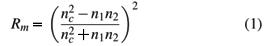Abstract
Crystalline films of pure titanium oxide have been prepared on soda lime slide glasses by sol-gel process and dip-coating. The definition of various parameters such as chemical concentration, viscosity, catalyst type and withdrawal speed led to the preparation of transparent, crystalline and adherent coatings with hydrophobic characteristics. Their crystalline structure was evaluated as anatase phase by low angle X-ray diffraction. Thicknesses were measured by perfilometry, and the refractive indices were determined from transmittance spectrum taking into consideration the layers deposited onto the two sides of the substrate. Porosity was also estimated by UV-visible spectroscopy by using the Lorentz - Lorenz equation. The average grain size was evaluated by atomic force microscopy. The thicker and denser films presented hydrophobicity, which decreased when the film porosity increased.
Titanium oxide thin films; Sol-gel process
SURFACES, INTERFACES, AND THIN FILMS
Nanocrystalline titanium oxide thin films prepared by sol-gel process
Marcelo M. Viana; Tarik D. S. Mohallem; Gabriel L. T. Nascimento; Nelcy D. S. Mohallem
Laboratório de Materiais Nanoestruturados, Departamento de Química, ICEx UFMG, 31270-901 Belo Horizonte-MG, Brazil
ABSTRACT
Crystalline films of pure titanium oxide have been prepared on soda lime slide glasses by sol-gel process and dip-coating. The definition of various parameters such as chemical concentration, viscosity, catalyst type and withdrawal speed led to the preparation of transparent, crystalline and adherent coatings with hydrophobic characteristics. Their crystalline structure was evaluated as anatase phase by low angle X-ray diffraction. Thicknesses were measured by perfilometry, and the refractive indices were determined from transmittance spectrum taking into consideration the layers deposited onto the two sides of the substrate. Porosity was also estimated by UV-visible spectroscopy by using the Lorentz - Lorenz equation. The average grain size was evaluated by atomic force microscopy. The thicker and denser films presented hydrophobicity, which decreased when the film porosity increased.
Keywords: Titanium oxide thin films; Sol-gel process
I. INTRODUCTION
The deposition of thin films on glass, ceramics, and metal substrates is one of the most important applications of the sol-gel process [1]. Single and multi-component oxide coatings can be obtained by spray, spin or dip-coating process in large scale at a cost lower than those of other methods. The colloidal solution used as a precursor must have appropriated physical-chemical properties to produce transparent, adherent and homogeneous films with a large variation in their characteristics [2-4]. This process facilitates the monitoring of the precursor solution parameters in such way that the obtained films have a good control on the chemical composition, thickness, morphology and texture. The dry films obtained by this process can be amorphous or crystalline, porous or dense, depending on the thermal treatment, and can be deposited onto various kinds of substrates.
Titanium oxide film is a very important material due to its multifunctional application in photocatalysis, hydrophobic material, photovoltaic cells, photochromic and electrochromic devices, gas sensor, biosensor, corrosion protection, bactericide, optical device, among others [5-8]. TiO2 can occur in a number of crystalline forms, the most important of which are anatase, rutile and brokite. Nowadays, it is one of the most extensive studied metal oxide, both as particulate and thin film form. Their properties depend on the crystalline phase, roughness, porosity, and particle size and distribution. When the particle size is sufficiently small it is possible to observe quantum size effects [9].
In this study, we used a colloidal solution with titanium alkoxide dissolved in its equivalent alcohol to prepare transparent, homogeneous, adherent and crystalline titanium oxide films. The films, deposited in glass plates, Pyrex and quartz, were thermally treated and morphologically, optically, structurally, and texturally characterized.
II. EXPERIMENTAL
Titanium oxide films have been prepared by sol-gel process from an alcoholic solution containing tetraisopropyl orthotitanate (Merck), isopropanol (Merck), and chloride acid (Vetec). The precursor solution was maintained under agitation at room temperature for one hour, and at rest for 4 hours for the viscosity to reach the desired values. To ensure uniform coating, surface pre-treatment was carried out by cleaning with detergent, followed by deionised water and acetones rinses. The substrate was immersed vertically into the bath of the precursor solution and was withdrawal from the solution at 1 mm per sec., which is the necessary velocity to occur the hydrolysis and condensation of the liquid coatings, simultaneously. During the dipping process, thin liquid films stuck onto the two faces of the substrate, hydrolyze rapidly with the air humidity, and polycondensate, forming gel films. After each dip-coating step, the films were dried in air for 30 min to produce chemical bound between the layer and the substrate, and they were thermally treated between 100 and 400 ºC for 10 min to crystallization and densification of the films. The deposition was made also on the one side of the substrate.
The evolution of the crystalline structure were observed by low angle X-ray diffractometry (incidence angle of 5º) at the D12A-XRD1 beam of the Laboratório Nacional de Luz Síncrotron/Campinas using radiation of 1.5424 Å.
The transmittance and reflectance of the films were determined by optical transmission spectra obtained with an ultraviolet and visible spectrometer (U3010 - Hitachi). Thicknesses and refractive indices were obtained by the Fresnel's equation, taking into consideration the layers deposited on the one or two sides of the substrate [7]:
where Rm is the minimum reflection from the coated surface, nc, n1, and n2 are the refractive indices of coating, medium, an substrate, respectively.
Thicknesses were also measured by using a perfilometer (Alphastep 100, Tencor Instruments, USA), with an estimated 6% uncertainty, for confirmation of the results. Porosity was calculated by the Lorentz-Lorenz equation [3, 7,8]:
where P is the film porosity, nc and no are the refractive index of the obtained film and of the titania film totally dense, defined as 2.3. The average grain size and morphology of the films were analysed by atomic force microscopy (Nanoscope II and a Dimension 3000, Digital Instruments) equipped with an extended modulus for phase imaging. The images were generated through the intermittent contact mode, using a silicon probe tip with 5 nm of curvature radius.
Hydrophobicity tests were done, dropping water in glass plates and glass recovered with titanium oxide films treated at various temperatures, to measure the wettability of the surfaces. Contact angle measurements were made using the imageJ program.
III. RESULTS AND DISCUSSION
The films were prepared from solutions with pH between 2 and 4, viscosity between 2.0 and 5.0 cp, surface tension of 20.1 ± 0.1 mNm - 1, density of 0.79 ± 0.01 gcm - 3, and atmosphere relative humidity lower than 40%, since the films were opaque and non adherent for other pH and relative humidity values. The films presented cracks for viscosity larger than approximately 5.0 cp. The determination and utilization of these value ranges of the parameters was necessary to the preparation of transparent, homogeneous and adherent films.
The X-ray peaks of the films are in accordance with those of a typical anatase nanocrystalline TiO2 calcined at 300 ºC, with particle size of ~ 30 nm, also prepared by sol-gel process. The peak intensities, shown in Fig. 1, increase with increasing the heating temperature and present low crystallinity. The amorphous phase is due to the substrate.
Transmission spectra of the substrates with TiO2 films treated at 400C in function of the deposition number are shown in Fig. 2. Fig. 2a and 2b show substrates recovered on one side and substrates recovered on both sides, respectively. Refractive indices calculated from transmission spectra for samples treated between 100 at 400 ºC ranged from 1.9 and 2.2 (l = 550 nm), evidencing the changes in porosity between 20 and 5% for samples heated at 100 and 400 C, respectively.
After drying, the films were porous when treated at low temperature and denser at 400 ºC. Each layer thickness was between 50 and 100 nm and increased with either increasing withdrawal speed for constant sol viscosity or increasing viscosity for constant withdrawal speed. Film thickness increased with multiple depositions, for example, 1-layer and 5-layer films were ~ 50 and ~ 200 nm thick, that can be verified in Fig. 2b. According to literature [2, 3, 8], the measured thickness of the films is linearly related to the square root of the withdrawal rate and the square root of the solution viscosity, facilitating the reproducibly of the films obtained [8].
AFM analysis revealed that the thin films treated at 100 ºC (Fig. 3) are more porous than those treated at 400 ºC (Fig. 4), with a homogeneous distribution of pores. The average particle size was in the order of 20 ± 2 nm for both temperatures.
In the hydrophobicity tests (Fig. 5), we observed a smaller contact angle between the water drop and the film surface ~ 89º when compared with that of water and glass surface ~ 144º. The hydrophobicity of the films increased with the increase in thickness and decrease in porosity.
IV. CONCLUSION
The titanium oxide thin films obtained were pure, crystalline, adherent, transparent, homogeneous, and free of microcracks. Films thermally treated between 100 and 400 ºC presented low crystallinity when compared with TiO2 nanoparticles also prepared by sol-gel process. The crystallinity increased and the porosity decreased with the increasing in the thermal treatment temperature.
The thicker and denser films presented more hydrophobicity than the porous ones.
Acknowledgement
This work was supported by Capes and CNPq. The authors thank LNLS- Campinas/Brazil for AFM images and XRD analyses.
Received on 8 December, 2005
- [1] J. Brinker, Scherer, Sol-Gel Science, Academic Press, 2nd ed., 1999.
- [2] H. Schroeder, Phys. Thin Films 87, (1969).
- [3] N.D. S. Mohallem, M.A.Aegeter, J. Non-Cryst. Solids, 100, 526 (1988).
- [4] N.D. Mohallem, L. M. Seara, App. Surf. Sci. 214, 143 (2003).
- [5] F. L. Toma, G. Bertrand, S. O. Chwa, C. Meunier, D. Klein, and C. Coddet, Surf. Coat. Techn. 200, 5855 (2006).
- [6] W. A. Daoud and J. H. Xin, J. Sol-Gel Sci. Tech. 29, 25 (2004).
- [7] B. E. Yoldas, Appl. Opt. 19, 1425 (1980).
- [8] G. L. T. Nacimento, L. M. Seara, B. R. A. Neves, and N. D. S. Mohallem, Progr. Colloid Polym. Sci 128, 227 (2004).
- [9] Y. Liu, R. Claus, J. Am. Chem. Soc. 119, 5273 (1997).
Publication Dates
-
Publication in this collection
29 Nov 2006 -
Date of issue
Sept 2006
History
-
Received
08 Dec 2005