
-
Publication in this collection
04 June 2013 -
Date of issue
June 2013
History
-
Received
14 Feb 2013 -
Accepted
10 May 2013 -
Reviewed
12 Apr 2013