Abstract
Trypanosoma cruzi is a parasite that causes Chagas disease, which affects millions of individuals in endemic areas of Latin America. One hundred years after the discovery of Chagas disease, it is still considered a neglected illness because the available drugs are unsatisfactory. Aromatic compounds represent an important class of DNA minor groove-binding ligands that exhibit potent antimicrobial activity. This study focused on the in vitro activity of 10 aromatic dicationic compounds against bloodstream trypomastigotes and intracellular forms of T. cruzi. Our data demonstrated that these compounds display trypanocidal effects against both forms of the parasite and that seven out of the 10 compounds presented higher anti-parasitic activity against intracellular parasites compared with the bloodstream forms. Additional assays to determine the potential toxicity to mammalian cells showed that the majority of the dicationic compounds did not considerably decrease cellular viability. Fluorescent microscopy analysis demonstrated that although all compounds were localised to a greater extent within the kinetoplast than the nucleus, no correlation could be found between compound activity and kDNA accumulation. The present results stimulate further investigations of this class of compounds for the rational design of new chemotherapeutic agents for Chagas disease.
aromatic compounds; Trypanosoma cruzi; chemotherapy; Chagas disease
ARTICLES
The biological in vitro effect and selectivity of aromatic dicationic compounds on Trypanosoma cruzi
Cristiane França da SilvaI; Patrícia Bernadino da SilvaI; Marcos Meuser BatistaI; Anissa DaliryI; Richard R TidwellII; Maria de Nazaré Correia SoeiroI, + + Corresponding author: soeiro@ioc.fiocruz.br
ILaboratório de Biologia Celular, Instituto Oswaldo Cruz-Fiocruz, Av. Brasil 4365, 21045-900 Rio de Janeiro, RJ, Brasil
IIDepartment of Pathology and Laboratory Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
ABSTRACT
Trypanosoma cruzi is a parasite that causes Chagas disease, which affects millions of individuals in endemic areas of Latin America. One hundred years after the discovery of Chagas disease, it is still considered a neglected illness because the available drugs are unsatisfactory. Aromatic compounds represent an important class of DNA minor groove-binding ligands that exhibit potent antimicrobial activity. This study focused on the in vitro activity of 10 aromatic dicationic compounds against bloodstream trypomastigotes and intracellular forms of T. cruzi. Our data demonstrated that these compounds display trypanocidal effects against both forms of the parasite and that seven out of the 10 compounds presented higher anti-parasitic activity against intracellular parasites compared with the bloodstream forms. Additional assays to determine the potential toxicity to mammalian cells showed that the majority of the dicationic compounds did not considerably decrease cellular viability. Fluorescent microscopy analysis demonstrated that although all compounds were localised to a greater extent within the kinetoplast than the nucleus, no correlation could be found between compound activity and kDNA accumulation. The present results stimulate further investigations of this class of compounds for the rational design of new chemotherapeutic agents for Chagas disease.
Key words: aromatic compounds - Trypanosoma cruzi - chemotherapy - Chagas disease
Chagas disease is a neglected tropical illness caused by the protozoan Trypanosoma cruzi. Although Carlos Chagas described it 100 years ago (1909), it is still an important public health problem in Latin America (Rocha et al. 2007). The main clinical symptoms of Chagas disease are cardiac and/or digestive alterations and the overall prevalence of the disease is about 12-14 million cases, which makes it the major cause of cardiac infectious disease in endemic areas (Stewart et al. 2005, Dias 2007). In addition, despite fruitful attempts to control vectorial and blood transmission, Chagas disease still lacks prophylactic therapies and effective chemotherapeutic schemes (Rodrigues Coura & De Castro 2002, Dias 2007). Nifurtimox and benznidazole are used for the treatment of Chagas disease (Urbina 2002); although they are effective for the treatment of acute infections, they present moderate activity, exhibit undesirable side effects and require long dosing schedules for chronic infections, which frequently necessitate the cessation of treatment (Jannin & Villa 2007, Soeiro et al. 2009). In addition, the pharmaceutical industries have given little attention to the design and development of new anti-parasitic compounds aromatic dicationic compounds represent a class of DNA minor-groove binding ligands that exhibit high activity against a variety of pathogens, such as bacteria, fungi and protozoa (Werbovetz 2006, Wilson et al. 2008). Recent data showed that diamidines and related compounds, such as the reversed amidines, present considerable efficacy against T. cruzi both in vitro (De Souza et al. 2004, Silva et al. 2007a) and in vivo (De Souza et al. 2006a, da Silva et al. 2008) and induce striking alterations on the parasite mitochondrion-kinetoplast complex (De Souza et al. 2006b, Silva et al. 2007b). In this context, the present study investigated the activity of 10 newly synthesised aromatic dicationic compounds on trypomastigotes and intracellular amastigotes, the clinically relevant forms of T. cruzi and the toxicity of these compounds in cardiac cells. Due to the intrinsic fluorescent characteristics of these compounds, we also studied their sub cellular distributions to evaluate their preferred targets in T. cruzi.
MATERIALS AND METHODS
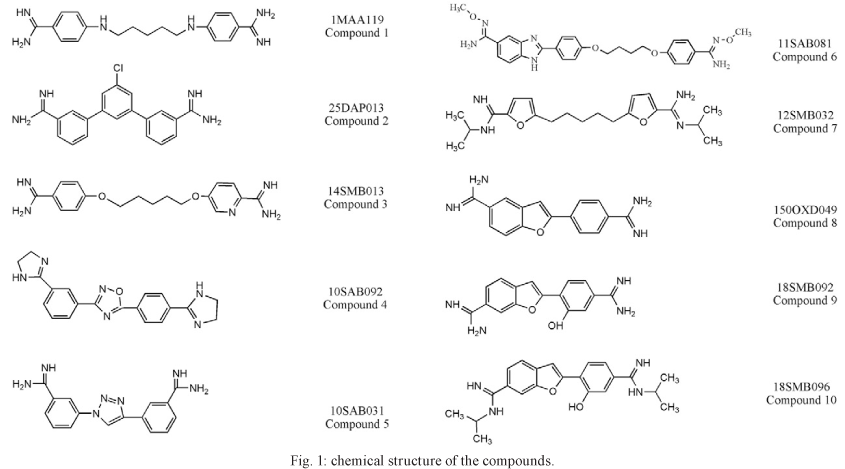
Compounds - The dicationic aromatic compounds 1MAA119 (Compound 1), 25DAP013 (Compound 2), 14SMB013 (Compound 3), 10SAB092 (Compound 4), 10SAB031 (Compound 5), 11SAB081 (Compound 6), 12SMB032 (Compound 7), 150OXD049 (Compound 8), 18SMB092 (Compound 9) and 18SMB096 (Compound 10) (Fig. 1) were synthesised in the laboratory of R.R.T. and the previously reported protocol (Daliry et al. 2009) was used to assess the effectiveness of aromatic compounds with different shapes, cationic centres and effective motifs. Stock solutions of the drugs (5 mM) were freshly prepared in dimethyl sulfoxide and the final solvent concentration in the assays never exceeded 0.6%, which is not toxic for either parasites or mammalian cells.
Cell cultures - Primary cultures of embryonic cardiomyocytes (CM) were obtained from Swiss mice as previously described (Meirelles et al. 1986). After purification, the CM were seeded at a density of 5 × 104 cells/well in 96-well microplates containing gelatin-coated cover slips and sustained in Dulbecco's modified medium (DMEM) supplemented with 10% horse serum, 5% foetal bovine serum (FCS), 2.5 mM CaCl2, 1 mM L-glutamine and 2% chicken embryo extract as described previously (Meirelles et al. 1986). The cultures were maintained at 37°C in an atmosphere of 5% CO2 and air and the assays were performed at least three times with duplicate samples. All procedures were carried out in accordance with the guidelines established by the Fiocruz Committee of Ethical for the Use of Animals (CEUA 0099/01).
Parasites - Bloodstream trypomastigotes from the Y strain of T. cruzi were harvested by heart puncture from infected Swiss mice at the parasitaemia peak (Meirelles et al. 1982).
Trypanocidal assays - For the analysis of the effect of the compounds on the bloodstream trypomastigotes, 5 x 106 parasites/mL were incubated for 24 h at 37°C in RPMI 1640 medium supplemented with 10% FCS, in the presence or absence of serial dilutions of the compounds (0.1-32 μM). Alternatively, the treatment was performed using trypomastigotes cultured in freshly isolated mouse blood at 4°C for 24 h with the drugs at concentrations up to 32 μM. The parasite death rates were determined through direct analysis by light microscopy using a Neubauer chamber and the IC50 values (the compound concentration that reduces the number of parasites by 50%) were calculated (Silva et al. 2007b).
Infection assays and effect on intracellular parasites - For the analysis of the effects of the drugs on intracellular parasites, after 24 h of parasite-host cell interaction (ratio of 10:1), the infected cultures were washed to remove free parasites and then maintained at 37°C in an atmosphere of 5% CO2 and air in the presence of the compounds (0.1 to 32 μM). The medium plus drug was replaced every 24 h. After 72 h of treatment, which corresponded to 96 h of infection, the supernatant was recovered, the number of released parasites was determined by direct quantification using light microscopy and a Neubauer chamber and the IC50 values were calculated.
Cytotoxicity assays - To measure the toxic effects on the host cell, uninfected CM were incubated with the compounds (up to 96 μM in DMEM) for 24 h and 72 h at 37ºC and then the cell morphology and viability were evaluated by light microscopy and the method of transcriptional and translational (MTT) colorimetric assay, respectively (Mosmann 1983). The absorbance was measured at 490 nm in a spectrophotometer (VERSAmax tunable, Molecular devices, USA) and was directly proportional to the cell viability, from which the LC50 values (the compound concentration that reduces cellular viability by 50%) were calculated.
Fluorescence microscopic analysis and fluorescent intensity determination - The bloodstream forms were treated for 30 min at 37ºC with 10 μg/mL of each compound, fixed with 4% paraformaldehyde and mounted with 2.5% 1.4-diazabicyclo-(2.2.2)octane (DABCO) on a slide covered with poly-L-lysine (Sigma Aldrich Corp). The fluorescence was analysed using a Zeiss photomicroscope equipped with epifluorescence (Zeiss Inc, Thornwood, NY). The fluorescence intensity of the treated parasites was determined using the program Image J 1.41 (NHI, Bethesda, Maryland) as the sum of the fluorescent pixel values in the selected regions (nucleus DNA - nDNA; kinetoplast DNA - kDNA). The results were expressed as the means and standard deviations of the kDNA/nDNA ratios, which reflect the partition of the kDNA and nDNA fluorescence measurements of at least 50 individual parasites.
RESULTS
We first evaluated the direct effect of the aromatic dicationic compounds on trypomastigotes, which represent the main infective stage of T. cruzi (Fig. 2). The most active compounds, Compounds 1, 2 and 3, displayed dose-dependent effects, with IC50 values of 2.3, 6.1 and 9.3 μM, respectively (Table I) and about 70, 85 and 97% parasite death at a dose of 32 μM (Fig. 2A-C). The other seven compounds displayed only modest activities, with IC50 values higher that 32 μM (Fig. 2D-J, Table I). However, when the bloodstream forms were exposed to Compounds 1, 2 and 3 in the presence of freshly isolated mouse blood, which tested the possible application of these compounds for the prophylaxis of banked blood, we observed a substantial decrease in the trypanocidal activities, with IC50 values higher than 32 μM (Table I).
Next, to evaluate the toxicity on mammalian host cells, uninfected cardiac cultures were incubated for 24 and 72 h with different doses of the compounds and then cellular viability was evaluated by both light microscopy and the MTT colorimetric assay. The compounds did not induce loss of cellular viability after incubation for 24 h with doses up to 96 μM (data not shown); however, most of the aromatic dicationic compounds displayed low toxicity after 72 h of incubation and Compounds 1 and 2 exhibited moderate toxicity, with LC50 values of 25 and 85 μM, respectively (Fig. 3).
Next, the anti-parasitic activity of the compounds against the intracellular forms of T. cruzi was assessed through the direct quantification of the number of parasites released in the supernatant of infected CM after 96 h of parasite interaction. Incubation for 72 h with Compounds 7, 4, 3, 6 and 5 resulted in dose-dependent effects that lead to considerable reductions in the number of parasites released into the supernatant, with micromolar and sub-micromolar IC50 values (Fig. 4C-G, Table I). On the other hand, Compounds 1, 8 and 9 exerted moderated activity while Compounds 2 and 10 were not active and had IC50 values higher that 32 μM (Fig. 4A-B, H-J, Table I). With the exceptions of Compounds 1 and 2, the other compounds displayed equal or better activity on intracellular parasites compared to the bloodstream parasites (Table I).
Based on the IC50 and LC50 values, the selectivity index (SI) of each compound was determined. This parameter reflects the quantity of compound that is active against the pathogen but is not toxic towards the host cell. For the bloodstream trypomastigotes, only one dicationic compound (Compound 1) showed a high SI value (> 40), but for the intracellular parasites, five out of 10 compounds displayed considerable selectivity: Compounds 7, 4, 3, 6 and 5 with SI ranging between > 43 and > 960. These five aromatic compounds also displayed higher anti-proliferative effects on the intracellular parasites.
Within the treated bloodstream parasites, all of the fluorescent compounds were localised in DNA-enriched structures, i.e., the kinetoplast and nucleus (Fig. 5). However, although there was consistently higher labelling within the kDNA compared to the nuclei (Fig. 5), the kDNA/nDNA ratios showed that the higher accumulation in the kDNA (ratios >1.28) did not correlate with compound efficacy: Compound 7, one of the less active compounds, showed the highest accumulation in the kinetoplast, with a 1.77 kDNA/nDNA ratio (Table II).
DISCUSSION
Diamidines and related dications are considered to be potential anti-parasitic agents due to their known activities against several pathogens (Soeiro et al. 2005). However, as they possess critical limitations regarding their poor oral bioavailability and considerable toxicity, new dicationic analogs have been synthesised to address this situation.
Our assays evaluated the effect of 10 aromatic dicationic compounds on trypomastigotes under different experimental conditions to explore their potential uses as chemotherapeutics (assays conducted at 37°C) and/or prophylactic compounds for banked blood (assays using whole blood at 4°C). Our data showed that although three compounds, Compounds 1, 2 and 3, induced high levels of parasite lysis and dose-dependent effects with low micromolar IC50 values when assayed at 37°C, all of them showed decreased activity in the presence of blood, possibly due to their association with and/or inactivation by serum components as reported previously (Santa-Rita et al. 2004, 2006, Silva et al. 2007a). Therefore, the decreased activity at 4°C in the presence of blood constituents demonstrated that the studied compounds are ineffective for the sterilisation of ex vivo blood batches to control Chagas disease.
In agreement with our previous studies showing that reversed amidines, also named arylimidamides, exhibited low toxicity to mammalian cells in vitro (Silva et al. 2007a), our present data showed that, except for Compounds 1 and 2, only high drug concentrations (> 96 μM) induced alterations in host cell viability.
We also found that five out of 10 Compounds (Compounds 7, 4, 3, 6 and 5) exerted considerable activity against the intracellular forms of T. cruzi at low micromolar and sub-micromolar doses and with high SI values (ranging between > 43 and > 960). This difference in activity on the intracellular forms compared to the bloodstream forms requires further analysis but could represent differences in drug uptake by these different parasite stages and/or different mechanisms of action upon non-dividing trypomastigotes and the highly multiplicative intracellular stages of the parasite.
Aromatic dicationic compounds, such as pentamidine, bind non-covalently and in a non-intercalative manner to the minor-groove of the DNA; however, their mechanism of action has not been fully elucidated and it has been proposed that they may possess multiple modes of action (Wilson et al. 2005). One of the long-hypothesised mechanisms of action of diamidines is related to their ability to bind to AT-rich regions of the DNA minor groove, but other mechanisms have also been proposed, such as inhibition of tyrosyl-DNA phosphodiesterase, topoisomerases, protein kinase A, proteases and polymerases (Tidwell & Boykin 2003, Soeiro et al. 2008, Soeiro & De Castro 2009).
According to our present results, we could not find any correlation between the localisation and higher accumulation of these dicationic fluorescent compounds within the T. cruzi kDNA and their trypanocidal activity, which we also found in another recent study of other dicationic compounds (Daliry et al. 2009). In fact, previous reports on African trypanosomes also could not correlate either intracellular accumulation or sub cellular localisation and distribution of aza analogs and diphenyl furans with their in vitro activities (Mathis et al. 2007).
Our present paper describes the potential effect of the aromatic dicationic compounds on T. cruzi, which supports further screening of new analogs that could be used alone or in combination with other drugs for the treatment of Chagas disease.
REFERENCES
Daliry A, Silva PB, Silva CF, Meuser MB, de Castro SL, Tidwell RR, Soeiro MNC 2009. In vitro analyses of the effect of aromatic diamidines upon Trypanosoma cruzi. J Antimicrob Chemother 64: 747-750.
da Silva CF, Batista MM, Batista D da G, de Souza EM, da Silva PB, de Oliveira GM, Meuser AS, Shareef AR, Boykin DW, Soeiro M de N 2008. Trypanocidal activity of a diarylthiophene diamidine against Trypanosoma cruzi: in vitro and in vivo studies. Antimicrob Agents Chemother 52: 3307-3314.
De Souza EM, Lansiaux A, Bailly C, Wilson WD, Hu Q, Boykin DW, Batista MM, Araújo-Jorge TC, Soeiro MN 2004. Phenyl substitution of furamidine markedly potentiates its antiparasitic activity against Trypanosoma cruzi and Leishmania amazonensis. Biochem Pharmacol 68: 593-600.
De Souza EM, Menna-Barreto R, Araújo-Jorge TC, Kumar A, Hu Q, Boykin DW, Soeiro, MNC 2006a. Antiparasitic activity of aromatic diamidines is related to apoptosis-like death in Trypanosoma cruzi. Parasitology 133: 75-79.
De Souza EM, Oliveira GM, Boykin DW, Kumar A, Hu Q, Soeiro MNC 2006b. Trypanocidal activity of the phenyl-substituted analogue of furamidine DB569 against Trypanosoma cruzi infection in vivo. J Antimicrob Chemother 58: 610-614.
Dias JC 2007. Southern Cone Initiative for the elimination of domestic populations of Triatoma infestans and the interruption of transfusion Chagas disease: historical aspects, present situation and perspectives. Mem Inst Oswaldo Cruz 102: 11-18.
Jannin J, Villa L 2007. An overview of Chagas disease treatment. Mem Inst Oswaldo Cruz 102: 95-97.
Mathis AM, Bridges AS, Ismail MA, Kumar A, Francesconi I, Anbazhagan M, Hu Q, Tanious FA, Wenzler T, Saulter J, Wilson WD, Brun R, Boykin DW, Tidwell RR, Hall JE 2007. Diphenyl furans and aza analogs: effects of structural modification on in vitro activity, DNA binding and accumulation and distribution in trypanosomes. Antimicrob Agents Chemother 51: 2801-2810.
Meirelles MN, de Araújo Jorge TC, de Souza W 1982. Interaction of Trypanosoma cruzi with macrophages in vitro: dissociation of the attachment and internalization phases by low temperature and cytochalasin B. Z Parasitenkd 68: 7-14.
Meirelles MN, de Araujo-Jorge TC, Miranda CF, de Souza W, Barbosa HS 1986. Interaction of Trypanosoma cruzi with heart muscle cells: ultrastructural and cytochemical analysis of endocytic vacuole formation and effect upon myogenesis in vitro. Eur J Cell Biol 41: 198-206.
Mosmann T 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55-63.
Rocha MO, Teixeira MM, Ribeiro AL 2007. An update on the management of Chagas cardiomyopathy. Expert Rev Anti Infect Ther 5: 727-743.
Rodriques Coura RJ, de Castro SL 2002. A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz 97: 3-24.
Santa-Rita RM, Barbosa HS, de Castro SL 2006. Ultrastructural analysis of edelfosine-treated trypomastigotes and amastigotes of Trypanosoma cruzi. Parasitol Res 100: 187-190.
Santa-Rita RM, Santos Barbosa H, Meirelles MN, de Castro SL 2004. Effect of the alkyl-lysophospholipids on the proliferation and differentiation of Trypanosoma cruzi. Acta Trop 75: 219-228.
Silva CF, Batista MM, Mota RA, de Souza EM, Stephens CE, Som P, Boykin DW, Soeiro MdeN 2007a. Activity of "reversed" diamidines against Trypanosoma cruzi in vitro. Biochem Pharmacol 73: 1939-1946.
Silva CF, Meuser MB, De Souza EM, Meirelles MN, Stephens CE, Som P, Boykin DW, Soeiro MN 2007b. Cellular effects of reversed amidines on Trypanosoma cruzi. Antimicrob Agents Chemother 51: 3803-3809. Soeiro MNC, De Castro SL, De Souza EM, Batista DGJ, Silva CF, Boykin DW 2008. Diamidine activity against trypanosomes: the state of the art. Curr Mol Pharmacol 1: 151-161.
Soeiro MN, Dantas AP, Daliry A, Silva CF, Batista DG, de Souza EM, Oliveira GM, Salomão K, Batista MM, Pacheco MG, Silva PB, Santa-Rita RM, Barreto RF, Boykin DW, Castro SL 2009. Experimental chemotherapy for Chagas disease: 15 years of research contributions from in vivo and in vitro studies. Mem Inst Oswaldo Cruz 104: 301-310.
Soeiro MN, De Castro SL 2009. Trypanosoma cruzi targets for new chemotherapeutic approaches. Expert Opin Ther Targets 13: 105-121.
Soeiro MN, De Souza EM, Stephens CE, Boykin DW 2005. Aromatic diamidines as antiparasitic agents. Expert Opin Investig Drugs 14: 957-972.
Stewart M, Krishna S, Burchmore RS, Brun R, de Koning HP, Boykin DW, Tidwell RR, Hall JE, Barrett MP 2005. Detection of arsenical drug resistance in Trypanosoma brucei with a simple fluorescence test. Lancet 366: 486-487.
Tidwell RR, Boykin DW 2003. Minor groove binders as antimicrobial agents. In M Demeunynck, C Bailly, WD Wilson (eds.), Small molecule DNA and RNA binder: synthesis to nucleic acid complexes, Wiley-VCH, New York, p. 416-460.
Urbina JA 2002. Chemotherapy of Chagas disease. Curr Pharm Des 8: 287-295.
Werbovetz K 2006. Diamidines as antitrypanosomal, antileishmanial and antimalarial agents. Curr Opin Investig Drugs 7: 147-157.
Wilson WD, Nguyen B, Tanious FA, Mathis A, Hall JE, Stephens CE, Boykin DW 2005. Dications that target the DNA minor groove: compound design and preparation, DNA interactions, cellular distribution and biological activity. Curr Med Chem Anticancer Agents 5: 389-408.
Wilson WD, Tanious FA, Mathis A, Tevis D, Hall JE, Boykin DW 2008. Antiparasitic compounds that target DNA. Biochimie 90: 999-1014.
Received 6 August 2009
Accepted 13 April 2010
Financial support: FAPERJ, Pensa Rio/FAPERJ, CNPq, PAPES V/Fiocruz, CPPD
- Daliry A, Silva PB, Silva CF, Meuser MB, de Castro SL, Tidwell RR, Soeiro MNC 2009. In vitro analyses of the effect of aromatic diamidines upon Trypanosoma cruzi J Antimicrob Chemother 64: 747-750.
- da Silva CF, Batista MM, Batista D da G, de Souza EM, da Silva PB, de Oliveira GM, Meuser AS, Shareef AR, Boykin DW, Soeiro M de N 2008. Trypanocidal activity of a diarylthiophene diamidine against Trypanosoma cruzi: in vitro and in vivo studies. Antimicrob Agents Chemother 52: 3307-3314.
- De Souza EM, Lansiaux A, Bailly C, Wilson WD, Hu Q, Boykin DW, Batista MM, Araújo-Jorge TC, Soeiro MN 2004. Phenyl substitution of furamidine markedly potentiates its antiparasitic activity against Trypanosoma cruzi and Leishmania amazonensis Biochem Pharmacol 68: 593-600.
- De Souza EM, Menna-Barreto R, Araújo-Jorge TC, Kumar A, Hu Q, Boykin DW, Soeiro, MNC 2006a. Antiparasitic activity of aromatic diamidines is related to apoptosis-like death in Trypanosoma cruzi Parasitology 133: 75-79.
- De Souza EM, Oliveira GM, Boykin DW, Kumar A, Hu Q, Soeiro MNC 2006b. Trypanocidal activity of the phenyl-substituted analogue of furamidine DB569 against Trypanosoma cruzi infection in vivo. J Antimicrob Chemother 58: 610-614.
- Dias JC 2007. Southern Cone Initiative for the elimination of domestic populations of Triatoma infestans and the interruption of transfusion Chagas disease: historical aspects, present situation and perspectives. Mem Inst Oswaldo Cruz 102: 11-18.
- Jannin J, Villa L 2007. An overview of Chagas disease treatment. Mem Inst Oswaldo Cruz 102: 95-97.
- Mathis AM, Bridges AS, Ismail MA, Kumar A, Francesconi I, Anbazhagan M, Hu Q, Tanious FA, Wenzler T, Saulter J, Wilson WD, Brun R, Boykin DW, Tidwell RR, Hall JE 2007. Diphenyl furans and aza analogs: effects of structural modification on in vitro activity, DNA binding and accumulation and distribution in trypanosomes. Antimicrob Agents Chemother 51: 2801-2810.
- Meirelles MN, de Araújo Jorge TC, de Souza W 1982. Interaction of Trypanosoma cruzi with macrophages in vitro: dissociation of the attachment and internalization phases by low temperature and cytochalasin B. Z Parasitenkd 68: 7-14.
- Meirelles MN, de Araujo-Jorge TC, Miranda CF, de Souza W, Barbosa HS 1986. Interaction of Trypanosoma cruzi with heart muscle cells: ultrastructural and cytochemical analysis of endocytic vacuole formation and effect upon myogenesis in vitro Eur J Cell Biol 41: 198-206.
- Mosmann T 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55-63.
- Rocha MO, Teixeira MM, Ribeiro AL 2007. An update on the management of Chagas cardiomyopathy. Expert Rev Anti Infect Ther 5: 727-743.
- Rodriques Coura RJ, de Castro SL 2002. A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz 97: 3-24.
- Santa-Rita RM, Barbosa HS, de Castro SL 2006. Ultrastructural analysis of edelfosine-treated trypomastigotes and amastigotes of Trypanosoma cruzi Parasitol Res 100: 187-190.
- Santa-Rita RM, Santos Barbosa H, Meirelles MN, de Castro SL 2004. Effect of the alkyl-lysophospholipids on the proliferation and differentiation of Trypanosoma cruzi Acta Trop 75: 219-228.
- Silva CF, Batista MM, Mota RA, de Souza EM, Stephens CE, Som P, Boykin DW, Soeiro MdeN 2007a. Activity of "reversed" diamidines against Trypanosoma cruzi in vitro Biochem Pharmacol 73: 1939-1946.
- Silva CF, Meuser MB, De Souza EM, Meirelles MN, Stephens CE, Som P, Boykin DW, Soeiro MN 2007b. Cellular effects of reversed amidines on Trypanosoma cruzi Antimicrob Agents Chemother 51: 3803-3809.
- Soeiro MNC, De Castro SL, De Souza EM, Batista DGJ, Silva CF, Boykin DW 2008. Diamidine activity against trypanosomes: the state of the art. Curr Mol Pharmacol 1: 151-161.
- Soeiro MN, Dantas AP, Daliry A, Silva CF, Batista DG, de Souza EM, Oliveira GM, Salomão K, Batista MM, Pacheco MG, Silva PB, Santa-Rita RM, Barreto RF, Boykin DW, Castro SL 2009. Experimental chemotherapy for Chagas disease: 15 years of research contributions from in vivo and in vitro studies. Mem Inst Oswaldo Cruz 104: 301-310.
- Soeiro MN, De Castro SL 2009. Trypanosoma cruzi targets for new chemotherapeutic approaches. Expert Opin Ther Targets 13: 105-121.
- Soeiro MN, De Souza EM, Stephens CE, Boykin DW 2005. Aromatic diamidines as antiparasitic agents. Expert Opin Investig Drugs 14: 957-972.
- Stewart M, Krishna S, Burchmore RS, Brun R, de Koning HP, Boykin DW, Tidwell RR, Hall JE, Barrett MP 2005. Detection of arsenical drug resistance in Trypanosoma brucei with a simple fluorescence test. Lancet 366: 486-487.
- Tidwell RR, Boykin DW 2003. Minor groove binders as antimicrobial agents. In M Demeunynck, C Bailly, WD Wilson (eds.), Small molecule DNA and RNA binder: synthesis to nucleic acid complexes, Wiley-VCH, New York, p. 416-460.
- Urbina JA 2002. Chemotherapy of Chagas disease. Curr Pharm Des 8: 287-295.
- Werbovetz K 2006. Diamidines as antitrypanosomal, antileishmanial and antimalarial agents. Curr Opin Investig Drugs 7: 147-157.
- Wilson WD, Nguyen B, Tanious FA, Mathis A, Hall JE, Stephens CE, Boykin DW 2005. Dications that target the DNA minor groove: compound design and preparation, DNA interactions, cellular distribution and biological activity. Curr Med Chem Anticancer Agents 5: 389-408.
- Wilson WD, Tanious FA, Mathis A, Tevis D, Hall JE, Boykin DW 2008. Antiparasitic compounds that target DNA. Biochimie 90: 999-1014.
Publication Dates
-
Publication in this collection
21 May 2010 -
Date of issue
May 2010
History
-
Received
06 Aug 2009 -
Accepted
13 Apr 2010