Abstracts
Objective:
The aim of the present investigation was to determine whether the difference in inflammatory tissue reaction between the Riccinus communis (castor) polymer with calcium carbonate and the titanium implant is statistically significant.
Methods:
Thirty-two Cavia porcellus were allocated into four groups of eight animals each. We implanted the two types of materials in the retroperitoneal space of all the animals. They were euthanized at 7, 20, 30 and 40 days after surgery, and an histological study of the samples was conducted.
Results:
All implants showed characteristics of chronic inflammation regardless of the material and timepoint of evaluation. There was no statistically significant difference between Pm+CaCO3 and Ti with regard to the presence of granulation tissue, tissue congestion, histiocytes, lymphocytes, neutrophils, giant cells, and fibrosis (P> 0.05).
Conclusion:
The castor oil polymer plus calcium carbonate implant was not statistically different from the titanium implant regarding inflammatory tissue reaction.
Bioprosthesis materials; Heart, artificial Guinea pigs; Implants, Experimental; Ricinus communis ; Boiocompatible; Inflammation
Objetivo:
Determinar se a reação tecidual do implante retroperitoneal do polímero de óleo de mamona com acréscimo de carbonato de cálcio (Pm+CaCO3) é significativa, por meio de análise histopatológica, tendo como controle o implante de titânio não tratado (Ti).
Métodos:
Estudo experimental, intervencionista e randomizado com 32 cobaias. Os animais foram separados em quatro grupos iguais e eutanasiados com 7, 20, 30 e 40 dias após o ato cirúrgico. Foram confeccionadas lâminas em hematoxilina-eosina e em tricrômio de Masson. Em relação a variáveis qualitativas dicotômicas, para análise da diferença entre o Pm+CaCO3 e o Ti em cada momento de avaliação foi usado o teste binomial. Considerando os materiais separadamente, a comparação dos quatro grupos foi feita utilizandose o teste exato de Fisher. Valores de P<0,05 indicaram significância estatística.
Resultados:
Todos os implantes apresentaram características de inflamação crônica, independente do material e do momento de avaliação. Não houve diferença estatisticamente significativa entre o Pm+CaCO3 e o Ti considerando a presença de tecido de granulação, congestão tecidual, histiócitos, linfócitos, neutrófilos, células gigantes e fibrose (P>0,05).
Conclusão:
Não foi encontrada diferença significativa entre a reação tecidual do Pm+CaCO3 e a do Ti.
Implante de Prótese; Coração Artificial; Cobaias; Implantes Experimentais; Ricinus communis ; Biocompatível; Inflamação
| Abbreviations, acronyms & symbols | |
|---|---|
| CaCO3 | Calcium carbonate |
| CAD | Circulatory assist device |
| CO2 | Carbon dioxide |
| COBEA | Brazilian College of Animal Experimentation |
| CHF | Congestive heart failure |
| HE | Hematoxilin-eosin |
| Pm | Castor oil polymer |
| Pm+CaCO3 | Castor oil polymer with added calcium carbonate |
| PVP-I | Polyvinylpirrolidone-iodine solution |
| Ti | Titanium |
INTRODUCTION
Cardiovascular diseases remain as the leading cause of morbidity and mortality worldwide[11. Roscani MG, Matsubara LS, Matsubara BB. Review Article Heart Failure with Normal Ejection Fraction. Arq Bras Cadiol. 2009;94(5):652-60.]. As life expectancy increases, the prevalence of these comorbidities is expected to become even greater. Congestive heart failure (CHF) is one of these diseases and accounts for an average of 400,000 new cases per year in the United States[22. Kubrusly LF, Madeira AF, Savytzky S, Wollman D, Melhem A, Adam R, et al. Dispositivo de assistência circulatória mecânica intraventricular de fluxo axial: estudo in vitro. Rev Bras Cir Cardiovasc. 2000;15(2):169-72.].
End-stage CHF is a clinical condition for which few therapeutic options exist. Heart transplant is still an option in advanced CHF; however, it is a limited course of action due to the small number of donors and the high surgical risk involved[22. Kubrusly LF, Madeira AF, Savytzky S, Wollman D, Melhem A, Adam R, et al. Dispositivo de assistência circulatória mecânica intraventricular de fluxo axial: estudo in vitro. Rev Bras Cir Cardiovasc. 2000;15(2):169-72.]. In view of this, developing new support measures for these patients is a necessity.
This prompted the conception of a Circulatory Assist Device (CAD). These devices, which are analogous to a human heart, are today a key component of the therapeutic support for patients in cardiogenic shock or end-stage heart failure[44. Dinkhuysen JJ, Andrade A, Contreras C, Paulista PP, Manrique R. Estudo experimental da aplicação do ventrículo artificial eletromecânico pulsátil implantável. Rev Bras Cir Cardiovasc. 2011;26(1):76-85.,55. Moreira LFP, Benício A. Assistência circulatória mecânica: uma grande lacuna na cirurgia cardíaca brasileira. Rev Bras Cir Cardiovasc. 2010;25(4):X-XII.].
In 1997, we began to develop an affordable CAD for the Brazilian public health system. We conducted tests in vitro33. Portner PM, Oyer PE, Pennington DG, Baumgartner WA, Griffith BP, Frist WR, et al. Implantable electrical left ventricular assist systems: Bridge to transplantation and future. Ann Thorac Surg. 1989;47(1):142-50.] and designed a device made of titanium, as is the great majority of such devices available worldwide. Experimental studies in vivo with sheep were performed and proved the efficacy of the device. In 2011, Kubrusly et al.[66. Kubrusly LF, Graça YLSDS, Sucharski EE, Sobral ACL, Olandoski M, Kubrusly FB. Biocompatibility of Ricinus comunnis polymer compared to titanium implant used in artificial hearts. Experimental study in guinea pigs. Rev Bras Cir Cardiovasc. 2012;27(3):392-400.] initiated studies with a biocompatible material of national origin for the prospective production of a lower-cost device-this marked the beginning of the studies on the use of castor oil polymer (Pm) in the production of CADs. This polymer is formed by the reaction, in equal amounts by weight, between a polyol (OH) and a castor oil prepolymer[77. Celeste SA, Rahal SC, Câmara O, Pereira-júnior M, Lopes J, Macedo S, et al. Resposta tecidual a implantes de discos de poliuretana de mamona nas formas pré-moldada ou biomassa moldada. Pesq Vet Bras. 2010;30(12):1089-95.].
In keeping with our primary objective, which is to develop a Brazilian-made device suited to the resources of our country, we are at the stage of replacing costly mechanical parts for national-hence, less expensive-materials.
Castor oil is a plant product extracted from the beans of Ricinus communis, which is found in tropical and subtropical areas. The oil is a viscous liquid obtained by hot or cold pressing of the seeds or by solvent extraction[88. Cangemi MJ, Santos AM, Neto S. A Revolução Verde da Mamona. Quím Nov Esc. 2010;32(1):3-8.]. The studies on the use of castor oil and its by-products for polyurethane synthesis started in the late 1940's with the preparation of polyurethane films for surface coating[99. Saunders JH, Frisch KC. Part 1: chemistry. Polyurethanes Chem Technol. New York; 1962. p.368.]. The first reports of the use of polyurethanes in medical applications date back to 1959, when Mandarino and Salvatore implanted a rigid polyetherurethane foam for bone fixation in situ. Castor oil polyurethanes can be produced with a range of different characteristics, from the most flexible (e.g., elastomers) to the most rigid ones (e.g., bone cements).
The Pm polymer can be prepared pure or with added calcium carbonate (CaCO3) at a range of ratios by weight between the polyol, prepolymer, and CaCO3. Adding CaCO3 to the implant increases its mechanical strength and further reduces the production costs of the material[1010. Junqueira R, Carlo D, Kawata D, Isabel M, Viloria V, Rizzo D, et al. Polímero derivado de mamona acrescido de cálcio , associado ou não à medula óssea autógena na reparação de falhas ósseas. Ciênc Rural. 2003;33(6):1081-8.]. In the present study, the ratio of 1/3 polyol, 1/3 prepolymer and 1/3 CaCO3 was used according to the mechanical tests performed by the manufacturer, Poliquil (Araraquara, SP), which point to that ratio as the optimal for medical use. In biocalcification studies, both in vivo and in vitro, Pm was regarded as a useful material not only for manufacturing implants, but also for the construction of accessories and devices such as pumps and valves for extracorporeal circulation[99. Saunders JH, Frisch KC. Part 1: chemistry. Polyurethanes Chem Technol. New York; 1962. p.368.].
The objective of the present study was to evaluate Pm with the addition of 33% CaCO3 (Pm+CaCO3) in order to gather sufficient histological data to ascertain low inflammatory reaction in the retroperitoneal space of guinea pigs compared with the reaction produced by titanium, thereby enabling the production of an even less expensive biomaterial, which would be affordable to the Brazilian population.
METHODS
This experimental, randomized interventional study was developed by the Denton Cooley Institute at the Histology and Cell Biology Laboratories, the Animal Colony, and the Clinical and Surgical Experimentation Laboratory of the Faculdade Evangélica do Paraná.
Ethical issues
The present study was submitted to the Research Ethics Committee of the Sociedade Evangélica Beneficente de Curitiba and approved under Technical Opinion 4277/11. This study was conducted in conformity with Law 6638 of May 8th, 1979-Regulations for the Didactic and Scientific Practice of Animal Vivisection. The standards established in the "Guide for the Care and Use of Laboratory Animals" (Institute of Laboratory Animal Resources, National Academy of Sciences, Washington, D.C., 1996) and the guidelines of the Brazilian College of Animal Experimentation (COBEA) were also met.
Sample
Thirty-two male guinea pigs (Cavia porcellus), Rodentia, Mammalia were used, with weights between 250-300 g (mean, 289.15±17.47 g), aged between 4-6 months, and previously healthy. The animals were obtained from the Instituto de Tecnologia do Paraná, and were raised and maintained under similar environmental and dietary conditions.
The animals were randomized into four groups (A, B, C and D), with eight guinea pigs in each group. They were euthanized in a CO2 gas chamber, four animals at a time, at 7 (group A), 20 (group B), 30 (group C), and 40 (group D) days after the implant surgery[1111. Brito KM de M, Pellizzon CH, Schellini SA, Galvão C, Neto T, Padovani CR. Inclusões de quitosana no subcutâneo de rato: avaliação clínica, histológica e morfométrica. Arq Bras Dermatol. 2009;84(1):35-40.].
Experimental setting
The guinea pigs were housed in the animal colony of the Faculdade Evangélica do Paraná in compliance with the "Manual of Technical Guidelines for Animal Experimentation Colonies" of the Department of Microbiology, Biomedical Sciences Institute, Universidade de São Paulo[1212. Kuramae M, Toledo L, Teixeira HM. Reação à resina vegetal de mamona sem carbonato de cálcio durante o processo de reparo em defeitos ósseos induzidos no corpo da mandíbula. Rev Odontol UNESP. 1999;28(1):63-72.]. The animals were acclimatized for seven days prior to the experiments, and were given filtered water and standard commercial chow ad libitum up to four hours before euthanasia. The operative procedures were conducted using sterile material and technique in a cooled environment at the Clinical and Surgical Experimentation Laboratory, Faculdade Evangélica do Paraná. Tramadol was administered intramuscularly at the dose of 1 mg/kg according to the analgesia protocol, with duration of 12-24 hours[1212. Kuramae M, Toledo L, Teixeira HM. Reação à resina vegetal de mamona sem carbonato de cálcio durante o processo de reparo em defeitos ósseos induzidos no corpo da mandíbula. Rev Odontol UNESP. 1999;28(1):63-72.].
Anesthesia
The animals were anesthetized as recommended by Radde et al.[1313. Radde GR, Hinson A, Crenshaw D, Toth LA. Evaluation of anaesthetic regimens in guineapigs. Lab Anim.1996;30(3):220-7.], using ketamine and xylazine (87 and 13 mg/kg, respectively), both administered intraperitoneally.
Operative technique
Following anesthesia, each animal was placed in the prone position and its hair was removed with scissors, bilaterally, along the dorsum. Subsequently, surgical field antisepsis was performed using a polyvinylpirrolidone-iodine solution (PVP-I) (Figure 1A).
A) Operative procedure surgical field antisepsis with polyvinylpirrolidone-iodine solution (PVP-I) and marking of the site of surgical incision; B) Implantation of the Pm+CaCO3 disc on the right-side hemibody; 1C) Implantation of the Ti disc on the left-side hemibody; 1D) Suture of the superficial plane with nylon 30
Local anesthesia was administered with 2% lidocaine diluted in normal saline. A 2-cm incision was subsequently made at 2 cm from the vertebral axis, to the right, in the region between the iliac crest and the last rib. The epithelium, the subcutaneous tissue and the fascia were penetrated, and the muscle layer was reached. This layer was carefully opened and the virtual retroperitoneal space was accessed, where the Pm+CaCO3 disc was implanted (Figure 1B). The procedure was repeated on the left side; however, the Ti disc was implanted this time (Figure 1C). The deep and superficial incision planes were sutured with nylon 30[1414. Ribeiro CMB, Silva Júnior VA, Silva Neto JC, Vasconcelos BC do E. Estudo clínico e histopatológico da reação tecidual às suturas interna e externa dos fios monofilamentares de nylon e poliglecaprone 25 em ratos. Acta Cir Bras. 2005;20(4):284-91.] in a running fashion (Figure 1D).
Collection of the material for histopathological study
The surgical specimen was removed en bloc and immediately immersed in 10% neutral buffered formalin, where it remained for 72 hours at room temperature. After fixation, the Ti and Pm+CaCO3 discs were removed through a lateral incision and the specimens were then rinsed in water for 24 hours, routinely processed and embedded in paraffin. The material was sectioned at 6 µm and stained by the hematoxylin-eosin (HE) technique for the cellular and capillary elements, and the technique of Masson's trichrome with Nile blue for the collagen fibers from fibrosis. The analysis was conducted using an Olympus® light microscope model DX 50.
The aim of microscopy was to evaluate the inflammatory process elicited by each material, i.e., congestion, formation of granulation tissue, and peri-implant fibrosis.
The inflammation characteristics were qualitatively assessed for every slide of each group, and graded as absent, mild, moderate, or marked. The analyzed cells were histiocytes, neutrophils, lymphocytes, and giant cells. Histiocytes were defined in the present histopathological study as a type of normally stationary and inactive macrophage originated in the reticuloendothelial system that can be activated on stimulation; neutrophils, polynuclear white blood cells with neutrophilic granules; lymphocytes, a type of white blood cells between 10-12 µm in diameter, having a round nucleus with condensed chromatin and scarce, faintly basophilic cytoplasm; giant cells were defined as those formed by the fusion of several distinct cells.
A x10 focusing eyepiece and x40 objective were used for the microscopy study. The cell types were counted in 10 fields under the microscope in the areas involved in the inflammatory process induced by the foreign body.
Preparation and source of implants
The control material was Ti and Pm+CaCO3, the test material. The Ti alloy, characterized as grade 2, was obtained from a business company in the form of cylindrical bars 5 mm in diameter. The bars were composed of 99.7% Ti; 0.009% C; 0.095 Fe; 0.0003% H; 0.0038% N, and 0.152% O2. The mechanical properties of the bar were the following: % elongation (IN) L=32; % reduction in area L= 52; ultimate tensile strength (UTS) Ksi L= 78.8, and yield tensile strength (YS) Ksi (0.2%) L= 57.
The Pm and the CaCO3 were obtained from a laboratory specialized in medical grade polymers (Poliquil, Araraquara, SP, Brazil). The preparation of the castor polymer implants began with equal amounts from one vial of polyol, one vial of vegetable polyurethane resin hardener, and an equal proportion by weight of CaCO3. These components were mixed at equal ratios by weight in a beaker using a glass stirring rod for 3 min until full homogenization was achieved. The homogenate thus obtained was placed in two insulin syringes. Following full polymerization, the syringes were sectioned with a scalpel blade into 3-mm-thick cross sections; subsequently, the plastic of the syringe barrel was removed[1515. Mastrantonio SDS, Ramalho LTDO. Resposta do Tecido Conjuntivo de Camundongos ao Poliuretano Vegetal de Óleo de Mamona. Rev Odontol UNESP. 2003;32(1):31-7.]. Thus, discs with a thickness of 3 mm and a diameter of 5 mm were obtained to be implanted retroperitoneally in the guinea pigs. Disc antisepsis was performed by immersing the discs in 70% alcohol for 15 min. The Ti implants were produced by a specialized manufacturer (Neodent, Curitiba, PR, Brazil). The Ti bar was attached to the plate of an engine lathe (Tormax 20A -ROMI) and cut with the aid of a cutting fluid (Quimatic oil). Subsequently, the discs were smoothed to be rid of rough spots or irregularities resulting from the first process. The Ti implants had the same dimensions as the Pm implants and were also sterilized in 70% alcohol.
Statistical analysis
Regarding the dichotomous qualitative variables, the test binomial was used in the analysis of the difference between the Pm+CaCO3 and Ti implants at each timepoint. When considering the materials separately, the four groups were compared using Fisher's exact test. Values of P<0.05 indicated statistical significance. The data were analyzed using the SPSS software version 20.0.
RESULTS
Regarding peri-implant tissue inflammation, 100% of the implants showed histological characteristics of chronic inflammation; therefore, no statistically significant difference was found between the Pm+CaCO3 and the Ti group regardless of the evaluation timepoint (P=1 across comparisons). The presence of lymphocytes, neutrophils, giant cells, and peri-implant tissue congestion was consistently not marked over time for either implant material (P=1 across comparisons). For some variables, the time effect was significant. The analysis of both Pm+CaCO3 and Ti implants showed a progressive and linear decrease in the granulation process and in the presence of histiocytes. For both materials, these factors appeared markedly in the group evaluated in the first 7 days of the study (group A) whereas they were predominantly graded as not marked in the last two groups (C and D) (P<0.05). However, there was no statistically significant difference in the behavior of these variables between the two types of material (P=1 across comparisons). Albeit also without statistical differences between the materials, peri-implant fibrosis was found to be marked in groups A and B, and not marked in groups C and D. A slight tendency was noted for the polymer implant to exhibit less fibrosis than the Ti in groups C and D, although this was not statistically significant (Figures 2 and 3).
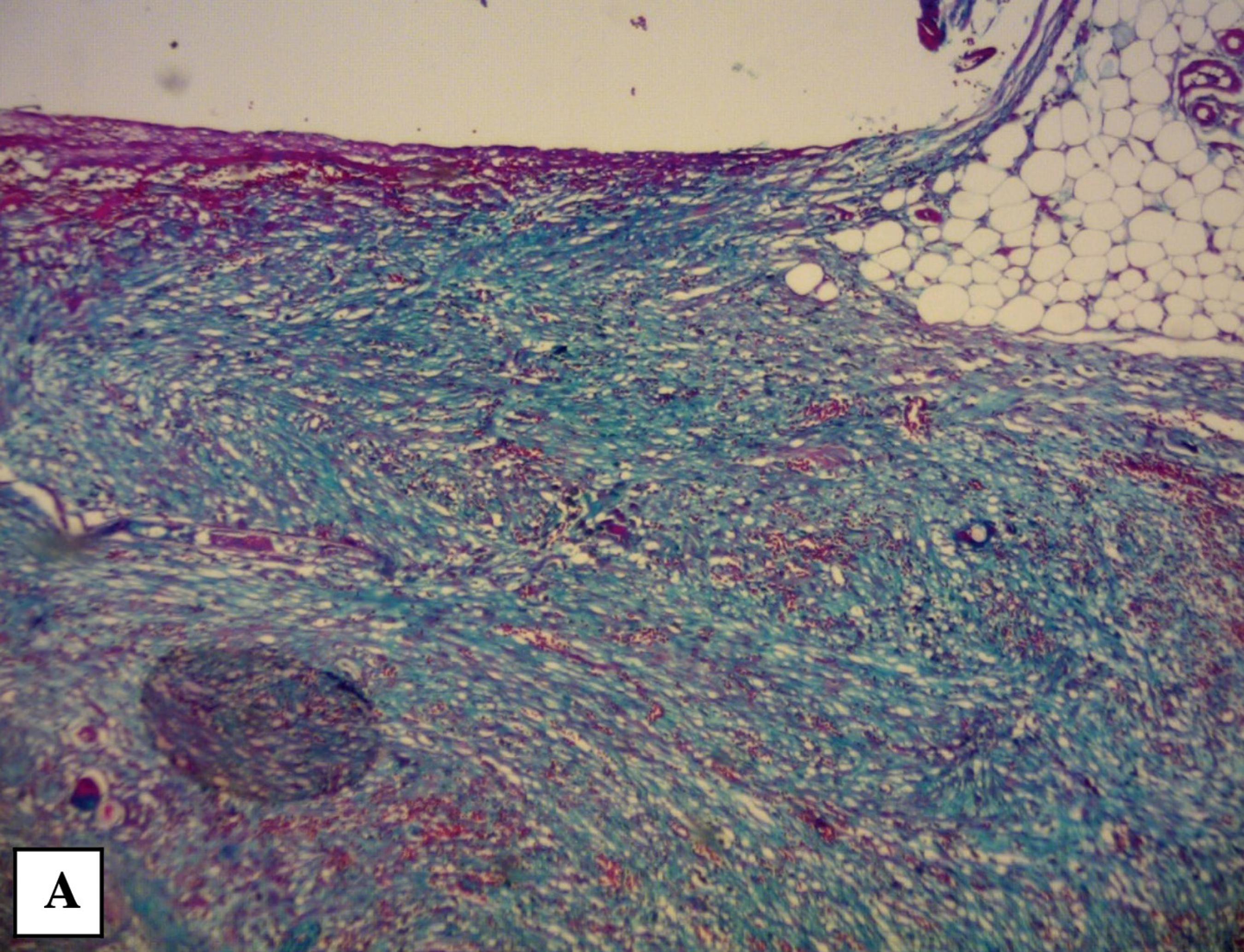
Light microscopy – Titanium-associated fibrosis. Staining by Masson’s Trichrome with Nile Blue
Light microscopy – Pm+CaCO3 -associated fibrosis. Staining by Masson’s Trichrome with Nile Blue
Analysis of the difference in marked fibrosis between groups Pm+CaCO3 and Ti according to the evaluation timepoints
DISCUSSION
As established in the previous study in the same line of research, we maintained the methodology[66. Kubrusly LF, Graça YLSDS, Sucharski EE, Sobral ACL, Olandoski M, Kubrusly FB. Biocompatibility of Ricinus comunnis polymer compared to titanium implant used in artificial hearts. Experimental study in guinea pigs. Rev Bras Cir Cardiovasc. 2012;27(3):392-400.] in order to obtain materials that are low-cost, abundant in nature, and with an acceptable biocompatibility profile that would ideally replace the time-honored materials in medical practice, as these are expensive and difficult to be obtained.
The favorable characteristics of Pm were first reported by such authors as Ohara et al.[1616. Ohara GH, Kojima KE, Rossi JC, Telles M, Soares TVC, Salomão C, et al. Estudo experimental da biocompatibilidade do polímero poliuretano da mamona implantada. Acta Ortop Bras. 1995;3(2):1-7.], Carvalho et al.[1717. Carvalho T, Araújo C, Teófilo J, Brentegani L. Histologic and histometric evaluation of rat alveolar wound healing around polyurethane resin implants. Int J Oral Maxillofac Surg. 1997;26(2):149-52.], Ignácio et al.[1818. Carlos J, Márcio C, Castro B. Poliuretana de mamona (Ricinus communis) para desvio da crista tibial no cão. Ciênc Rural. 2004;34(3):821-7.], Silva[1919.Silva MJA. Estudo radiográfico das imagens convencionais e digitalizadas do comportamento do tecido ósseo frente ao implante do polímero de mamonas em coelhos [Tese de mestrado]. Universidade de São Paulo; 1997. p.135.], and Kubrusly[66. Kubrusly LF, Graça YLSDS, Sucharski EE, Sobral ACL, Olandoski M, Kubrusly FB. Biocompatibility of Ricinus comunnis polymer compared to titanium implant used in artificial hearts. Experimental study in guinea pigs. Rev Bras Cir Cardiovasc. 2012;27(3):392-400.], thereby opening the way to the use of this polymer in orthopedics, orthodontics, bucomaxilar surgery, neurosurgery, and cardiovascular surgery. Furthermore, the production process of Pm is very attractive, as it needs no catalysts and shows simple processability and flexibility in formulation, thereby enabling the addition of other components- in the present case, CaCO3- without interfering with the chemical reaction.
The Pm material shows versatility in terms of curing temperature, with a maximum exothermic peak at 45ºC and no production of post-reaction free residual monomers-an undesirable occurrence for assisted circulation devices, common among polymers, which prompted researchers to use titanium for this purpose. Dias et al.[2020. Dias PCDJ, Granato L, Pretel H. Avaliação histológica da biocompatibilidade do polímero da mamona no dorso nasal de macacos-pregos (Cebus apella). Braz J Otorhinolaryngol. 2009;75(3):350-5.] conducted a study with four capuchin monkeys (Cebus apella), wherein the investigators placed a Pm implant in a bone defect previously created in the nasal dorsum of the animals; later, the histological analysis revealed no foreign body granulomas or phagocytic cells. Although no bone tissue analysis was performed in the present study, no foreign body granulomas were found on histological analysis. A study by Mastrantonio and Ramalho[1515. Mastrantonio SDS, Ramalho LTDO. Resposta do Tecido Conjuntivo de Camundongos ao Poliuretano Vegetal de Óleo de Mamona. Rev Odontol UNESP. 2003;32(1):31-7.] evaluated the response of the connective tissue of mice to Pm over a period of 60 days and, again, biocompatibility results were favorable.
Titanium is considered to be an inert material; authors such as Van Noort et al.[2121. Van Noort R. Titanium: The implant material of today. J Mater Sci. 1987;22(11):3801-11.] have extensively demonstrated its applicability in medical grade alloys since mid-20th century. Alloys of Ti have been widely used as bone substitutes, in implantable materials in the human body such as screws and plates, and are present today in reconstructive surgery as well. Likewise, Pm has been expanding its range of applications.
In the present study, it was noted that the predominant type of inflammation in both study materials (Pm+CaCO3 and Ti) was chronic inflammation, i.e., predominance of monomorphonuclear cells regardless of the assessment timepoint. The initial seven-day period is directly related to the type of suture used in the surgery. Siqueira and Dantas[2222. Siqueira JRJ. Inflamação - Aspectos biodinâmicos das respostas inflamatória e imunologica. Rio de Janeiro: Editora Pedro Primeiro LTDA; 1996.] have defined this length of time as the period of deposition of hyaluronic acid and collagen, both of which are produced in an attempt to reconstitute the injured tissues. In the current study, the presence of granulation tissue and histiocytes was marked in the first seven days, albeit without statistical significance when the two types of material were compared.
The lower amount of fibrosis found in groups C and D, i.e., at 30 and 40 postoperative days, diverged from the results of Costa et al.[2323. Costa CA de S, Marcantonio RAC, Hebling J, Teixeira HM, Kuramae M. Biocompatibilidade do polímero de poliuretana vegetal derivada do óleo de mamona em estudo comparativo com cimento de óxido de zinco e eugenol: avaliação histopatológica de implantes subcutâneos de ratos. Odonto 2000.1997;1(1):44-8.], who noted the formation of dense fibrous tissue surrounding the Pm implant. In the present study, as in the previous study by Kubrusly and co-workers[66. Kubrusly LF, Graça YLSDS, Sucharski EE, Sobral ACL, Olandoski M, Kubrusly FB. Biocompatibility of Ricinus comunnis polymer compared to titanium implant used in artificial hearts. Experimental study in guinea pigs. Rev Bras Cir Cardiovasc. 2012;27(3):392-400.], for both materials, the adjacent connective tissue showed normal histological characteristics and a tendency toward decreasing tissue reaction with increasing post-implantation timepoints.
Furthermore, macro- and microscopically, no polymer structure degradation occurred when it was subjected to the body temperature ranges of the guinea pigs. This can be reinforced by the fact that the polyurethanes exhibited thermal stability up to 210ºC, which demonstrates that these polymers will not undergo thermal decomposition at ambient temperature[2424. Pereira PHL. Estudo das propriedades físico-químicas da poliuretana derivada do óleo de mamona com potencial aplicação na área médica. [Tese de mestrado].Universidade de São Paulo; 2010. p.75.].
In addition to showing stability at high temperatures, Pm+CaCO3 is a Brazilian technology and represents the possibility of providing a lighter implantable device for the patient. Our CAD in study, the K-pump, made of steel, weighs 194 g. A titanium-made device with the same dimensions is estimated to weigh between 85-95 g. The weight of the Pm+CaCO3 implant will be approximately 45-55 g, which is below the average of most currently available fully implantable CADs and suitable for pediatric patients and small-built adults.
In line with the data from the study with pure Pm[66. Kubrusly LF, Graça YLSDS, Sucharski EE, Sobral ACL, Olandoski M, Kubrusly FB. Biocompatibility of Ricinus comunnis polymer compared to titanium implant used in artificial hearts. Experimental study in guinea pigs. Rev Bras Cir Cardiovasc. 2012;27(3):392-400.], Pm+CaCO3 also has identical biocompatibility properties to those of the Ti implant. In addition to the preserved biocompatibility, the new combination confers greater mechanical strength and a smaller proportion of polymer raw material is used in its preparation. Therefore, production costs are further reduced, since smaller amounts of polyol and prepolymer are needed to produce our CAD. This economy of 33 % in raw material affords a 10-20% reduction in the production costs of the core structure of our CAD; this represents a further step toward our goal.
CONCLUSION
No statistically significant difference was found between the Pm+CaCO3 and the Ti implants with respect to the induced tissue reaction in guinea pigs.
| Authors’ roles & responsibilities | |
|---|---|
| YLSG | Review of the literature; participation in the experimental surgeries; drafting of the manuscript |
| ACO | Review of the literature; participation in the experimental surgeries; drafting of the manuscript |
| BEGB | Review of the literature; participation in the experimental surgeries; drafting of the manuscript |
| BOE | Review of the literature; participation in the experimental surgeries; drafting of the manuscript |
| CCM | Review of the literature; participation in the experimental surgeries; drafting of the manuscript |
| FCK | Review of the literature; participation in the experimental surgeries; drafting of the manuscript |
| EES | Review of the literature; participation in the experimental surgeries; drafting of the manuscript |
| LFK | Review of the literature; participation in the experimental surgeries; drafting of the manuscript |
ACKNOWLEDGMENTS
We would like to thank the Denton Cooley Research Institute, particularly Professor Dr. Luiz Fernando Kubrusly for his support and encouragement in the present study; the Vita Research Center, which made possible the undertaking of the present study; POLIQUIL (Araraquara, SP, Brazil), particularly Mr. Antonio Carlos Rossi and Mr. Alexandre Guillen, who helped supply the castor polymer; Neodent company (Curitiba, PR, Brazil), particularly Mr. Adailton Becker, for their assistance handling and cutting the titanium pieces; Dr Ana Cristina Lira Sobral for Reading and Report of the blades of pathological analysis; Dr Marcia Olandoski for Statistical analysis; Dr Fernando Bermudez Kubrusly for his support in the present study and the Faculdade Evangélica do Paraná for the helpfulness and support throughout the study.
-
No financial support.
-
This work was carried out at Denton Cooley Research Institute; Faculdade Evangélica do Paraná; Hospital Vita-InCor Education and Research Institute.
REFERENCES
-
1Roscani MG, Matsubara LS, Matsubara BB. Review Article Heart Failure with Normal Ejection Fraction. Arq Bras Cadiol. 2009;94(5):652-60.
-
2Kubrusly LF, Madeira AF, Savytzky S, Wollman D, Melhem A, Adam R, et al. Dispositivo de assistência circulatória mecânica intraventricular de fluxo axial: estudo in vitro. Rev Bras Cir Cardiovasc. 2000;15(2):169-72.
-
3Portner PM, Oyer PE, Pennington DG, Baumgartner WA, Griffith BP, Frist WR, et al. Implantable electrical left ventricular assist systems: Bridge to transplantation and future. Ann Thorac Surg. 1989;47(1):142-50.
-
4Dinkhuysen JJ, Andrade A, Contreras C, Paulista PP, Manrique R. Estudo experimental da aplicação do ventrículo artificial eletromecânico pulsátil implantável. Rev Bras Cir Cardiovasc. 2011;26(1):76-85.
-
5Moreira LFP, Benício A. Assistência circulatória mecânica: uma grande lacuna na cirurgia cardíaca brasileira. Rev Bras Cir Cardiovasc. 2010;25(4):X-XII.
-
6Kubrusly LF, Graça YLSDS, Sucharski EE, Sobral ACL, Olandoski M, Kubrusly FB. Biocompatibility of Ricinus comunnis polymer compared to titanium implant used in artificial hearts. Experimental study in guinea pigs. Rev Bras Cir Cardiovasc. 2012;27(3):392-400.
-
7Celeste SA, Rahal SC, Câmara O, Pereira-júnior M, Lopes J, Macedo S, et al. Resposta tecidual a implantes de discos de poliuretana de mamona nas formas pré-moldada ou biomassa moldada. Pesq Vet Bras. 2010;30(12):1089-95.
-
8Cangemi MJ, Santos AM, Neto S. A Revolução Verde da Mamona. Quím Nov Esc. 2010;32(1):3-8.
-
9Saunders JH, Frisch KC. Part 1: chemistry. Polyurethanes Chem Technol. New York; 1962. p.368.
-
10Junqueira R, Carlo D, Kawata D, Isabel M, Viloria V, Rizzo D, et al. Polímero derivado de mamona acrescido de cálcio , associado ou não à medula óssea autógena na reparação de falhas ósseas. Ciênc Rural. 2003;33(6):1081-8.
-
11Brito KM de M, Pellizzon CH, Schellini SA, Galvão C, Neto T, Padovani CR. Inclusões de quitosana no subcutâneo de rato: avaliação clínica, histológica e morfométrica. Arq Bras Dermatol. 2009;84(1):35-40.
-
12Kuramae M, Toledo L, Teixeira HM. Reação à resina vegetal de mamona sem carbonato de cálcio durante o processo de reparo em defeitos ósseos induzidos no corpo da mandíbula. Rev Odontol UNESP. 1999;28(1):63-72.
-
13Radde GR, Hinson A, Crenshaw D, Toth LA. Evaluation of anaesthetic regimens in guineapigs. Lab Anim.1996;30(3):220-7.
-
14Ribeiro CMB, Silva Júnior VA, Silva Neto JC, Vasconcelos BC do E. Estudo clínico e histopatológico da reação tecidual às suturas interna e externa dos fios monofilamentares de nylon e poliglecaprone 25 em ratos. Acta Cir Bras. 2005;20(4):284-91.
-
15Mastrantonio SDS, Ramalho LTDO. Resposta do Tecido Conjuntivo de Camundongos ao Poliuretano Vegetal de Óleo de Mamona. Rev Odontol UNESP. 2003;32(1):31-7.
-
16Ohara GH, Kojima KE, Rossi JC, Telles M, Soares TVC, Salomão C, et al. Estudo experimental da biocompatibilidade do polímero poliuretano da mamona implantada. Acta Ortop Bras. 1995;3(2):1-7.
-
17Carvalho T, Araújo C, Teófilo J, Brentegani L. Histologic and histometric evaluation of rat alveolar wound healing around polyurethane resin implants. Int J Oral Maxillofac Surg. 1997;26(2):149-52.
-
18Carlos J, Márcio C, Castro B. Poliuretana de mamona (Ricinus communis) para desvio da crista tibial no cão. Ciênc Rural. 2004;34(3):821-7.
-
19Silva MJA. Estudo radiográfico das imagens convencionais e digitalizadas do comportamento do tecido ósseo frente ao implante do polímero de mamonas em coelhos [Tese de mestrado]. Universidade de São Paulo; 1997. p.135.
-
20Dias PCDJ, Granato L, Pretel H. Avaliação histológica da biocompatibilidade do polímero da mamona no dorso nasal de macacos-pregos (Cebus apella). Braz J Otorhinolaryngol. 2009;75(3):350-5.
-
21Van Noort R. Titanium: The implant material of today. J Mater Sci. 1987;22(11):3801-11.
-
22Siqueira JRJ. Inflamação - Aspectos biodinâmicos das respostas inflamatória e imunologica. Rio de Janeiro: Editora Pedro Primeiro LTDA; 1996.
-
23Costa CA de S, Marcantonio RAC, Hebling J, Teixeira HM, Kuramae M. Biocompatibilidade do polímero de poliuretana vegetal derivada do óleo de mamona em estudo comparativo com cimento de óxido de zinco e eugenol: avaliação histopatológica de implantes subcutâneos de ratos. Odonto 2000.1997;1(1):44-8.
-
24Pereira PHL. Estudo das propriedades físico-químicas da poliuretana derivada do óleo de mamona com potencial aplicação na área médica. [Tese de mestrado].Universidade de São Paulo; 2010. p.75.
Publication Dates
-
Publication in this collection
Apr-Jun 2014
History
-
Received
11 Mar 2013 -
Accepted
13 Jan 2014