Abstract
Purpose:
To evaluate the effect of human amniotic membrane (hAM) fragment on inflammatory response, proliferation of fibroblast and organization of collagen fibers in injured tendon.
Methods:
Sixty rats were divided into 3 groups: C - surgical procedures without tendon lesion and with simulation of hAM application; I - surgical procedures, tendon injury and simulation of hAM application; T - surgical procedures, tendon injury and hAM application. These groups were subdivided into four experimental times (3, 7, 14 and 28 days). The samples underwent histological analysis and ATR-FTIR spectroscopy.
Results:
Histological analysis at 14 days, the T group showed collagen fibers with better alignment. At 28 days, the I group presented the characteristics described for the T group at 14 days, while this group presented aspects of a mature connective tissue. FT-IR analysis showed a clear distinction among the three groups at all experimental times and groups T and I presented more similarities to each other than to group C.
Conclusion:
Acute injury of tendon treated with human amniotic membrane fragment showed a faster healing process, reduction in inflammatory response, intense proliferation of fibroblasts and organization of collagen fibers.
Key words:
Achilles Tendon; Amnion; Inflammation; Collagen; Rats
Introduction
The largest tendon in the body, the Achilles tendon, is also one of the most affecteds by many pathological and/or traumatic injuries. These injuries are more common among athletes, but the incidence in the general population is growing as a result of the increase in physical activities. Overload of the tendon appears to be a risk factor for the development of tendon disorders, like acute tendonitis, especially in the lower extremities in athletes11. de Jonge S, van den Berg C, de Vos RJ, van der Heide HJ, Weir A, Verhaar JA, Bierma-Zeinstra SM, Tol JL. Incidence of midportion Achilles tendinopathy in the general population. Br J Sports Med. 2011 Oct;45(13):1026-8. doi: 10.1136/bjsports-2011-090342.
https://doi.org/10.1136/bjsports-2011-09...
. This condition is characterized by inflammatory signs and can occur by a mechanism that has not been clarified yet, and includes repetitive motion, chafing and pressure tendon overload22. Millar NL, Hueber AJ, Reilly JH, Xu Y, Fazzi UG, Murrell GA, McInnes IB. Inflammation is present in early human tendinopathy. Am J Sports Med. 2010 Oct;38(10):2085-91. doi: 10.1177/0363546510372613.
https://doi.org/10.1177/0363546510372613...
.
Treatment of these injuries continues to be a challenge for orthopedic surgeons in part due to nature of the native tendon tissue. Tendons consist of collagen (mostly type I collagen) and elastin embedded in a proteoglycan-water extracellular matrix (ECM) with collagen accounting for 65-80% and elastin approximately 1-2% of the dry mass of the tendon. These elements are produced by tenoblasts and tenocytes, which are the elongated fibroblasts and fibrocytes that lie between the collagen fibers33. Kannus, P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000 Dec;10(6):312-20. PMID: 11085557., aligned parallel to the longitudinal tendon axis and surrounded by a tendon sheath also consisting of extracellular matrix components. While the longitudinally linear organization of collagen fibers in the tendons results in optimal stiffness and strength at low strains under tensile load, this organization makes the repair process of the injured tendons extremely difficult, even under the best conditions. Additionally, due to hypocellularity and hypovascularity, the natural healing ability of tendons is extremely low and inefficient44. James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008 Jan;33(1):102-12. doi: 10.1016/j.jhsa.2007.09.007.
https://doi.org/10.1016/j.jhsa.2007.09.0...
.
In general, the healing or repair process of an injured tendon passes throughout three main phases containing distinctive cellular and molecular cascades. The initial inflammatory stage is characterized by inflammatory cells such as neutrophils, monocytes and macrophages are attracted to the injury site by pro-inflammatory cytokines. Fibroblasts and local tenocytes recruited to the site begin to synthesize various components of the extracellular matrix. Angiogenic factors are released during this phase and initiate the formation of a vascular network. In the proliferative stage, the continued recruitment of fibroblasts and their rapid proliferation at the wound site are responsible for the synthesis of collagens, proteoglycans, and other components of the ECM. These components are initially arranged in a random manner within the ECM, which at this point is composed largely of type III collagen. Finally, the tendon enters the remodeling phase, which is characterized by a decrease in cellularity, reduced matrix synthesis, decrease in type III collagen, and an increase in type I collagen that start to organize along the longitudinal axis of the tendon. However tendon repair is a slow process, and characterized by a scar with mechanically inferior tissue, at least initially. The lack of use or morbidity leads to extensive scarring and adhesions. Therefore, after the repair process injured tendons rarely have the same biological and mechanical properties as the original tissue44. James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008 Jan;33(1):102-12. doi: 10.1016/j.jhsa.2007.09.007.
https://doi.org/10.1016/j.jhsa.2007.09.0...
.
Considering the difficulty of repair process and the high incidence of tendinopathies, studies have been shown, both in animal and patients, many therapeutic approaches, including nitric oxides patches, anti-inflammatory drugs (steroids and non-steroids), eccentric exercises, ultrasound and laser55. Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008 Jul;466(7):1539-54. doi: 10.1007/s11999-008-0260-1.
https://doi.org/10.1007/s11999-008-0260-...
. However, none of these treatments are completely effective in tissue regeneration and pain reduction. Currently, the studies are investigating strategies for enhancing tendon repair by biological means, mainly of applying growth factors, stem cells, and biomaterials, alone or cell-loaded, at the site of tendon damage4. In line with this current strategies, the innermost layer of the of the fetal membranes, the human amniotic membrane (hAM), which is usually discarded after birth as part of placenta, has gained attention because of its ability to modulate adult wound healing by promoting epithelialization while suppressing inflammation and scarring. Additionally, hAM is a biomaterial that can be easily obtained, processed, transported, stored and minimal ethical concerns66. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue. Eur Cell Mater. 2008 Apr;15:88-99. PMID: 18446690..
Amniotic membrane (AM) is composed of three histologically distinct layers: the epithelial layer, the basement membrane and avascular mesenchymal/stromal layer. The epithelial layer comprises a flat, cuboidal and columnar cells uniformly disposed on the basement membrane, that consists mainly of collagen IV, elastin, fibronectin, laminin and proteoglycans. The mesenchymal layer in turn consists of three regions: an acellular compact layer, that forms the main fibrous skeleton of the AM composed of collagens I, III and fibronectin; a network of dispersed fibroblast-like mesenchymal cells; and a spongy layer of loosely arranged collagen fibers that separates the amniotic membrane from the chorion77. Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012 Aug;349(2):447-58. doi: 10.1007/s00441-012-1424-6.
https://doi.org/10.1007/s00441-012-1424-...
. Both cell types isolated from hAM, amniotic epitelial cells (AEC) and amniotic mesenchymal stromal cells (AMSC), express stem cell markers and have the ability to differentiate toward all three germ layers88. Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005 Nov-Dec;23(10):1549-59. doi: 10.1634/stemcells.2004-0357.
https://doi.org/10.1634/stemcells.2004-0...
.
Studies showed that AM have several properties that are beneficial for tissue repair and regeneration of various tissues99. Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007 Nov;105(3):215-28. PMID: 17986813., including anti-inflammatory action, protease inhibiting, scar formation-reducing, antibacterial and antipain actions, wound protecting and anti-adhesive effect1010. Manuelpillai U, Moodley Y, Borlongan CV, Parolini O. Amniotic membrane and amniotic cells: potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta. 2011 Oct;32 Suppl 4:S320-5. doi: 10.1016/j.placenta.2011.04.010.
https://doi.org/10.1016/j.placenta.2011....
. Another, well documented, property of the intact hAM is its ability to promote re-epithelialization. This membrane has been used as a basement membrane to promote epithelial cell migration, differentiation, and prevent epithelial cell apoptosis, and also produces factors that can stimulate epithelialization, such as bFGF, HGF, and TGFβ. The intact hAM has been show to produce an array of anti-angiogenic factors, that is important for corneal surface reconstruction, but also pro-angiogenic factors, which can be considered important contributors to its wound healing and regenerative capabilities1111. Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000 Mar;20(3):173-7. doi: 10.1076/0271-3683(200003)2031-9FT173.
https://doi.org/10.1076/0271-3683(200003...
. Furthermore, this biomaterial not induces rejection after transplantation because their cells do not express the histocompatibility antigens HLA, A, B, C or DR, and contain some immunomodulatory factors, causing minimal or no inflammatory response66. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue. Eur Cell Mater. 2008 Apr;15:88-99. PMID: 18446690.. In addition to these biological properties, others such as biocompatibility, permeability, highly flexibility, transparency, and stability make the MA a tissue with reasonable mechanical properties and, thus a potential scaffold the growth, cell adhesion and migration, characteristics required for tissue engineering66. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue. Eur Cell Mater. 2008 Apr;15:88-99. PMID: 18446690..
Pre-clinical studies have successfully used the amniotic epithelial cells (AECs)1212. Barboni B, Russo V, Curini V, Mauro A, Martelli A, Muttini A, Bernabò N, Valbonetti L, Marchisio M, Di Giacinto O, Berardinelli P, Mattioli M. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012 Apr;21(11):2377-95. doi: 10.3727/096368912X638892.
https://doi.org/10.3727/096368912X638892...
-1313. Muttini A, Mattioli M, Petrizzi L, Varasano V, Sciarrini C, Russo V, Mauro A, Cocciolone D, Turriani M, Barboni B. Experimental study on allografts of amniotic epithelial cells in calcaneal tendon lesions of sheep. Vet Res Commun. 2010 Jun;34 Suppl 1:S117-20. doi: 10.1007/s11259-010-9396-z.
https://doi.org/10.1007/s11259-010-9396-...
to accelerate the repair process in tendinopathies. However, cell culture can result in a loss of some of its own cellular factors, such as cytokines as well as growth and differentiation factors, which are present in the AM microenvironment. The results obtained in studies that utilized intact amniotic membrane, amniotic-derived cells, or their conditioned medium, showed that intact hAM seems to primarily function as a matrix and as a source of bioactive soluble factors, rather than by virtue of its functional cells1010. Manuelpillai U, Moodley Y, Borlongan CV, Parolini O. Amniotic membrane and amniotic cells: potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta. 2011 Oct;32 Suppl 4:S320-5. doi: 10.1016/j.placenta.2011.04.010.
https://doi.org/10.1016/j.placenta.2011....
. In addition harvesting hAM is a simple procedure and does not require special experience or equipment and it is readily available, easily stored and inexpensive66. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue. Eur Cell Mater. 2008 Apr;15:88-99. PMID: 18446690.. Considering these arguments, we used in the present study, the intact hAM fragment, maintaining the microenvironment of this biomaterial essential for the tissue repair process. Then, we could hypothesize that amniotic membrane patches may be useful to accelerate the healing process in the rat Achilles tendon.
Methods
The research was approved by Research Ethics Committee (350.427) and Animal Ethics Committee (A03/CEAU/2013).
Sixty rats (Rattus norvegicus albinus-Wistar, male, 60 days, 200 ± 20g) were individually housed in polypropylene plastic cages with feed and water ad libitum. The animals were maintained in a controlled temperature environment (20 ± 2°C) and a light cycle of 12:12 h light: dark. The rats were divided into three groups of control (C), injured (I) and treated (T), and then subdivided according to the experimental periods of 3, 7, 14 and 28 days (n=5 in each group). Group C: rats subjected to surgical procedures without tendon lesion and hAM application simulation; Group I: animals subjected to surgical procedures, including tendon injury, and hAM application simulation; and Group T: animals subjected to surgical procedures, including tendon injury, and hAM application. The tendon injury was induced by direct trauma in animals of groups I and T using a mini guillotine (Physiology Laboratory at the University of Paraíba Valley), according to the protocol used by Neves et al.1414. Neves MA, Pinfildi CE, Wood VT, Gobbato RC, Silva FM, Parizotto NA, Hochman B, Ferreira LM. Different power settings of LLLT on the repair of the calcaneal tendon. Photomed Laser Surg. 2011 Oct;29(10):663-8. doi: 10.1089/pho.2010.2919.
https://doi.org/10.1089/pho.2010.2919...
.
The sample size of 5 subjects per group was validated by calculating the power (beta), obtaining values of 0.86 for the inflammatory cell counts and 0.99 for fibroblast count. Considering that a power above 0.8 is suitable, we conclude that our experiment has statistical validity.
The placentas were harvested at the Municipal Hospital of São José dos Campos after signing the informed consent form by the parturient. For experimental procedures, the AM fragments from three human placentas (hAM), were used following the inclusion criteria described by Sant'Anna et al.1515. Sant'Anna LB, Cargnoni A, Ressel L, Vanosi G, Parolini O. Amniotic membrane application reduces liver fibrosis in a bile duct ligation rat model. Cell Transplant . 2011 Aug;20(3):441-53. doi: 10.3727/096368910X522252.
https://doi.org/10.3727/096368910X522252...
: a) placentas obtained from cesareans; b) gestational age equal or superior to 37 weeks (related to the degree of maturity of the placenta); c) healthy medical history of the mother; d) negative serological tests for syphilis, HIV-1 and Hepatitis B and C.
Immediately after cesareans, the placenta was inspected by the doctor responsible for the surgery, and instead of being discarded, it was placed in a sterile plastic bag under refrigeration at 10-15°C until the time of collection by the researcher (maximum 12 hours after birth). The placenta was transported (from hospital to the laboratory) according to standardized rules cited in previous studies1515. Sant'Anna LB, Cargnoni A, Ressel L, Vanosi G, Parolini O. Amniotic membrane application reduces liver fibrosis in a bile duct ligation rat model. Cell Transplant . 2011 Aug;20(3):441-53. doi: 10.3727/096368910X522252.
https://doi.org/10.3727/096368910X522252...
-1616. Cargnoni A, Di Marcello M, Campagnol M, Nassuato C, Albertini A, Parolini O. Amniotic membrane patching promotes ischemic rat heart repair. Cell Transplant . 2009 Jun;18(10):1147-59. doi: 10.3727/096368909X12483162196764.
https://doi.org/10.3727/096368909X124831...
. The biomaterial was accommodated in an insulated box with temperature control (10-15°C) in the private car of the researcher. All procedures performed for processing the hAM were done under sterile conditions at the laboratory in a laminar flow hood.
Following the proceeding described by Sant'Anna et al.1515. Sant'Anna LB, Cargnoni A, Ressel L, Vanosi G, Parolini O. Amniotic membrane application reduces liver fibrosis in a bile duct ligation rat model. Cell Transplant . 2011 Aug;20(3):441-53. doi: 10.3727/096368910X522252.
https://doi.org/10.3727/096368910X522252...
, preparation of the biomaterial included manual separation of the hAM from the chorionic membrane and washed with phosphate buffered saline (PBS) (Sigma, St. Louis, MO, USA) containing 100 U/ml penicillin, 100 μg/ml streptomycin and amphotericin (Lonza, Basel, Switzerland). Using a scalpel, the hAM was then cut into adequately sized pieces (1 cm2), being larger than the injured area. The mesenchymal side of the hAM was flagged with a cut at the top right of each fragment to facilitate identification of the correct side (mesenchymal). The fragments were then stored separately at room temperature, as reported by Hennerbichler et al.1717. Hennerbichler S, Reichl B, Pleiner D, Gabriel C, Eibl J, Redl H. The influence of various storage conditions on cell viability in amniotic membrane. Cell Tissue Bank. 2007 Mar;8(1):1-8. doi: 10.1007/s10561-006-9002-3.
https://doi.org/10.1007/s10561-006-9002-...
, in 50 ml vials filled with serum-free and phenol red-free DMEM in sterile conditions until application. The hAM fragments were used within 24 h.
For experimental procedures, the animals were anesthetized with a combination of xylazine hydrochloride (0.01 ml/kg) and ketamine hydrochloride (0.005 ml/kg) administered via intramuscular route. Animals from groups I and T were submitted to tendon injury induction by a non-surgical method according previous studies1818. Oliveira FS, Pinfildi CE, Parizoto NA, Liebano RE, Bossini PS, Garcia EB, Ferreira LM. Effect of low level laser therapy (830 nm) with different therapy regimes on the process of tissue repair in partial lesion calcaneus tendon. Lasers Surg Med. 2009 Apr;41(4):271-6. doi: 10.1002/lsm.20760.
https://doi.org/10.1002/lsm.20760...
. The right leg of the animal was positioned on the base of the mini guillotine, keeping the ankle in dorsiflexion. A weight of 20 g was released from a fixed height, over the flexed leg of the rat, causing a transverse crush on the tissue fibers relative to the long axis of the tendon. The weight was removed immediately after lesion.
The surgical procedure began with a longitudinal incision at the posterior lower portion of the right hindpaw of the rat. In the specimens of group C (SHAM), the Achilles tendon was exposed and handled to simulate hAM application, and the skin tissues were repositioned and sutured. After the induced tendon injury with the mini guillotine, the group I rats were subjected to the same surgical procedure described for group C. Immediately after the tendon injury, rats in group T also underwent the previously described surgical procedures, followed by circumferentially applying an hAM fragment (1 cm2) to the tendon at the injured region. For this procedure, intense manipulation of the tendon was necessary to separate it from surrounding tissues, generating adequate space for hAM application. The rats in groups C and I also were submitted to hAM application simulation, considering that the manipulation of the tendon for hAM application might influence the results.
It is important to highlight that hAM was applied to the mesenchymal face in contact with the injured tissue, in consideration of previous studies that reported better results when this protocol was followed1919. Niknejad H, Yazdanpanah G. Opposing effect of amniotic membrane on angiogenesis originating from amniotic epithelial cells. J Med Hypotheses Ideas. 2014 Jan;8:39-41. doi: 10.1016/j.jmhi.2013.08.002.
https://doi.org/10.1016/j.jmhi.2013.08.0...
. For security, the extremities of the membrane were only connected with a drop of methacrylate glue (Loctite®) in order to keep the hAM fragment around the region of interest.
After surgery, animals from all groups received the following treatments: antibiotic - Amoxicillin (0.001 ml/kg, intramuscular, single dose); anti-inflammatory - Flunixin meglumine (0.001 ml/kg subcutaneously at 24-h intervals for 3 days); and analgesic - Metamizole (0.001 mg/kg orally for 3 days).
The rats were euthanatized with an overdose of the same anesthetic at the end of each experimental period (3, 7, 14 and 28 days). Two incisions were performed on the right paw: one in the muscular junction and the other in the tendon insertion, allowing for careful removal of the Achilles tendon. Each tendon was hemi-sectioned in its long axis, so that injury was observed in both fragments. The first fragment was fixed in 10% neutral buffered formalin (Synth, Diadema-SP, Brazil) for 48 hours at room temperature. The second fragment was stored in a cryogenic tube (1.2 ml) in a freezer (-80°C).
Samples in formalin formaldehyde underwent routine histological processing and embedded in paraffin (Paraplast, Oxford, St. Louis, MO, USA). Five micron thick sections were longitudinally cut in the direction of the tendon fibers and were stained with hematoxylin-eosin (HE) for histological and quantitative analysis by light microscopy using a Leica DM 2500 microscope coupled to a Leica DFC 425 camera and the Leica Application Suite LAS v3.7 program.
Images were captured and digitized at 1024x768 pixels with 24 bit/pixel and magnification of 20x. Digital images were analyzed with ImageJ software to quantify the number of inflammatory cells and fibroblasts, determined according to their histologic appearance observed during histological analysis. The program allowed the elaboration of a square grid and the intersection points between lines were considered. Only cells that were at the grid intersection were counted. Cell nuclei were individually marked using the manual counting tool (cell counter) in the program in the region of interest.
Data from the histomorphometric analysis were statistically analyzed by comparison tests using Minitab 17 software. We applied the normal Kolmogorov-Smirnov two-way ANOVA test for presenting two distinct factors: more than one experimental time (3, 7, 14 and 28 days) and different experimental protocols (Control/Injury/Treated) with a significance level of 0.05. Next, we separately studied variable dependence by applying the one-way ANOVA test with post Tukey test (significance level of 0.05).
Organization of the collagen fibers was used to generate a score in order to evaluate the alignment of these fibers in the different groups and experimental times based on the score system used by Barboni et al.1212. Barboni B, Russo V, Curini V, Mauro A, Martelli A, Muttini A, Bernabò N, Valbonetti L, Marchisio M, Di Giacinto O, Berardinelli P, Mattioli M. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012 Apr;21(11):2377-95. doi: 10.3727/096368912X638892.
https://doi.org/10.3727/096368912X638892...
. The analysis was made on the injured tendon area with an increase of x20, totaling 5 samples per group of all experimental times studied. Thus, three different scores were adopted to indicate:
-
Disorganized collagen fibers;
-
Collagen fibers started to acquire a parallel orientation;
-
Parallel fibers, oriented along the longitudinal axis of the healthy portion of the tendon.
Regarding the ATR-FTIR-Spectroscopy measurements, the samples stored in a freezer (-80°C) were defrosted and dried using a 5301 Eppendorf concentrator, which has advantages such as faster sampling with no preparation and thicker samples2020. Oberle J, Dighton J, Arbuckle-Keil G. Comparison of methodologies for separation of fungal isolates using Fourier transform infrared (FTIR) spectroscopy and Fourier transform infrared-attenuated total reflectance (FTIR-ATR) microspectroscopy. Fungal Biol. 2015 Nov;119(11):1100-14. doi: 10.1016/j.funbio.2015.08.007.
https://doi.org/10.1016/j.funbio.2015.08...
. The samples were analyzed on a Diamond ATR Module coupled to a Perkin Elmer Spectrum 400 FTIR-spectrometer. The absorption spectrum of the measured samples was in the range of 4,000-900 cm-1 with 64 scans and 4 cm-1 of spectral resolution. This technique indicates the vibrations of molecular radical in the medium infrared range. These molecular vibrations (e.g. bending, stretching, scissoring, etc.) are unique to the type and spatial arrangement of the atoms in a sample2020. Oberle J, Dighton J, Arbuckle-Keil G. Comparison of methodologies for separation of fungal isolates using Fourier transform infrared (FTIR) spectroscopy and Fourier transform infrared-attenuated total reflectance (FTIR-ATR) microspectroscopy. Fungal Biol. 2015 Nov;119(11):1100-14. doi: 10.1016/j.funbio.2015.08.007.
https://doi.org/10.1016/j.funbio.2015.08...
.
The spectra were inserted into OPUS software version 4.2, for cluster analysis with the following parameters: second derivative, smoothing 9, Ward's algorithm and scaling to 1st range. Cluster analysis (CA) was used for conducting statistical analysis, which consists in analyzing the data of the sample spectra through its particularities. CA is the classification of objects into groups, shown by an image called a dendrogram, which presents the obtained data through branches. A cluster is a collection of objects that are similar to each other according to some preset similarity criteria, and of different objects which belong to other clusters2121. Rousseeuw PJ. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Computational Appl Mathematics. 1987 Nov; 20:53-65. doi: 10.1016/0377-0427(87)90125-7.
https://doi.org/10.1016/0377-0427(87)901...
.
Results
Histological and quantitative analysis
3 days
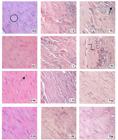
Analysis of histological sections showed an inflammatory infiltration in all groups, and the specimens of groups I and T also presented fragmented and disorganized collagen fibers in the region where the tendon injury was induced (Table 1). The granulation tissue (Figure 1) observed in all specimens presented inflammatory cells (neutrophils with multilobulated nucleus and condensed chromatin), macrophages (abundant cytoplasm and large nuclei) and fibroblasts (ovoid shape, large nucleus centrally located and high synthesis activity).
Histologic aspects of the repair process of the Achilles tendon at the experimental times of 3, 7, 14 and 28 days, including inflammatory response and collagen fibers in the region of tendon injury in the following groups C (SHAM), I (Injuried) and T (Injured+AMh) (HE, 20x). Collagen fibers (
 ); Inflammatory cell (
); Inflammatory cell ( ); Fibrocyte (
); Fibrocyte ( ); Activated fibroblasts ( ).
); Activated fibroblasts ( ).
There was a significant increase in inflammatory cells in groups I and T compared to the control group (p<0.01). However, there was no significant difference between I and T groups (Figure 2). Furthermore, histological analysis of the injured region in group T showed the beginning of interaction between the hAM fragment and the injured tendon (Figure 3A).
Mean and standard deviation of inflammatory cell count in the experimental groups at the experimental times of 3, 7, 14 and 28 days. The statistical significance values are indicated by ** (p <0.01) and ns (not significant).
Histologic aspects of the AMh interface with the tendon of group T (Injured+AMh) at experimental times of 3 (A), 7 (B), 14 (C) and 28 (D) days after injury induction (HE, x20).
7 days
A small number of inflammatory cells was observed in the histological sections of group C, while the specimens of groups I and T showed intense inflammatory infiltrate with the presence of numerous macrophages. There were also a great number of fibroblasts and thin and non-aligned neoformed collagen fibers in group I, while group T showed the beginning of the alignment of collagen fibers along the longitudinal axis of the tendon (Table 1). Histomorphometric analysis revealed that the increase of inflammatory cells in groups I and T was statistically significant (p<0.01) compared to group C (Figure 2).
Spatial distribution of the inflammatory infiltrate presented distinct patterns in the specimens of groups I and T. In group T, the inflammatory cells were at the central region of the lesion, while the inflammatory cells in group I were scattered all over the wounded area. The presence of hAM fragments around the tendon in histological sections of group T is noteworthy. The interface region between the hAM and the tendon showed interaction with the biomaterial (hAM), with a high number of fibroblasts and the presence of several immature collagen fibrils (Figure 3B).
14 days
All groups showed a reduction of inflammatory cells when compared to the previous experimental time (Figure 1). The increase in fibroblast cells and the presence of immature collagen fibers in groups I and T were visible. Collagen fibers were more organized in rats treated with hAM (T) (Table 1). Furthermore, small fragments of hAM were still visible (Figure 3C) in group T. Specimens of group I still exhibited several inflammatory cells in the central region of the lesion, similar to the pattern already presented by group T at 7 days. The inflammatory response showed statistically significance differences (Figure 2) between group C and I (p<0.05), and between groups I and T (p<0.01).
28 days
At 28 days, the histological sections of group T presented better alignment and organization of collagen fibers (Table 1). It was possible to observe a large number of fibrocytes, evidentiated by its elongated core and dark nuclei, a small number of fibroblasts (Figure 1), aspects that characterize mature connective tissue. Human AM fragments were no longer visible, indicating the degradation of this biomaterial by the organism. Collagen fibers showed better organization and orientation at the interface hAM/Tendon region (Figure 3D).
The inflammatory cells decreased in all experimental groups (Figure 2), but the difference was statistically significant only between groups C and I (p<0.01). Regarding fibroblasts, these cells increased in I group at 28 days while their number remained stable in T group. Data analysis showed significant difference (p<0.01) in the number of fibroblasts between all groups (Figure 4). Table 2 presents the statistically significant values of intra-group changes observed in inflammatory cells and fibroblasts at different experimental times.
Mean and standard deviation of fibroblasts count in the experimental groups at experimental times of 3, 7, 14 and 28 days. The statistical significance values are indicated by ** (p <0.01) and ns (not significant).
Cluster analysis of the data obtained by FTIR- ART spectroscopy showed that the best results were obtained using the spectral ranges of 1213 cm-1 - 1263 cm1 and 1421 cm-1 - 1477 cm-1. According to the literature, the major biochemical attributes of the sample are present in these spectral ranges. The bands in the spectral region of 1480-1355 cm-1 are assigned to (C-H) of CH2 and CH3, which represent the proteins and carbohydrates, and the bands in the spectral region of 1274-1189 cm-1 are assigned to the amide III region2222. Maquelin K, Kirschner C, Choo-Smith LP, Braak N, Endtz HP, Naumann D, Puppels GJ. Identification of medically relevant microorganisms by vibrational spectroscopy. J Microbiol Methods. 2002 Nov;51(3):255-71. PMID: 12223286.-2323. Belbachir K, Noreen R, Gouspillou G, Petibois C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal Bioanal Chem. 2009 Oct;395(3):829-37. doi: 10.1007/s00216-009-3019-y.
https://doi.org/10.1007/s00216-009-3019-...
.
The dendrogram generated by the FT-IR analysis in the experimental time of 3 days showed a clear distinction among the three groups. However, although the biochemical composition presented similarities between all groups, T and I groups were closer to each other compared to group C. The dendrograms from all groups remained separate at 7 and 14 days, but the distance between groups I and T increased. The groups continued to be separated at 28 days, although the dendrograms of groups T and I were closer to each other (Figure 5).
Dendrogram generated by cluster analysis of the groups C, I and T spectra as a function of the experimental time.
Discussion
Considering that several studies showed the AM properties in the repair process and the increase incidence of tendinopathy in the general population, the present study aimed to evaluate the effectiveness of this biomaterial on the inflammatory response and organization of collagen fibers at different stages of the acute injury healing process in the Achilles tendon. The results showed that the inflammatory response in groups T and I was similar in the beginning of the healing process regarding the number of inflammatory cells. However, at 14 days, in the T group specimens the number of inflammatory cells showed significant reduction and the newly formed collagen fibers already showed early organization and alignment when compared to group I.
In experimental studies on animal tendons, partial or total surgical tenotomy is the most commonly used method for inducing injury2424. Joensen J, Gjerdet NR, Hummelsund S, Iversen V, Lopes-Martins RAB, Bjordal JM. An experimental study of low-level laser therapy in rat Achilles tendon injury. Lasers Med Sci. 2012 Jan;27(1):103-11. doi: 10.1007/s10103-011-0925.
https://doi.org/10.1007/s10103-011-0925...
. Tallon et al.2525. Tallon C, Maffulli N, & Ewen SWB. Ruptured Achilles tendons are significantly more degenerated than tendinopathic tendons. Med Sci Sports Exerc. 2001 Dec;33(12):1983-90. PMID: 11740288. showed that the most prominent features detected in the samples from ruptured Achilles tendons were a remarkable degeneration and disorganization of the collagen fibers, increased cellularity and fibrocytes with rounding of nuclei. Similar results were observed in areas of degeneration in tendons with tendinopathy.
In this study, we chose to use a mini guillotine to induce injury in the calcaneal tendon in attempt to mimic a tendon disorder with inflammatory components and disorganized collagen fibers, considering previous studies in rodents1818. Oliveira FS, Pinfildi CE, Parizoto NA, Liebano RE, Bossini PS, Garcia EB, Ferreira LM. Effect of low level laser therapy (830 nm) with different therapy regimes on the process of tissue repair in partial lesion calcaneus tendon. Lasers Surg Med. 2009 Apr;41(4):271-6. doi: 10.1002/lsm.20760.
https://doi.org/10.1002/lsm.20760...
. According to Joensen et al.2424. Joensen J, Gjerdet NR, Hummelsund S, Iversen V, Lopes-Martins RAB, Bjordal JM. An experimental study of low-level laser therapy in rat Achilles tendon injury. Lasers Med Sci. 2012 Jan;27(1):103-11. doi: 10.1007/s10103-011-0925.
https://doi.org/10.1007/s10103-011-0925...
, significant increase in the thickness of injured tendons justifies the appropriateness of this methodology. They also state that this experimental model appears useful for investigating the effect of therapies in acute tendon injuries. This model has the advantage of being easier to use than overuse models such as the treadmill running model of Soslowsky et al.2626. Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996 Sep-Oct;5(5):383-92. PMID: 8933461..
Experimental evidences have shown the great potential of cells and AM fragments in the regeneration process of various tissues. Human amniotic membrane can be an alternative for accelerate the tendon repair, mainly for its anti-inflammatory, pró-angiogenic, antiscaring properties and capacity for preventing peritendinous adhesions99. Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007 Nov;105(3):215-28. PMID: 17986813.-1010. Manuelpillai U, Moodley Y, Borlongan CV, Parolini O. Amniotic membrane and amniotic cells: potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta. 2011 Oct;32 Suppl 4:S320-5. doi: 10.1016/j.placenta.2011.04.010.
https://doi.org/10.1016/j.placenta.2011....
.
The results obtained in the present study, regarding to rats of group C, demonstrated that the tendon was characterized by a dense and regular connective tissue composed of bundles of collagen fibers aligned parallel to the longitudinal tendon axis and elongated nuclei of fibrocytes between them, typical histological characteristics of the normal tendon33. Kannus, P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000 Dec;10(6):312-20. PMID: 11085557.-44. James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008 Jan;33(1):102-12. doi: 10.1016/j.jhsa.2007.09.007.
https://doi.org/10.1016/j.jhsa.2007.09.0...
. However, in this group, was also observed the presence of few inflammatory cells, which decreased over time becoming significantly reduced after 28 days. This finding is probably related to the simulation of the hAM application around the tendon, considering that it was necessary to separate the tendon from adjacent tissues. Furthermore, the methodology used in this study to induce injury was validated by the intense neutrophilic inflammatory infiltrate and by the disruption of the pre-existing collagen fibers observed in groups I and T at 3 days, confirming that the injury was effectively induced. Similar results for the inflammatory response observed in groups I and T at 3 days indicate that application of the hAM fragment did not induce an increase in the inflammatory response in the lesion area, which confirms the low immunogenicity of hAM reported in several studies99. Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007 Nov;105(3):215-28. PMID: 17986813..
During tendon repair process, after the inflammatory phase, the proliferative stage takes place accompanied by the synthesis of abundant ECM components which are arranged in a random manner, and an increased in cellularity44. James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008 Jan;33(1):102-12. doi: 10.1016/j.jhsa.2007.09.007.
https://doi.org/10.1016/j.jhsa.2007.09.0...
.
Barboni et al.1212. Barboni B, Russo V, Curini V, Mauro A, Martelli A, Muttini A, Bernabò N, Valbonetti L, Marchisio M, Di Giacinto O, Berardinelli P, Mattioli M. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012 Apr;21(11):2377-95. doi: 10.3727/096368912X638892.
https://doi.org/10.3727/096368912X638892...
investigated the efficiency of AECs allotransplantation for tendon healing, in lesions of the Achilles tendon in sheep, and showed similar results. Particularly, they observed that AECs induced a centripetal repair process. In the treated tendons, the cellularity distribution gradient presented higher values in the central area of the defect and lower values in the periphery. However, increased cellularity was more generalized in the control injured group. These results are in agreement with the histological analysis described in the present study up to the experiment time of 7 days. Human AM fragment applied around the injured region possibly generated a stimulus for migration, and cell proliferation circumscribed to that region. In addition, Barboni et al.1212. Barboni B, Russo V, Curini V, Mauro A, Martelli A, Muttini A, Bernabò N, Valbonetti L, Marchisio M, Di Giacinto O, Berardinelli P, Mattioli M. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012 Apr;21(11):2377-95. doi: 10.3727/096368912X638892.
https://doi.org/10.3727/096368912X638892...
explain that the beneficial effects of cells derived from AM on healing process can be related to the stimulus generated by growth factors (TGF-β1 and VEGF) and/or the recruitment of progenitor cells of the host, and can also be a consequence of AECs’ differentiation, resulting in tendon regeneration.
In our study, during the proliferative phase (7 to 14 days), the number of fibroblasts increased in all groups. However, histomorphometric analysis showed a high increase of fibroblast cells in group T, probably related to the presence of various growth factors in the epithelium of the membrane, such as epidermal growth factor (EGF), transforming growth factor (TGF), β1, β2, β3, keratinocyte growth factor (KGF), basic fibroblast growth factor (b-FGF) and hepatocyte growth factor (HGF), which act in facilitating cell migration2727. Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004 Jan-Feb;49(1):51-77. PMID: 14711440.. This is a very important finding, considering that the recruitment of fibroblasts to the wound bed is essential for wound healing44. James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008 Jan;33(1):102-12. doi: 10.1016/j.jhsa.2007.09.007.
https://doi.org/10.1016/j.jhsa.2007.09.0...
.
Previous studies reported better results by applying hAM mesenchymal face in contact with the injured tissue1919. Niknejad H, Yazdanpanah G. Opposing effect of amniotic membrane on angiogenesis originating from amniotic epithelial cells. J Med Hypotheses Ideas. 2014 Jan;8:39-41. doi: 10.1016/j.jmhi.2013.08.002.
https://doi.org/10.1016/j.jmhi.2013.08.0...
. Niknejad et al.1919. Niknejad H, Yazdanpanah G. Opposing effect of amniotic membrane on angiogenesis originating from amniotic epithelial cells. J Med Hypotheses Ideas. 2014 Jan;8:39-41. doi: 10.1016/j.jmhi.2013.08.002.
https://doi.org/10.1016/j.jmhi.2013.08.0...
reported that hAM has both angiogenic and anti-angiogenic factors, and that the potential of the angiogenic effect is dependent on the side of the amniotic membrane. These authors assert that the mesenchymal side is responsible for an increase in angiogenesis. Since angiogenesis is important for the survival of the newly forming fibrous tissue at the injury site44. James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008 Jan;33(1):102-12. doi: 10.1016/j.jhsa.2007.09.007.
https://doi.org/10.1016/j.jhsa.2007.09.0...
, we can speculate that one possible mechanism which could account for the action of AM in favoring the evolution of tissue repair, in our study, might be its pro-angiogenic property. However, this supposition requires a further investigation.
There was sharp decrease in the number of inflammatory cells at 14 days in group T, while this decrease was slower in group I, similar to the results observed by Muttini et al.1313. Muttini A, Mattioli M, Petrizzi L, Varasano V, Sciarrini C, Russo V, Mauro A, Cocciolone D, Turriani M, Barboni B. Experimental study on allografts of amniotic epithelial cells in calcaneal tendon lesions of sheep. Vet Res Commun. 2010 Jun;34 Suppl 1:S117-20. doi: 10.1007/s11259-010-9396-z.
https://doi.org/10.1007/s11259-010-9396-...
. These findings may be related to the anti-inflammatory properties of hAM cited in the study of Manuelpillai et al.1010. Manuelpillai U, Moodley Y, Borlongan CV, Parolini O. Amniotic membrane and amniotic cells: potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta. 2011 Oct;32 Suppl 4:S320-5. doi: 10.1016/j.placenta.2011.04.010.
https://doi.org/10.1016/j.placenta.2011....
. These studies have associated this property to the presence of various factors in hAM stroma, such as interleukin-10 (IL-10), interleukin-1 receptor antagonist (IL-1), hyaluronic acid and prostaglandin E2 (PGE2). Tseng et al.2828. Tseng SC, Espana EM, Kawakita T, Di Pascuale MA, Li W, He H, Liu TS, Cho TH, Gao YY, Yeh LK, Liu CY. How does amniotic membrane work? Ocul Surf. 2004 Jul;2(3):177-87. PMID: 17216089., related that the anti-inflammatory action of hAM requires a close contact with its stromal matrix. Thus, in our study, reduced number of inflammatory cells in group T supports the anti-inflammatory effect of intact hAM as used as a patch.
Similar to our study, others have demonstrated the great potential of using hAM fragments on the treatment of other tissue injuries. Cargnoni et al.1616. Cargnoni A, Di Marcello M, Campagnol M, Nassuato C, Albertini A, Parolini O. Amniotic membrane patching promotes ischemic rat heart repair. Cell Transplant . 2009 Jun;18(10):1147-59. doi: 10.3727/096368909X12483162196764.
https://doi.org/10.3727/096368909X124831...
applied a hAM fragment to reduce the induced necrosis in rat hearts and Sant'Anna et al.1515. Sant'Anna LB, Cargnoni A, Ressel L, Vanosi G, Parolini O. Amniotic membrane application reduces liver fibrosis in a bile duct ligation rat model. Cell Transplant . 2011 Aug;20(3):441-53. doi: 10.3727/096368910X522252.
https://doi.org/10.3727/096368910X522252...
demonstrated that hAM application resulted in reduced liver fibrosis induced by bile duct ligation when it was applied around the rat liver. Furthermore, these studies related that hAM patching preserves the micro-environment, with all the growth factors and differentiation factors present, providing action of these chemical mediators in the injured tissue repair process.
Reduction of the inflammatory process at an early stage of tendon repair facilitates the organization and the increase in the diameter of collagen fiber, as reported by Alaseirlis et al.2929. Alaseirlis DA, Li Y, Cilli F, Fu FH, Wang JHC. Decreasing inflammatory response of injured patellar tendons results in increased collagen fibril diameters. Connect Tissue Res. 2005 Aug;46(1):12-7. doi: 10.1080/03008200590935501.
https://doi.org/10.1080/0300820059093550...
. Thus the decrease in the inflammatory cells, observed in our study, may have contributed to the alignment and the beginning of collagen fiber organization in specimens of group T at 14 days. As days passed, the collagen fibers of this group showed a more organized arrangement, indicating the "maturity" of the newly formed tendon tissue, agreeing with the findings of Barboni et al.1212. Barboni B, Russo V, Curini V, Mauro A, Martelli A, Muttini A, Bernabò N, Valbonetti L, Marchisio M, Di Giacinto O, Berardinelli P, Mattioli M. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012 Apr;21(11):2377-95. doi: 10.3727/096368912X638892.
https://doi.org/10.3727/096368912X638892...
. These authors reported that immunohistochemical and biochemical tests showed that the extracellular matrix remodeling was faster in the group treated with AECs.
These results show that the repair process phases seem to have evolved more quickly in group T, being one of the aims of this research into new alternatives for the treatment of tendinopathy. Additionally, the presence of collagen-synthesizing fibroblasts at the hAM/injured tissue region, especially at 14 days, confirms biomaterial interacting with tendon tissue. Our results confirm that, despite hAM being a xenogeneic biomaterial (meaning from different species), she presented biocompatibility and its application stimulated and accelerated the healing process of the injured tissue.
In order to understand the changes on biochemical composition of the tissue, Fourier Transform Infrared (FT-IR) Spectroscopy was applied to obtain the biochemical information of the sample such as proteins, fatty acids, carbohydrates, nucleic acids and lipopolysaccharides. FT-IR has been studied as an alternative technique, which is based on interaction between the infrared beam (in the medium infrared range) with the tissue sample; the energy absorption of molecular radicals are associated with molecular vibrations that are dependent of molecule chains of the tissue structure. Thus, the result of this measurement is an absorption spectrum that allows for determining these biochemical changes2020. Oberle J, Dighton J, Arbuckle-Keil G. Comparison of methodologies for separation of fungal isolates using Fourier transform infrared (FTIR) spectroscopy and Fourier transform infrared-attenuated total reflectance (FTIR-ATR) microspectroscopy. Fungal Biol. 2015 Nov;119(11):1100-14. doi: 10.1016/j.funbio.2015.08.007.
https://doi.org/10.1016/j.funbio.2015.08...
.
Cluster analysis showed that the three groups were separated into different branches, confirming the histological results. The presence of hAM can justify the separation between the T and I groups, and the tendon injury may explain the separation of both from the control group. Considering the characteristics of the samples analyzed and the IR range, modification of collagen fibers can be associated to changes on pyrrolidine rings of proline and hydroxyproline through CH bonds at 1450 cm-11. de Jonge S, van den Berg C, de Vos RJ, van der Heide HJ, Weir A, Verhaar JA, Bierma-Zeinstra SM, Tol JL. Incidence of midportion Achilles tendinopathy in the general population. Br J Sports Med. 2011 Oct;45(13):1026-8. doi: 10.1136/bjsports-2011-090342.
https://doi.org/10.1136/bjsports-2011-09...
2323. Belbachir K, Noreen R, Gouspillou G, Petibois C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal Bioanal Chem. 2009 Oct;395(3):829-37. doi: 10.1007/s00216-009-3019-y.
https://doi.org/10.1007/s00216-009-3019-...
.
Dendrograms generated by cluster analysis at the 3-day experimental time showed groups I and T to be closer when compared to group C. This indicates that the spectra of these groups (which are in the same branch) have greater spectral similarity; in other words, the chemical compositions of different samples have relatively low differences. The spectra of group T distanced themselves from the other groups at 7 and 14 days. Group I approached group T only after 28 days, but it still remained in distinct clusters. We suggest that the correlation between the cluster analysis and the histological findings is related to collagen formation at the hAM/injured tendon interface region observed in group T at 7 days. Histological sections of this group showed the formation of thin and new immature collagen fibers, which were probably produced by the stimulus generated by hAM.
At 28 days, during the remodeling phase, the collagen fibers in group I began to align in parallel, but these were less aligned and organized than in group T, which had already acquired these aspects at 14 days. This tissue maturity refers to a larger number of collagen fibers aligned in a single direction and a significantly smaller number of fibroblast nuclei, when compared with group I. These histological results would justify the rapprochement of clusters observed between these two groups, in FT-IR analysis.
Similar to our result, Barboni et al.1212. Barboni B, Russo V, Curini V, Mauro A, Martelli A, Muttini A, Bernabò N, Valbonetti L, Marchisio M, Di Giacinto O, Berardinelli P, Mattioli M. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012 Apr;21(11):2377-95. doi: 10.3727/096368912X638892.
https://doi.org/10.3727/096368912X638892...
observed, in Achilles tendon, that immature collagen fibers were completely replaced by mature ones in 28 days. And, stated that the increase in maturity of tendon was due to the stimulus induced by cells derivate from amniotic membrane, which provide a positive paracrine environment, thus accelerating the recovery of normal tendon microstructure and biomechanical properties.
It is important to notice that the use of amniotic membrane as a treatment for Achilles tendon injury offers the evident advantages of being in plentiful supply and being immediately applicable without the need for any cell isolation, selection, or culture steps, which makes it a low-cost approach. In addition, amniotic membrane has good preservability1717. Hennerbichler S, Reichl B, Pleiner D, Gabriel C, Eibl J, Redl H. The influence of various storage conditions on cell viability in amniotic membrane. Cell Tissue Bank. 2007 Mar;8(1):1-8. doi: 10.1007/s10561-006-9002-3.
https://doi.org/10.1007/s10561-006-9002-...
and is immunologically tolerated in both allogeneic and xenogeneic conditions, both in vitro and in vivo66. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue. Eur Cell Mater. 2008 Apr;15:88-99. PMID: 18446690..
Conclusions
Human amniotic membrane fragment application on acute lesion in the Achilles tendon of rats favored the evolution of tissue repair, reduced the inflammatory response, induced the proliferation of fibroblasts and collagen fibers, and allowed a reduction in healing process time. Future studies with longer experimental times and biomechanical tests for tendinopathy induced in calcaneus and treated with hAM will confirm whether the newly formed tissue will present the same characteristics and properties of normal tendon tissue.
References
-
1de Jonge S, van den Berg C, de Vos RJ, van der Heide HJ, Weir A, Verhaar JA, Bierma-Zeinstra SM, Tol JL. Incidence of midportion Achilles tendinopathy in the general population. Br J Sports Med. 2011 Oct;45(13):1026-8. doi: 10.1136/bjsports-2011-090342.
» https://doi.org/10.1136/bjsports-2011-090342 -
2Millar NL, Hueber AJ, Reilly JH, Xu Y, Fazzi UG, Murrell GA, McInnes IB. Inflammation is present in early human tendinopathy. Am J Sports Med. 2010 Oct;38(10):2085-91. doi: 10.1177/0363546510372613.
» https://doi.org/10.1177/0363546510372613 -
3Kannus, P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000 Dec;10(6):312-20. PMID: 11085557.
-
4James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008 Jan;33(1):102-12. doi: 10.1016/j.jhsa.2007.09.007.
» https://doi.org/10.1016/j.jhsa.2007.09.007 -
5Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008 Jul;466(7):1539-54. doi: 10.1007/s11999-008-0260-1.
» https://doi.org/10.1007/s11999-008-0260-1 -
6Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue. Eur Cell Mater. 2008 Apr;15:88-99. PMID: 18446690.
-
7Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012 Aug;349(2):447-58. doi: 10.1007/s00441-012-1424-6.
» https://doi.org/10.1007/s00441-012-1424-6 -
8Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005 Nov-Dec;23(10):1549-59. doi: 10.1634/stemcells.2004-0357.
» https://doi.org/10.1634/stemcells.2004-0357 -
9Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007 Nov;105(3):215-28. PMID: 17986813.
-
10Manuelpillai U, Moodley Y, Borlongan CV, Parolini O. Amniotic membrane and amniotic cells: potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta. 2011 Oct;32 Suppl 4:S320-5. doi: 10.1016/j.placenta.2011.04.010.
» https://doi.org/10.1016/j.placenta.2011.04.010 -
11Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000 Mar;20(3):173-7. doi: 10.1076/0271-3683(200003)2031-9FT173.
» https://doi.org/10.1076/0271-3683(200003)2031-9FT173 -
12Barboni B, Russo V, Curini V, Mauro A, Martelli A, Muttini A, Bernabò N, Valbonetti L, Marchisio M, Di Giacinto O, Berardinelli P, Mattioli M. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012 Apr;21(11):2377-95. doi: 10.3727/096368912X638892.
» https://doi.org/10.3727/096368912X638892 -
13Muttini A, Mattioli M, Petrizzi L, Varasano V, Sciarrini C, Russo V, Mauro A, Cocciolone D, Turriani M, Barboni B. Experimental study on allografts of amniotic epithelial cells in calcaneal tendon lesions of sheep. Vet Res Commun. 2010 Jun;34 Suppl 1:S117-20. doi: 10.1007/s11259-010-9396-z.
» https://doi.org/10.1007/s11259-010-9396-z -
14Neves MA, Pinfildi CE, Wood VT, Gobbato RC, Silva FM, Parizotto NA, Hochman B, Ferreira LM. Different power settings of LLLT on the repair of the calcaneal tendon. Photomed Laser Surg. 2011 Oct;29(10):663-8. doi: 10.1089/pho.2010.2919.
» https://doi.org/10.1089/pho.2010.2919 -
15Sant'Anna LB, Cargnoni A, Ressel L, Vanosi G, Parolini O. Amniotic membrane application reduces liver fibrosis in a bile duct ligation rat model. Cell Transplant . 2011 Aug;20(3):441-53. doi: 10.3727/096368910X522252.
» https://doi.org/10.3727/096368910X522252 -
16Cargnoni A, Di Marcello M, Campagnol M, Nassuato C, Albertini A, Parolini O. Amniotic membrane patching promotes ischemic rat heart repair. Cell Transplant . 2009 Jun;18(10):1147-59. doi: 10.3727/096368909X12483162196764.
» https://doi.org/10.3727/096368909X12483162196764 -
17Hennerbichler S, Reichl B, Pleiner D, Gabriel C, Eibl J, Redl H. The influence of various storage conditions on cell viability in amniotic membrane. Cell Tissue Bank. 2007 Mar;8(1):1-8. doi: 10.1007/s10561-006-9002-3.
» https://doi.org/10.1007/s10561-006-9002-3 -
18Oliveira FS, Pinfildi CE, Parizoto NA, Liebano RE, Bossini PS, Garcia EB, Ferreira LM. Effect of low level laser therapy (830 nm) with different therapy regimes on the process of tissue repair in partial lesion calcaneus tendon. Lasers Surg Med. 2009 Apr;41(4):271-6. doi: 10.1002/lsm.20760.
» https://doi.org/10.1002/lsm.20760 -
19Niknejad H, Yazdanpanah G. Opposing effect of amniotic membrane on angiogenesis originating from amniotic epithelial cells. J Med Hypotheses Ideas. 2014 Jan;8:39-41. doi: 10.1016/j.jmhi.2013.08.002.
» https://doi.org/10.1016/j.jmhi.2013.08.002 -
20Oberle J, Dighton J, Arbuckle-Keil G. Comparison of methodologies for separation of fungal isolates using Fourier transform infrared (FTIR) spectroscopy and Fourier transform infrared-attenuated total reflectance (FTIR-ATR) microspectroscopy. Fungal Biol. 2015 Nov;119(11):1100-14. doi: 10.1016/j.funbio.2015.08.007.
» https://doi.org/10.1016/j.funbio.2015.08.007 -
21Rousseeuw PJ. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Computational Appl Mathematics. 1987 Nov; 20:53-65. doi: 10.1016/0377-0427(87)90125-7.
» https://doi.org/10.1016/0377-0427(87)90125-7 -
22Maquelin K, Kirschner C, Choo-Smith LP, Braak N, Endtz HP, Naumann D, Puppels GJ. Identification of medically relevant microorganisms by vibrational spectroscopy. J Microbiol Methods. 2002 Nov;51(3):255-71. PMID: 12223286.
-
23Belbachir K, Noreen R, Gouspillou G, Petibois C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal Bioanal Chem. 2009 Oct;395(3):829-37. doi: 10.1007/s00216-009-3019-y.
» https://doi.org/10.1007/s00216-009-3019-y -
24Joensen J, Gjerdet NR, Hummelsund S, Iversen V, Lopes-Martins RAB, Bjordal JM. An experimental study of low-level laser therapy in rat Achilles tendon injury. Lasers Med Sci. 2012 Jan;27(1):103-11. doi: 10.1007/s10103-011-0925.
» https://doi.org/10.1007/s10103-011-0925 -
25Tallon C, Maffulli N, & Ewen SWB. Ruptured Achilles tendons are significantly more degenerated than tendinopathic tendons. Med Sci Sports Exerc. 2001 Dec;33(12):1983-90. PMID: 11740288.
-
26Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996 Sep-Oct;5(5):383-92. PMID: 8933461.
-
27Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004 Jan-Feb;49(1):51-77. PMID: 14711440.
-
28Tseng SC, Espana EM, Kawakita T, Di Pascuale MA, Li W, He H, Liu TS, Cho TH, Gao YY, Yeh LK, Liu CY. How does amniotic membrane work? Ocul Surf. 2004 Jul;2(3):177-87. PMID: 17216089.
-
29Alaseirlis DA, Li Y, Cilli F, Fu FH, Wang JHC. Decreasing inflammatory response of injured patellar tendons results in increased collagen fibril diameters. Connect Tissue Res. 2005 Aug;46(1):12-7. doi: 10.1080/03008200590935501.
» https://doi.org/10.1080/03008200590935501
-
Financial source: CAPES
-
1
Research performed at Laboratories of Immunology, Photodynamic Therapy and Nanosensors, Universidade do Vale do Paraíba (UNIVAP), São José dos Campos-SP, Brazil. Part of PhD degree thesis, Postgraduate Program in Biomedical Engineering. Tutor: Profa. Emilia Ângela Loschiavo Arisawa
Publication Dates
-
Publication in this collection
Feb 2017
History
-
Received
28 Oct 2016 -
Reviewed
23 Dec 2016 -
Accepted
26 Jan 2017