Abstract
The vancomycin dose for hemodialysis (HD) patients should be adjusted by monitoring drug serum concentrations. However, this procedure is not available in most health services in Brazil, which usually adopts protocols based on published studies. The trials available are controversial, and several have not been conducted with current dialyzers. This study aimed at assessing the suitability of vancomycin serum concentrations in HD patients at a public hospital. Blood samples of HD patients were collected from November 2006 to May 2007, at time intervals of 48, 96, 120, or 168 hours after vancomycin administration. Drug measurement was performed with polarized light immunofluorescence. Approximately 86% of trough vancomycin serum concentrations were below the recommended value, indicating exposure to subtherapeutic doses and a higher risk for selecting resistant microorganisms.
vancomycin; chronic renal failure; hemodialysis
BRIEF COMMUNICATION
Vancomycin: the need to suit serum concentrations in hemodialysis patients
Lívia Luize MarengoI; Fernando de Sá Del FiolII; Sara de Jesus OliveiraII; Celso NakagawaIII; Eduardo Leite CrocoIII; Silvio Barberato-FilhoII; Marcela Pellegrini PeçanhaIV; Douglas Felix da SilvaII; Maria Inês de ToledoII,V
IPharmacy School of Universidade de Sorocaba PIBIC-CNPq
IIResearcher of the Reference Center on Information About Antibiotics (CRIA) of Universidade de Sorocaba
IIIConjunto Hospitalar de Sorocaba
IVFaculdade de Ciências Médicas e da Saúde - PUC São Paulo
VFaculdade de Tecnologia de Sorocaba
Correspondence Correspondence to: Profa. Dra. Maria Inês de Toledo Rua Silvério E. Oliveira, 160 - Jd. Saira Sorocaba - São Paulo - Brazil CEP: 18085-645 Phone: 55 15 3238 5266 E-mail: mitoledo@yahoo.com
ABSTRACT
The vancomycin dose for hemodialysis (HD) patients should be adjusted by monitoring drug serum concentrations. However, this procedure is not available in most health services in Brazil, which usually adopts protocols based on published studies. The trials available are controversial, and several have not been conducted with current dialyzers. This study aimed at assessing the suitability of vancomycin serum concentrations in HD patients at a public hospital. Blood samples of HD patients were collected from November 2006 to May 2007, at time intervals of 48, 96, 120, or 168 hours after vancomycin administration. Drug measurement was performed with polarized light immunofluorescence. Approximately 86% of trough vancomycin serum concentrations were below the recommended value, indicating exposure to subtherapeutic doses and a higher risk for selecting resistant microorganisms.
Keywords: vancomycin, chronic renal failure, hemodialysis.
INTRODUCTION
Vancomycin, an amphoteric and high-molecular-weight complex tricyclic glycopeptide antibiotic, available in the form of water-soluble hydrochloride, is often used for treating severe hospital infections.1 Vancomycin concomitantly inhibits the cell wall synthesis of susceptible microorganisms or bacteria, altering cytoplasm membrane permeability and interfering with RNA synthesis.2 Its most significant adverse effects are ototoxicity and nephrotoxicity.Marinho (2005) has reported that approximately 30% of patients have experienced some adverse reaction to vancomycin, such as renal failure (18%), thrombocytopenia (7%), neutropenia (2%), red man syndrome (2%), and ototoxicity (1%).3
The increased use of vancomycin has been attributed to the increase of nosocomial infections caused by methicillin-resistant Staphylococcus aureus (MRSA), which increased from 2% in 1974 to more than 50% in 2000.4
Bacterial infections most often occur in hemodialysis (HD) patients. This is most probably related to frequent violation of normal skin and mucosa barriers than to immune dysfunction. Bacterial infections in HD patients progress more rapidly and resolve less promptly than those in non-uremic patients.5 Venous access site is the source of 50% to 80% of the bacteremia episodes in dialysis patients. Bacteremia can lead to endocarditis, meningitis, osteomyelitis, paraspinal abscess, or formation of septic embolus. Arteriovenous fistulas do not have an exit site, thus being associated with lower infection rates.6
In HD patients, infections caused by Grampositive bacteria can be treated with vancomycin, mainly due to its activity against most Gram-positive pathogens, especially MRSA. Moreover, vancomycin has a long half life in renal failure, which allows using intermittent doses regimes.7 However, the pharmacokinetic characteristics of this antibiotic should be respected to maintain adequate levels, minimize the appearance of resistant strains, and avoid toxicity.8 Changes in renal function associated with normal aging or with diseases can have a deep effect on the pharmacology of antibiotics, which require their doses to be adjusted.9,10,11
The amount of vancomycin removed by HD is negligible when conventional dialyzers are used; however, when using high-flow membrane dialyzers, a significant reduction in vancomycin levels can occur.5,11
Measurement of vancomycin serum levels in patients with renal failure is required to assure adequate bactericidal levels. According to Lentino and Leehey (2001), vancomycin maximum and minimum serum levels are 30-40 µg/mL and 5-10 µg/mL, respectively.5
Due to the high variability of the minimum inhibitory concentrations (MIC) for vancomycin-susceptible staphylococcus strains, a recent study has recommended that vancomycin levels range from 5 to 10 times the MIC value.12
The suggested dose of vancomycin for HD patients is 15 mg/kg every 7 to 10 days.13 However, when high-flow membranes are used in HD, additional vancomycin doses are recommended at every HD session.14 Lentino and Leehey (2001) have recommended that the interval between vancomycin doses in HD patients should be enlarged, but treatment should begin with an attack dose of 20 mg/kg and continued with subsequent doses of 15 mg/kg every seven days after the HD session.5
Other authors have suggested changes in dosing and interval according to the administration regimen and the type of dialyzer used. However, reviewing studies on the pharmacokinetics of vancomycin in HD patients showed several limitations, different conclusions and suggestions of dose adjustment could be identified as shown in Table 1. The divergences and limitations of the studies published have motivated the conduction of this study that aimed at monitoring the serum levels of vancomycin in HD patients and checking if the concentrations were adequate.
METHOD
The study was approved by the Research Ethics Committee of the Universidade de Sorocaba (Protocol 001/05) and by the Teaching and Research Committee of the Conjunto Hospitalar de Sorocaba (state of São Paulo), a state public hospital that attends approximately 120 HD patients. All patients included in this study used vancomycin during the period studied (from November 2006 to May 2007) and provided written informed consent. The exclusion criteria were as follows: report of allergy to vancomycin, liver disease, or previous use of vancomycin up to 15 days before sample collection.
Data collection from medical records included the following: gender, age, origin, and ethnicity. The results of culture and antibiogram were obtained from the Hospital Infection Control Service of the Conjunto Hospitalar de Sorocaba.
Vancomycin (Vancocina CP - ABL Antibióticos do Brasil) was given in dose of 1 g diluted into 100 mL of 0.9% sodium chloride solution infused during the last hour of HD, according to the service's protocol, which recommends the administration of 1 g every seven days. Blood samples were collected by the nurse team in tubes containing ethylene diamine tetraacetic acid (EDTA) for hemogram and in dry tubes for measuring vancomycin and albumin. Then, they were centrifuged and frozen. The collections for measuring vancomycin were obtained in single samples at the time points 48, 96, 120 or 168 hours after administering the first dose of the drug.
Hemogram was performed in the automated ABX Pentra60® device, at the Instituto Diagnóstico de Sorocaba. The endpoint colorimetric method was used for measuring albumin with a kit containing bromocresol green (BCG) of Laborlab, at the Universidade de Sorocaba. Vancomycin serum levels were measured by using 150 µL of serum and polarized light immunofluorescence with monoclonal antibodies (ABBOTT Laboratories kits) in the automated AxSym® device (ABBOTT), in the sector of serology of the Hemonúcleo of the Conjunto Hospitalar de Sorocaba.
The results were reported regarding the percentage of patients with inadequate serum concentrations and need for dose adjustment. Statistical analyses (ANOVA) were performed in the correlation of the results of vancomycin and albumin concentrations and hematocrit levels. The significance level of 5% was adopted.
RESULTS
The study comprised 17 patients, five of whom used vancomycin more than once during the period studied, at a mean interval of 72 days, adding up to the collection of 22 samples. Their mean age was 56.9 ± 6.0 years. Most patients (56%) were female, married (65%), white (65%), and came from the municipalities of the Sorocaba region (59%).
The following clinical conditions related to HD procedure stand out: double-lumen catheter as the most common venous access (65%); dialysis capillary reuse time ranging from 1-19 days, median of three days; association of diabetes mellitus and arterial hypertension as the most frequent etiology of chronic renal failure (41%); mean HD time of 16 months. All patients used polysulfone dialyzer membrane.
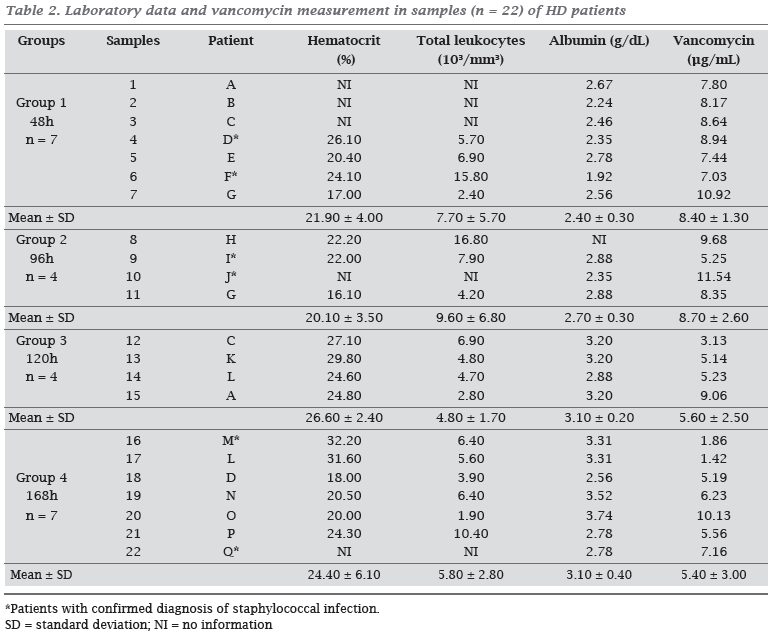
Patients' laboratory data presented in Table 2 showed that albumin (reference values: 3.4-4.8 g/dL)21 and hematocrit (reference values: women: 39-49% and men: 35-45%)21 were below normal values, but compatible with the setting of chronic renal failure. Neither statistical difference between groups (ANOVA), nor correlation between the patient's plasma level of albumin and serum concentration of vancomycin were observed (p > 0.05).
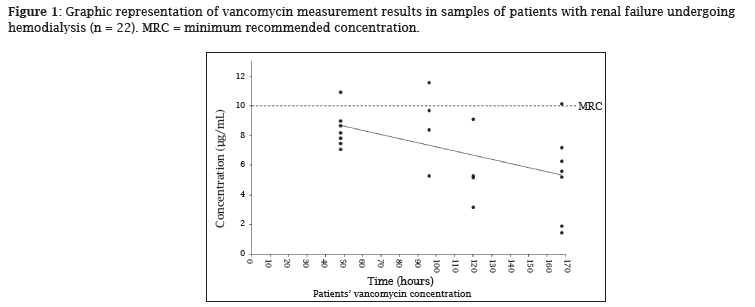
Regarding to vancomycin serum concentrations , no statistical difference (ANOVA, p > 0.05) between the values of the four groups was observed (Table 2). However, at least 14% of the results were inadequate when considering the minimum trough concentration greater than 5 µg/mL, and 86% were inadequate when considering the minimum trough concentration greater than 10 µg/mL (Figure 1).
Staphylococci were identified in one third of the cases, and approximately 25% of the microorganisms isolated were Staphylococcus aureus. Of those, 50% were oxacillinresistant, and 100% were sensitive to vancomycin.
DISCUSSION
Several studies used for establishing the current empirical protocols of vancomycin dosing in patients with renal failure undergoing HD are outdated with regards to dialysis equipments. The manufacturer of the reference drug (Vancocin®) reports that it is not dialyzable and its half life is 7.5 days in patients with severe renal failure. However, the various trials have different case series and several limitations, such as the small number of patients, unstable patients (with sepsis), patients with no infection, and patients using different types of dialyzers. Launay-Vaucher et al. (2002) have discussed the use of several dialysis membranes and systems aiming at establishing protocols for monitoring serum concentration. In fact, that aspect has been seldom explored in the international literature, since most hospitals in developed countries monitor vancomycin concentration in patients at risk (neonates, patients with large burns, patients with renal failure, and the elderly). In Brazil, however, few hospitals do this on a regular basis. The cost of that automated test is considered a limiting factor and is equivalent to the mean price of one drug vial.10
Currently, approximately 80% of the hospital staphylococcal infections are treated with glycopeptides, such as vancomycin and teicoplanin. This is still the antimicrobial class of choice for treating infections caused by MRSA, although strains with reduced sensitivity to vancomycin have already been isolated in Brazil.22
The accessory gene regulator (agr) of Staphylococcus aureus modulates the expression of numerous virulence factors in the phase-dependent growth. According to Rose et al. (2007), all agr groups develop intermediate resistance to vancomycin with subtherapeutic exposures.23
The study by Sakoulas et al. (2006) has shown that the exposure to subtherapeutic vancomycin concentrations is associated with the development of clinically hetero-GISA (glycopeptide intermediate-resistant S. aureus) isolates. These authors consider that the constant exposure of microorganisms to a vancomycin concentration >10 mg/L is important in preventing the appearance of intermediate resistance to glycopeptides.24
Johnson and Woodford (2002), studying glycopeptide resistant S. aureus (GRSA), have reported that GRSA isolated in Michigan with one vanA gene, along with other genes, encode a high level of resistance to glycopeptides.25
Due to the increasing use of vancomycin, worrisome vancomycin-resistant strains of E. faecium and E. faecalis have emerged. The determinant of this resistance is located in a transposon that is part of a plasmid; thus, the vancomycin-resistant enterococcus can transfer its resistance to other Gram-positive bacteria, such as staphylococci, worsening even more the occurrence of multi-resistant bacteria.3
The GISA and GRSA resistances,although still rare,should be under constant multidisciplinary surveillance. The following patients are at risk for GISA: patients receiving prolonged vancomycin therapy; patients previously colonized by MRSA or vancomycin-resistant enterococci (VRE),particularly those with peritoneal catheters; patients with documented MRSA infection; and HD patients, who frequently have MRSA and undergo therapy with glycopeptides.26
Because VRE are usually resistant to other antibiotics commonly used in hospitals, severe exposure to other drugs is associated with a high risk of acquiring VRE.27
Similarly to beta-lactam antibiotics, vancomycin has negligible post-antibiotic effects and requires the maintenance of concentrations equivalent to four to five times MIC during prolonged periods to obtain an efficient antibacterial effect.8
In clinical practice in Brazilian hospitals, dosing for most patients with chronic renal failure is 1 g every seven days. However, in a study by González-Martin et al. (1996), the vancomycin level seven days after administration was lower than expected and a subtherapeutic level was probably achieved days before.17
Zoer et al. (1997) have recommended an initial vancomycin dose of 1 g, to which 500 mg should be added during each subsequent HD session in patients with a positive culture for S. aureus. The ideal time for plasma monitoring would occur 48h, and 96h or 120h after vancomycin infusion.18 Other authors have recommended the determination of vancomycin serum concentration before the first HD session subsequent to the administration and before 72-hour of HD session. If the results are below the recommended values (between 5 and 10 µg/mL), a complementary 500-mg dose of vancomycin can be used.5,28
It is worth noting that, in this study, 86% of the patients had concentrations below 10 µg/mL and 14% of the patients had concentrations below 5 µg/mL. When the collection times are considered, one can infer that the number of patients reaching trough concentrations below 5 µg/mL would be greater. It is also worth noting that vancomycin has been administered during the HD session, but supplementary doses are not administered according to the recommendation of Mason et al. (2003) who have compared three regimens of vancomycin dosing.7
In conclusion, the patients included in this study were exposed to antibiotic subtherapeutic doses. Considering the increased risk of selecting resistant microorganisms, monitoring of vancomycin serum concentration in HD patients is recommended.
ACKNOWLEDGEMENTS
The authors thank the support of the Nephrology Service, the nurse Yuriko Miyamoto, Edênia M. C. Fronteira, and Ivanilda S. Aquino of the Service of Hospital Infection Control and Blood Center of Conjunto Hospitalar de Sorocaba. They also thank the Instituto de Diagnóstico de Sorocaba and the Unimed Hospital de Sorocaba.
The authors also thank the CNPq for financial support (references 402722/2005-1 and 117276/2006-5).
Submitted on: 02/16/2009
Approved on: 10/15/2009
This study received financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)
- 1. Klasco RK editor. Martindale: the extra pharmacopoeia. Base de dados na Internet. Greenwood Village: Thomson MICRO-MEDEX; 1974-2006.Disponível: http://www.periodicos.capes.gov.br Acesso em 20 de maio de 2008.
- 2. Ge M, Chen Z, Onishi HR, Kohler J, Silver LL et al Vancomycin derivatives that inhibit peptidoglycan biosynthesis without binding D-ala-D-ala. Science 1999; 284:507-10.
- 3. Marinho, DS Vancomicina, estudo de utilização com ênfase em suas reações adversas. 2005. 120 f. Dissertação (Mestrado) - Fundação Oswaldo Cruz, Rio de Janeiro, 2005.
- 4. Bartlett JG Antibiotic selection for infections involving methicillin-resistant Staphylococcus aureus. 2004. Disponível em: http://www.medscape.com/viewprogram/3124 Acesso em 03 de dezembro 2007.
- 5. Lentino JR, Leehey DJ. Infecções. In: Daugirdas JT, Blake PG, Ing TS. Manual de diálise. 3. ed. Rio de Janeiro: Guanabara Koogan, 2001.
- 6. Marcondes CRR, Biojone CR, Cherri JM, Takachi P, Carlos E. Complicações precoces e tardias em acesso venoso central: análise de 66 implantes. Acta Cirúrgica Brasileira 2007; 15:73-5.
- 7. Mason NA,Neudeck BL,Welage LS,Patel JA,Swartz RD.Comparison of 3 vancomycin dosage regimens during hemodialysis with cellulose triacetate dialyzers: post-dialysis versus intradialytic administration. Clinical Nephrology 2003; 60:96-104.
- 8. Silva LAM, Pansard HM, Rosa DB, Botton SR, Mezzomo NF et al Níveis sanguíneos de vancomicina e adequação de seu uso em pacientes com insuficiência renal em tratamento dialítico. Jornal Brasileiro de Nefrologia 1998; 20:411-8.
- 9. Tavares W. Glicopeptídeos e Lipopeptídeos. In: Antibióticos e quimioterápicos para o clínico. São Paulo: Atheneu, 2007.
- 10. Launay-Vacher V, Izzedine H, Mercadal L, Deray G. Clinical review: use of vancomycin in haemodialysis patients. Critical Care 2002; 6(4):313-6.
- 11. Livornese LL, Slavin D, Gilbert B, Robbins P, Santoro J. Use of antimicrobial agents in renal failure. Infectious Disease Clinics of North America 2004; 18(3):551-79.
- 12. Bingen E, Mariani-Kurkdjian P, Nebbad B. Optimal vancomycin serum level in Staphylococcus aureus infections? Medecine et Maladies Infectieuses 2006; 36(9):439-42.
- 13. Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett´s: principles and pratice of infectious diseases. 6 ed. Philadelfia: Elsevier, 2005.
- 14. Stryjewski ME, Szczech LA, Benjamin DK, Inrig JK, Kanafani ZA et al Use of vancomycin or first-generation cephalosporins for the treatment of hemodialisys-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clinical Infectious Diseases 2007; 20:190-6.
- 15. Nielsen HE, Hansen HE, Korsager B, Skov PE. Renal excretion of vancomycin in kidney disease. Acta Medica Scandinavica 1975; 197(4):261-4.
- 16. Masur H, Francioli P, Ruddy M, Murray HW. Vancomycin serum levels and toxicity in chronic hemodialysis patients with Staphylococcus aureus bacteremia. Clinical Nephrology 1993; 2:85-8.
- 17. Gonzalez-Martin G, Acuna V, Perez C, Labarca J, Guevara A, Tagle R. Pharmacokinetics of vancomycin in patients with severely impaired renal function. International Journal of Clinical Pharmacology and Therapeutics 1996; 34(2): 71-5.
- 18. Zoer J, Schrander-Van Der Meer AM, Van Dorp WT. Dosage recommendations of vancomycin during haemodialysis with highly permeable membranes. Pharmacy World & Science 1997; 19(4):191-6.
- 19. Foote EF, Dreitlein WB, Steward CA, Kapoian T, Walker JA, Sherman RA. Pharmacokinetics of vancomycin when administered high flux hemodialysis. Clinical Nephrology 1998; 50(1):51-5.
- 20. Lucksiri A, Scott MK, Mueller BA, Hamburger RJ, Sowinski KM. CAHP-210 dialyzer influence on intra-dialytic vancomycin removal. Nephrology Dialysis Transplantation 2002; 17(9):1649-54.
- 21. Goldman L, Ausiello D. Cecil: tratado de medicina interna. 22. ed. Rio de Janeiro: Elsevier, 2005.
- 22. Bernardes RC, Jorge AOC, Leão MVP. Sensibilidade à oxacilina, vancomicina e teicoplanina de Staphylococcus coagulasepositivo isolados de pacientes hospitalizados de São José dos Campos. Revista Biociências 2004; 10(1-2):73-8.
- 23. Rose WE, Rybak MJ, Tsuji BT, Kaatz GW, Sakoulas G. Correlation of vancomycin and daptomycin susceptibility in Staphylococcus aureus infererence to accessory gene regulator (agr) polymorphism and function. Journal of Antimicrobial Chemotherapy 2007; 59:1190-3.
- 24. Sakoulas G, Howard SG, Cohen RA, Venkataraman L, Moellering RC, Eliopoulos GM. Effects of prolonged vancomycin administration on methicillin-resistant Staphylococcus aureus (MRSA) in a patient with recurrent bacteraemia. Journal of Antimicrobial Chemotherapy 2006; 57(4):699-704.
- 25. Johnson AP, Woodford N. Glycopeptide-resistant Staphylococcus aureus Journal of Antimicrobial Chemotherapy 2002; 50:621-23.
- 26. Center for Disease Control and Prevention. Laboratory capacity to detect antimicrobial resistance. 1998; 48:1167-71.
- 27. Finch RG, Eliopoulos GM. Safety and efficacy glicopeptide antibiotics. Journal of Antimicrobial Chemotherapy 2005; 55(S2):ii5-ii13.
- 28. Santos CR, Feferbaum R, Paula LSA, Bertoline MA, Omosako CEK, Santos SRC. Micrométodo para quantificação da vancomicina em plasma através da cromatografia líquida de alta eficiência: monitorização plasmática de vancomicina na sustentação farmacológica de neonatos com sepse. Revista Brasileira de Ciências Farmacêuticas 2001; 37(1):87-93.
Publication Dates
-
Publication in this collection
11 June 2010 -
Date of issue
Apr 2010
History
-
Accepted
15 Oct 2009 -
Received
16 Feb 2009