Abstract
Purpose:
To evaluate histopathological and ultrastructural changes and expression of proteins related to apoptosis CASPASE 3 and XIAP after experimental induction of temporary focal cerebral ischemia (90 minutes) due to obstruction of the middle cerebral artery in alcoholism model.
Methods:
Forty adult Wistar rats were used, subdivided into 5 experimental groups: control group (C); Sham group (S); Ischemic group (I); Alcoholic group (A); and Ischemic and Alcoholized group (I+A): animals submitted to the same treatment of group A and after four weeks were submitted to focal cerebral ischemia during 90 minutes, followed by reperfusion of 48 hours. Were processed for histopathological analysis and immunohistochemistry (for the protein expression of CASPASE -3 and XIAP).
Results:
Greater histopathological changes were observed in the animals of groups I and I+A in the three areas analyzed. The neuronal loss was higher in the medial striatum region of the animals of groups I and I + A. The protein expression of CASPASE -3 was higher than that of XIAP in the groups I and I + A for both proteins.
Conclusion:
The expression of XIAP was slightly higher where the histopathological changes and expression of CASPASE -3 was less evident.
Key words:
Brain Ischemia; Apoptosis; Alcoholism; Morphology; Rats
Introduction
Cerebral ischemia is one of the most threatening diseases in humanity with high mortality and morbidity rates worldwide11 Conway EM, Zwerts F, Eymen VV, Devriese A, Nagai N, Luo W, Collen D. Survivin-dependent angiogenesis in ischemic brain molecular mechanisms of hipoxia-induced up-regulation. Am J Pathol. 2003;163(3):935-46. doi: 10.1016/S0002-9440(10)63453-0.
https://doi.org/10.1016/S0002-9440(10)63...
,22 Shalak L, Perlman JM. Hipoxic-ischemic brain injury in the term infant-current conceps. Early Hum Dev. 2004;80(2):125-41. doi: 10.1016/j.earlhumdev.2004.06.003.
https://doi.org/10.1016/j.earlhumdev.200...
. This is a frequent condition in clinical practice which has a difficult therapeutic solution, because the physiopathological study, among other factors, is more difficult due to the great diversity in its anatomical location, etiology and clinical manifestations33 Bratina P, Greenberg L, Pasteur W, Grotta JC. Cruenta emergency department management of stroke in Houston, Texas. Stroke. 1995;26(3):409-14. PMID: 7886715.
4 Colli BO, Silva MN, Carlotti Jr Cg. Isquemia cerebral experimental. In: Silva Jr OC, Zucoloto S, Beer Jr A. Modelos experimentais de pesquisa em cirurgia. São Paulo: Editora Robe; 1998. p.643-62.-55 Carlotti Jr CG, Colli BO, Kazuo JY. Avaliação da isquemia cerebral pela respiração mitocondrial. Arq Neuropsiquiatr. 2001;59(2-B):365-71. PMID: 11460181.. Researches and studies performed on trial animals as well as on men concluded that excessive alcohol consumption harms specifically the Central Nervous System66 Clair HR St. Recognizing alcoholism and its effects: a mine-guide. Basel: S. Ed. Karger; 1991. p.105-21.. Alcohol is incorporated into various body fluids and its concentration in the tissues is directly proportional to the water content77 Kalant H. Absorption, diffusion, distribution and elimination of ethanol: effects on biological membranes. In: Kissin B, Begleiter H. The biology of alcoholism. Switzerland: Springer Nature; 1971; p.1-62.. As a consequence, in high concentrations, alcohol reaches the brain88 Strong R, Grotta JC, Aronowski J. Combination of low dose ethanol and caffeine protects brain from damage produced by focal ischemia in rats. Neuropharmacology. 2000;39(3):515-22. PMID: 10698017.. The event of cerebral ischemia / reperfusion (I/R) causes intense immune response, inflammation and cell death99 Kriz J. Inflammation in ischemia brain injury: timing is important. Crit Rev Neurobiol. 2006;18(1-2):145-57. PMID: 17725517.. Several families of proteases (calpain, cathepsins, caspases, metalloproteinases) are involved in different forms of cell death caused by cerebral ischemia1010 Chaitanya GV, Schwaninger M, Alexander JS, Babu PP. Granzyme-B is involved in mediating post-ischemic neuronal death during focal cerebral ischemia in rat model. Neuroscience. 2010;165(4):1203-16. doi: 10.1016/j.neuroscience.2009.10.067.
https://doi.org/10.1016/j.neuroscience.2...
. Several studies have described neuronal death a few days after transient cerebral ischemia1111 Kamestu Y, Osuga S, Hakim AM. Apoptosis occurs in the penumbra zone during short-duration focal ischemia in the rat. J Cereb Blood Flow Metab. 2003;23:416-22. doi: 10.1097/01.WCB.0000052281.23292.7C.
https://doi.org/10.1097/01.WCB.000005228...
,1212 Renolleau S, Aggoun-Zouaoui D, Ben-Ari Y, Charriaut-Marlan-Gue C. A model of transient unilateral focal ischemia with reperfusion in the P7 neonatal rat: morphological changes indicative of apoptosis. Stroke. 1998;29(7):1454-60. PMID: 9660403.. However, it remains controversial whether apoptosis or necrosis are involved in late neuronal death. Namura et al.1313 Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli Kj, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659-68. PMID: 9570797. evaluated the activity of CASPASE 3 in the brain neurons of adult mice by immunohistochemical study and Western blot after two hours of middle cerebral artery occlusion (MCA). By the Tunel technique, labeled cells were detected 6-24 hours after reperfusion, suggesting the existence of a time dependent evolution from the characterization of the ischemic lesion, by the close correlation observed between the activation of caspase as enzyme and the associated increase in immunoreactive product (CASPASE 3) several hours later, through the morphological and biochemical characteristics of apoptosis.
Considering the evaluation of the XIAP protein expression in the cerebral tissue of rats submitted to MCA occlusion ischemia, Shibata et al.1414 Shibata M, Hattori H, Sasaki T, Gotoh J, Hamada J, Fukuuchi Y. Subcellular localization of a promoter and an inhibitor of apoptosis (Smac/DIABLO and XIAP) during brain ischemia/reperfusion. Neuroreport. 2002;13:1885-88. PMID: 12395105. found that after one hour of ischemia followed by reperfusion between two to 47 hours, immunoreactivity with diffuse expression pattern inside neuronal cells was observed. Quantitative analysis by Western blot demonstrated a minor change in nerve tissue in animals submitted to ischemia/reperfusion Therefore, as the comparison between morphological studies related to apoptosis in experimental cerebral ischemia and alcoholism is limited, the present aimed to analyze the morphological alterations of the cerebral territory irrigated by the middle cerebral artery (MCA) and the role of apoptosis through the immunohistochemical study of Expression of CASPASE 3 and XIAP in ischemic cell death after experimental induction of transient focal cerebral ischemia with reperfusion and chronic alcoholism.
Methods
The experiments were carried out according to the Ethical Principles for Experimental Animals (COBAO) and the study was approved by the Animal Experimentation Committee (CETEA), Medical School, USP, Ribeirao Preto-SP.
During the preoperative period, the animals were kept in the Laboratory of Surgical Technique and Experimental Surgery, Department of Surgery and Anatomy, Medical School of Ribeirao Preto, USP), placed in plastic cages (38 x 23 x 15cm) with metal lid type grill, lined with wood and with free access to the balanced feed (Nuvilab CR1 - Nuvital®) and water. The temperature of the vivarium was maintained at about 250C with continuous ambient air exhaustion and 12 hour light / dark cycles. Throughout the experiment, the norms recommended by the Brazilian College of Animal Experimentation were respected, aiming at always maintaining an ethical procedure in all stages of the research.
After reperfusion, the stability of vital parameters was performed by continuous suturing of the skin with 5-0 mononylon thread, the inhalation anesthetic was suspended, allowing the recovery of the animal. After spontaneous breathing resumed, the orotracheal cannula was removed and the animal was returned to its plastic cage, with free supply of water and feed without the use of anesthetics.
Forty young adult male rats of the Wistar strain (Rattus norvegicus) weighing approximately 200 grams (6-7 weeks) at the beginning of the experiment and 280-310 grams at the end of the experiment were used. Animals were randomly divided into five experimental groups: control (C), eight animals were sacrificed without being through surgical procedures; sham (S), animals submitted to complete simulation of the surgical procedure but without ischemia and then euthanized; ischemic (I), animals submitted to focal ischemia for 90 minutes followed by reperfusion for 48 hours, and then euthanized; alcoholic (A), animals that received 20% ethanol for four weeks and they were euthanized, and, ischemic and alcoholic (IA): animals subjected to the same treatment from group A and they were submitted to focal cerebral ischemia for 90 minutes followed by reperfusion for 48 hours.
The groups of animals A and I + A had a gradual adaptation to the consumption of ethanol, which consisted in giving them a progressive concentration of 5%, 10% and 20%, which was weekly increased, and that the experimental phase was started after the third week of this treatment. The water with ethanol was laid out at will for the animals. This 20% dose was stipulated for the animals of the group Alcoholism and Ischemia + Alcoholism without stipulating a daily dose, since the animals ingest the 20% ad libitum.
Induction of cerebral ischemia
All animals were anesthetized by inhalation of halothane and, when spontaneous movement ceased, it was then intubated with an orotracheal cannula. For blood collection, the ventral artery of the tail of the animals was dissected and channeled with a PE 10 caliber catheter, which in turn was connected with a pressure transducer for continuous recording of heart rate and mean arterial artery pressure (Biomotor model 78339A - Hewlett Packard Company, USA). After fifteen minutes of mechanical ventilation, a blood sample from the artery was collected for gasometry: paCO2, paO2, pH plus glycemic, hemoglobin and hematocrit dosages (Portable Gasometer Model I-STAT Portable Clinical Analysis - ABBOTT Laboratories Inc).
A new blood collection was performed in the last fifteen minutes of ischemia only in groups I and I + A for the dosing of the same parameters described above. When pCO2 values were not acceptable (between 34 and 42 mmHg), a new blood collection was performed to determine the blood gas parameters. Considering the results, changes were made in the endotracheal pressure or the respiration rate. Body temperature was maintained between 37 and 38°C by applying a heat source (220V incandescent lamp) as required by a digital thermometer positioned in the animal’s rectum.
After the stable condition of the animal, the surgical procedure for the induction of ischemia was started by trichotomy of the anterior cervical region, incisions of the medial region of the skin, subcutaneous tissue and left lateral musculature of the neck to visualize the surgical field containing the left common carotid artery (ACCE) until its bifurcation into the left external carotid artery) And left internal carotid artery (ICA). The ACE was sectioned for the retrograde introduction of the 4-0 mononylon clogging wire, 2.5 cm long, having a thickened end with a 5 mm extension silicone mixture until the resistance sensation in its progression, technique proposed by Koiosumi et al.1515 Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke. 1989;20(12):1627-42. PMID:2688195, modified by Carlotti Jr. et al.55 Carlotti Jr CG, Colli BO, Kazuo JY. Avaliação da isquemia cerebral pela respiração mitocondrial. Arq Neuropsiquiatr. 2001;59(2-B):365-71. PMID: 11460181.. Then, measurements were made to ensure that the thread penetrated enough to occlude the MCA origin ostium.
After the ischemia period, the obstructive strand was removed for reperfusion and the skin and subcutaneous tissue were closed. The animals were placed in the boxes with water and food ad libitum for postoperative recovery. After reperfusion of 48 hours they were submitted to euthanasia.
Evaluation of cerebral ischemia
For the study by light microscopy (LM), transmission electron microscopy (TEM) and immunohistochemistry (IH), only one of the coronal sections (2mm) was selected. This cut was performed in the anterior or rostral region passing (1) inferiorly through the optic chiasm; (2) immediately ahead of the anterior commissure and third ventricle; (3) superiorly through the body of the corpus callosum and central portion of the lateral ventricle. This region was selected for analysis because it allowed visualization of the three main ischemic areas after MCA obstruction.
For the accomplishment of the histopathological analysis by LM, these coronal sections of cerebral tissue of all animals were immersed in the fixing solution of 4% paraformaldehyde for 24 hours and then washed with ethanol 70% solution, to aid in removal of excess liquid binder. Subsequently, these sections were submitted to dehydration, diaphanization and were embedded in paraffin.to be the cut.
The sections were stained by the Luxol Fast Blue technique to emphasize the cellular elements (neurons) that are stained in pink-violet and the blue-green myelin on the same slide1616 Kluver H, Barrera E. A method for the combined staining of cells and fibers in the central nervous system. J Neuropath Exp Neurol. 1953;12:400-3. PMID: 13097193..
Histopathological alterations were evaluated by LM, such as: neuronal injury, neuronal necrosis, edema and inflammatory infiltrate using increases of 50 and x400. The analysis was performed in the regions irrigated by MCA (global evaluation of the following areas: dorsolateral cortex, lateral cortex and striatum) of the left cerebral hemisphere in six animals of each group.
For ultrastructural evaluation by TEM in the areas described above, two animals were used per group. After reduction of the material from the coronal sections, small samples of brain tissue were initially fixed by immersion in 2.5% glutaraldehyde solution for four hours at 40°C and washed in 0.1M phosphate buffer solution for 24 hours. They were then post-fixed in 2% osmium tetroxide in 0.2 M phosphate buffer for four hours at 40°C and washed with 0.1 M phosphate buffer for 24 hours. Subsequently, dehydration was performed using a growing series of acetone (30 to 100%). The samples were then embedded in epoxy resin for inclusion, and after polymerization, the blocks were cut into sections of 0.5μm for the choice of the best areas. Of these, cuts of 600 Å of thickness were realized in razor of diamond. The sections were stained with uranyl acetate and transferred to copper grids. A Philips EM208 transmission electron microscope (Royal Philips Electronics, Amsterdam, The Netherlands) was used to analyze the ultrastructural changes of brain tissue in the ACM irrigation region.
The IH analysis was performed on six animals from each group. Coronal sections of the brain tissue were fixed by immersion in 4% paraformaldehyde embedded in paraffin and submitted to avidin-biotin-peroxidase immunohistochemical analysis (Novostain Super ABC Kit - universal, NCL-ABCu, Novocastra Laboratories Ltd, Newcastle upon Typo, UK) - and incubation of brain tissue with antibodies to the apoptosis mechanism: CASPASE 3, pro-apoptotic (CP 229-B, Biocare Medical) (pro-apoptotic) and XIAP, Anti-apoptotic (H-202 / sc-11426 / Rabbit Polyclonal IgG / Santa Cruz).
The parameter analyzed was percentage of positively labeled cells (neurons and neurorocyte cells) in the 3 main areas of MCA irrigation in the left cerebral hemisphere. For the counting, three fields with 400x magnification were chosen in the regions with the highest positive mark (hot spot regions) for the respective protein (CASPASE 3 and XIAP). From the count of the total number of positive and negative cells, the percentage of positive cells was calculated.
Statistical analysis
For the evaluation of the protein expression, the statistical analysis was performed using the Kruskal-Wallis test and multiple comparison post-test of Dunns. GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego - California USA) was used, with values of p<0.05 being considered statistically significant.
Results
Histopathology
The histopathological evaluation was performed globally in the three main regions (dorsolateral cortex, lateral cortex and striatum) of the nervous tissue, irrigated by MCA, in the left cerebral hemisphere, in all experimental groups.
For the animals of groups C and S, no histological changes were observed in the evaluated regions. In all animals of I and I+A groups was observed the presence of neurons with diffuse interstitial edema in distinct regions of the dorsolateral and lateral cortex, mainly located in its deeper layers (III to VI), also in this region, the presence of neurons with picnotic nuclei, an important histological characteristic of cells in the process of necrosis, was identified. Mainly in groups I and I + A, diffuse neurons were observed in the layers of the cortex and/or forming some neuronal foci, presenting some characteristic histological lesions: neurons with loss of the contour of their cellular membranes; with the presence of cytoplasmic edema and a clear-appearing foamy nucleus (Figure 1).
Photomicrographs of the dorsolateral region of the frontal cortex of group C (A - mouse 3) with the molecular (I) and fusiform (VI) molecules. Corpus callosum (CC); longitudinal cleft of the brain (*); pia mater (→) and choroid plexus (thick arrow) of the lateral ventricle (VL); and of group I (B - mouse 5). An area surrounded by arrows shows interstitial edema of the cortex with pycnotic nuclei. Above normal cortical tissue (CO). Pia mater (thick arrow); lateral ventricle (*). Luxol fast blue; x50.
In the striatum, diffuse interstitial edema was less evident in its lateral region (near the corpus callosum). The neurons of this area, presented the diffuse presence of neurons with cytoplasmic edema and no clear contour of their cell membrane was described, as well as neurons with pyknotic nuclei.
Another change observed in the animals of groups I and I+A was the presence of leukocyte or inflammatory infiltration, with the presence of neutrophils inside the capillaries, in diapedesis and also forming areas of interstitial infiltration in the cortical tissue, mainly in the dorsolateral cortex. Changes observed in cortical tissue were more evident in the dorsolateral cortex; But similar for groups I and I+A. In the striatum region, fewer inflammatory infiltrates were observed when compared to the cerebral cortex (Figure 2).
Photomicrographs of the striatum of a group C animal (A - mouse 2), with homogeneous distribution of (→) pink - violet and myelin (in blue - green) neurons; and striatum in the I + A group (B - mouse 2). Note interstitial edema (E), the presence of pycnotic nuclei (thick arrows) and regions of inflammatory infiltration (thin arrows), also observed in detail above and to the right, with the presence of neutrophils in the interstitial tissue (thick arrow) and in the interior of two capillaries (thin arrows). Luxol fast blue; x400.
Group A presented few changes and focal, characteristics of the neuronal lesions described previously for the animals of group I and I+A, mainly in the two cortical regions. The histological findings of interstitial edema and inflammatory infiltration were not observed in the animals of this experimental group.
Ultrastructural evaluation by transmission electron microscopy
No changes were observed in the animals of groups C and S. In the animals of groups I and I + A, the ultrastructural changes were observed mainly in the region called neuropil (Figure 3). The presence of degeneration in some myelin axons, presence of mitochondrial edema formation, increased endoplasmic reticulum volume in the cytoplasm of neural cells and neuropilus, presence of cytoplasmic vacuoles in neural cells and interstitial edema.
Electromicrography of the cortex of a group I animal, showing in detail in the neuropile, the presence of myelinated electrodens axons (*), of axons in degeneration with the characteristic presence of concentric membranes similar to myelinic figures (major arrows). Some axons are observed in more advanced stage of degeneration (Ax), as well as the presence of edemaciadas mitochondria inside (small arrows). Mild edema is seen among these elements of the neuropil (E). x8000.
The number of neural cells with lesions was large in the animals of groups I and I + A, some presenting cytoplasmic vacuoles and even the occasional presence of secondary lysosomes, suggestive of cellular degradation (Figures 4 and 5). Few of the described changes were observed in the animals of group A. These animals presented characteristics such as: well evident nucleolus, nucleus with loose chromatin and condensations near the nuclear membrane and mild degree of mitochondrial edema.
Electromyrography of the cortex of a group I animal. Note a neuron with a high degree of lesion, presenting a large number of edemaciate mitochondria (*) that appear as clear vesicles in the cytoplasm, where a large amount of rough endoplasmic reticulum is also present. Volume (smaller arrows). Interstitial edema (E); neuropil (Ne) and myelinated axons (major arrows). x6700.
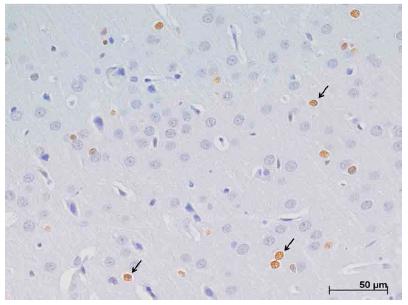
Photomicrography of the dorsolateral cortex in the left cerebral hemisphere for evaluation by the immunohistochemical technique, demonstrating nuclear positive protein expression (arrows) for CASPASE- 3 in group I. x400.
Immunohistochemical analysis of CASPASE-3
Positive nuclear marking for the CASPASE 3 protein was observed diffusely in the nervous tissue mainly in groups I and I + A. Compared to groups A, S and C, there was a statistically significant difference between: CxI, CxI + A, SxI, SxI + A (p ˂ 0,001), and AxI + A (p ˂ 0,05).
In the animals of groups I and I + A, the highest expression of this protein was located in the penumbra areas in the medial region of the dorsolateral cortex (Figure 6) and the medial region of the striatum. When the two cortical regions are analyzed, the greater positive marking was observed in the deeper layers (III to VI).
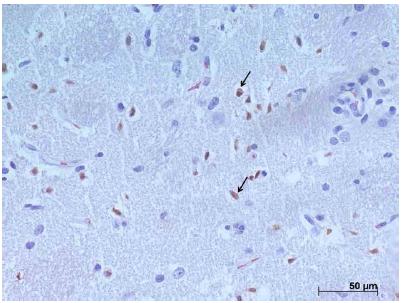
Photomicrograph of the striatum in the left cerebral hemisphere for evaluation by immunohistochemistry demonstrating positive nuclear protein expression (arrows) to XIAP, group I + A. x400.
Immunohistochemical analysis of XIAP
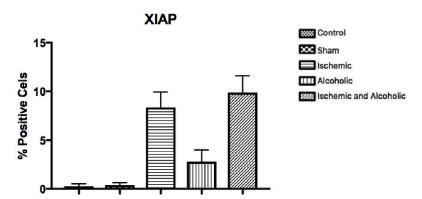
Figure 7 represents the expression of the XIAP protein. There is little positive and diffuse nuclear marking for this protein in nerve tissue and this was lower when compared to the expression of CASPASE-3 in all groups studied. In the regions of the three analyzed fields, when we compared the expression of XIAP, it was slightly larger in the striatum. It was observed higher expression of this protein in the animals of groups I and I + A when compared to groups A, C and S, without statistical difference (p = 0.4060, Kruskal-Wallis test (p> 0.05), post-test Of Dunns).
Average representation (± standard deviation) of the protein expression of XIAP in the three regions of nerve tissue between the groups [p = 0.4060, Kruskal-Wallis test (p>0.05), Dunns post-test].
Discussion
MCA occlusion is the most common model used for the study of focal cerebral ischemia and can be induced by an intraluminal filament enabling reperfusion55 Carlotti Jr CG, Colli BO, Kazuo JY. Avaliação da isquemia cerebral pela respiração mitocondrial. Arq Neuropsiquiatr. 2001;59(2-B):365-71. PMID: 11460181.,1515 Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke. 1989;20(12):1627-42. PMID:2688195. Focal ischemia causes lesions predominantly in the cortex and striatum. This model has been extensively used because of its relevance to human embolic stroke1717 Zhang LAN, Chopp M, Zheng G, Jiang Q, James R. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;22(1-2):83-92. PMID: 9359590..
The stroke eventually involves dysfunction or loss of brain cells. Late neuronal death exhibits distinct morphological characteristics of apoptosis1818 Chopp M, Li Y. Apoptosis in focal cerebral ischemia. Acta Neurochir Suppl. 1996;66:21-6. PMID: 8780792.. While in focal cerebral ischemia the majority of ischemic focus cells undergo necrosis, which is characterized by a sudden reduction of cellular energy, with the formation and rupture of cellular organelles; the margin of brain tissue that is hypoperfused around the ischemic focus is called the ischemic penumbra, a region that is capable of recovery if the perfusion is improved. Cell death in the penumbra is considered an active process and largely dependent on the activation of the cell death or apoptosis program1919 Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke. 1999;20(12):1627-42. PMID: 2688195..
The histologically visible changes resulting from ischemia in brain tissue are observed about 10 minutes after their occurrence, followed by reperfusion of 24 to 48 hours. These changes range from mild neuronal vacuolation, cytoplasmic edema, foam nuclei and presence of irregular chromatin until the presence of late infarction in the ischemic focus region, after longer periods of ischemia and reperfusion1111 Kamestu Y, Osuga S, Hakim AM. Apoptosis occurs in the penumbra zone during short-duration focal ischemia in the rat. J Cereb Blood Flow Metab. 2003;23:416-22. doi: 10.1097/01.WCB.0000052281.23292.7C.
https://doi.org/10.1097/01.WCB.000005228...
.
This article refers to the same regions already studied in previous studies. However, there was a greater severity in relation to the lesions since the ischemic animals were evaluated after a period of reperfusion (48 hours). The I + A group provided the possibility of observation of the most serious histological lesions in relation to the other groups. These lesions were mainly located in the neurons of the III to VI layers of the cortex, which are usually more susceptible to ischemic. Alterations such as cytoplasmic edema, loss of cell membrane boundaries, presence of foamy nuclei (clearer), cells with picnotic nuclei suggestive of necrosis, as well as the presence of interstitial edema and some foci of inflammatory infiltrate2020 Sun H, Zhao H, Sharpe GM, Arrick DM, Mayhan WG. Effect of chronic alcohol consumption on brain damage following transient focal ischemia. Brain Res. 2008;15:1173-80. doi: 10.1016/j.brainres.2007.11.061.
https://doi.org/10.1016/j.brainres.2007....
.
Huang et al.2121 Huang Y, Park YC, Rich RL, Segal D, Myszka DG, Wu H. Structural basis of caspase inhibition by XIAP: differencial roles of the linker versus the BIR domain. Cell. 2001;104(5):781-90. doi: 10.1016/S0092-8674(01)00273-2.
https://doi.org/10.1016/S0092-8674(01)00...
described by TEM the presence of proteins isolated or in the form of protein aggregates, located in the cytoplasm of neurons and in the neuropil area, as well as mitochondrial swelling, rough endoplasmic reticulum dilatation and polyribosomal disaggregation. These protein aggregates may contribute to the slow or late neurodegeneration observed after focal cerebral ischemia.
Functional alterations in brain capillaries after ischemia. This allows the release of vascular contents in the ischemic region, with the presence of leukocyte infiltrate in the neural tissue, favoring the pathogenesis of ischemia2222 Askalan R, Salweski R, Tuor UI, Hutchison J, Hawkins C. X-linked inhibitor of apoptosis protein expression after ischemic injury in the human and rat developing brain. Pediatric Res. 2009;1:21-6. doi: 10.1203/PDR.0b013e3181894a25.
https://doi.org/10.1203/PDR.0b013e318189...
. The presence of leukocyte infiltrate has been observed in ischemic lesions over one hour, followed by reperfusion between 24 hours and seven days2323 Dhodda VK, Saiolor KA, Bowen KK, Vemuganti R. Putative endogenous mediators of preconditioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. J Neurochem. 2004;89(1):73-89. PMID: 15030391..
Associated with edema, foci of inflammatory infiltrate were noticed in some animals of groups I and I + A, mainly in the dorsolateral and lateral cortex. The presence of neutrophils, as described previously, were also observed inside the capillaries and in the interstitial tissue, amid edema and often picnotic cells.
Brain ischemia/reperfusion (I/R) lesions trigger multiple and distinct cell pathways, but with overlapping cell signaling pathways, which can lead to cell survival or cell damage. Evidence shows that, alongside necrosis, apoptosis contributes significantly to cell death after I/R lesions. Apoptosis pathways play a vital role, and once initiated, recruit a cascade of apoptotic molecules to perform cell death. Caspases and members of the BCL2 family appear to be crucial in regulating the various pathways of cell death by apoptosis and various pathways initiated during I/R2424 Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54:34-66. doi: 10.1016/j.brainresrev.2006.11.003.
https://doi.org/10.1016/j.brainresrev.20...
.
Morphological and biochemical evidences of apoptosis have been well documented in experimental animal models of ischemic brain lesions. The most convincing morphological evidence of post-ischemic neuronal apoptosis was detected a few hours after the initiation of the ischemic insult, in the penumbra region and during reperfusion2525 Charriaut-Marlangue C, Margaill I, Represa A, Popovici T, Plotkine M, Ben-Ari Y. Apoptosis and necrosis after reversible focal ischemia: an in situ DNA fragmentation analysis. J Cereb Blood Flow Metab. 1996;16(2):186-94. PMID: 8594049. doi: 10.1097/00004647-199603000-00002.
https://doi.org/10.1097/00004647-1996030...
. CASPASE-3 is a signal of the apoptotic mechanism, since as an effector caspase, it is common in cell death due to its two pathways (intrinsic and extrinsic) and thus, the most evaluated in studies involving cerebral ischemia. In the present study, it is possible to determine the relationship between the number of species and the number of species that are present in the animal population2626 Chen J, GrahamSH, Zhu RL, Simon RP. Stress proteins and tolerance to focal cerebral ischemia. J Cereb Blood Flow Metab. 1996;16(4):566-77. doi: 10.1097/00004647-199607000-00006.
https://doi.org/10.1097/00004647-1996070...
.
Zhu et al.2727 Zhu HC, Gao XQ, Xing Y, Sun SG, Li HG, Wang YF. Inhibition of caspase-3 activation and apoptosis is involved in 3-nitropropionic acid-induced ischemic tolerance to transient focal cerebral ischemia in rats. J Mol Neurosci. 2004;24(2):299-305. doi: 10.1385/JMN:24:2:299.
https://doi.org/10.1385/JMN:24:2:299...
demonstrated that the death of cortical neurons and the striatum in the penumbra zone, after focal ischemia for two hours, followed by reperfusion of 24 hours; Involves apoptosis and is characterized by DNA damage and CASPASE 3 activation observed by flow cytometry and by the high number of TUNEL-positive cells in the rat brain.
Fei Wang et al.2828 Wang F1, Wang Y, Geng X, Asmaro K, Peng C, Sullivan JM, Ding JY, Ji X, Ding Y. Neuroprotective effect of acute ethanol administration in a rat with transient cerebral Ischemia. Stroke. 2012;43(1):205-10. doi: 10.1161/STROKEAHA.111.629576.
https://doi.org/10.1161/STROKEAHA.111.62...
demonstrated the role of acute ethanol administration in a model of cerebral ischemia by occlusion of MCA followed by reperfusion. The authors evaluated the expression of the pro-apothetic proteins CASPASE-3, BAX and AIF and the anti-apoptotic proteins BCL-2 and BCL-xl and observed that acute ethanol causes increased expression of anti-apoptotic proteins (BCL-2 and BCL-xl) and a decrease in pro-apothetic proteins expression (CASPASE-3, BAX and AIF).
In this study, the highest expression of CASPASE 3 was observed in the I + A group when compared to group I, demonstrating that when associated with ischemia, the lesions caused by chronic alcoholism are even more severe. The low expression regions of CASPASE 3 are sites corresponding to the ischemic focus, where there is a high index of the mechanism of cell death due to necrosis, in an initial phase after the installation of the ischemic insult. Therefore, these regions present a lower number of viable cells (alive), besides the alterations such as edema and inflammatory infiltrate (as previously described in the histopathological alterations), thus explaining the lower marking of the cells of these regions by the XIAP protein.
In the three analyzed areas, XIAP expression was higher in the groups with ischemic insult, associated or not to chronic alcoholism, because in these groups, after the period of 48 hours of reperfusion, the highest expression of XIAP in these three groups (A, I And I + A) may also reflect an adaptation to neural cell survival after ischemic insult associated or not with alcoholism. This higher expression of XIAP in these groups can be explained by the attempt of this protein in blocking the action of initiator (such as caspase 9) or effector caspases (such as caspases 3 and 7), and thus the mechanism of apoptosis.
Carvalho et al.2929 Carvalho CAM, Tirapelli DPC, Rodrigues AR, Lizarte Neto FS, Novais PC, Silva, JP, Carlotti CG Júnior, Colli BO, Tirapelli LF. Morphological and immunohistochemical analysis of apoptosis in the cerebellum of rats subjected to focal cerebral ischemia with or without alcoholism model. Acta Cir Bras. 2016;31:629-37. doi: 10.1590/S0102-865020160090000009.
https://doi.org/10.1590/S0102-8650201600...
reported that the histopathological changes were observed to a greater degree in the cerebellum of rats submitted to the same protocol of ischemia performed by the present study. In groups A and IA the expression of CASPASE-3 was greater than the expression of BCL-2 and XIAP, increased with groups A and IA, especially near the transition region between the granular and molecular layers.
This study observed increased expression of the anti-apoptotic CASPASE-3 protein in the ischemic group associated with chronic alcoholism, and this was not observed in the isolated groups. These results suggest that neuroprotection associated with decreased expression of CASPASE-3 protein is directly associated with acute alcoholism, since the chronic alcoholism model increased the expression of said pro-apoptotic protein. Since CASPASE-3 is an effector protease present in both the intrinsic and extrinsic pathways, it can be concluded that chronic alcoholism causes an increase in the rate of cell death by the mechanism of apoptosis.
Few studies have demonstrated the role of chronic alcoholism associated with cerebral ischemia. Sun et al.3030 Sun M, Zhao Y, Gu Y, Xu C. Neuroprotective actions of aminoguanidine involve reduced the activation of calpain and caspase-3 in a rat model of stroke. Neurochem Int. 2010;56:634-41. doi: 10.1016/j.neuint.2010.01.009.
https://doi.org/10.1016/j.neuint.2010.01...
through a model of cerebral ischemia by MCA occlusion and reperfusion observed increased expression of the PPARPγ protein that has been associated with neural differentiation and cell death in the nervous system, as well as mechanisms of inflammation and neurodegeneration .
The protein expression of CASPASE 3 was greater than the expression of the anti-apoptotic protein XIAP, taking into account its greater apoptotic effect in the period of 48 hours of reperfusion in groups I and I + A. The cells died from necrosis in the early stage of ischemia, with a higher incidence of apoptosis, especially in the penumbra area. Alcohol alone did not cause morphological lesions and increase in significant protein and gene expression; however, when associated with ischemia, lesions were usually more severe than those of group I animals.
Thus, the involvement of the CASPASE-3 protein in the model of cerebral ischemia associated with experimental chronic alcoholism was verified in our study as in previous studies. However, differences in expression levels thereof are observed with regard to the reperfusion period and dosage of ethanol administered. The expression of XIAP protein showed little involvement in our study and there are also few studies that evaluated its role associated with cerebral ischemia and even fewer alcoholics. Therefore, further studies are needed to clarify the role of these proteins associated with cerebral ischemia and alcoholism.
Conclusion
Chronic ethanol consumption associated with cerebral ischemia suggest changed in the resulted histopathological, ultrastructural alterations and also in the protein expression of CASPASE-3. We also observed the expression of XIAP was slightly higher where the histopathological changes and expression of CASPASE -3 was less evident.”
Acknowledgement
To Laboratory of Molecular Biology, Department of Surgery and Anatomy, Medical School, USP.
References
-
1Conway EM, Zwerts F, Eymen VV, Devriese A, Nagai N, Luo W, Collen D. Survivin-dependent angiogenesis in ischemic brain molecular mechanisms of hipoxia-induced up-regulation. Am J Pathol. 2003;163(3):935-46. doi: 10.1016/S0002-9440(10)63453-0.
» https://doi.org/10.1016/S0002-9440(10)63453-0 -
2Shalak L, Perlman JM. Hipoxic-ischemic brain injury in the term infant-current conceps. Early Hum Dev. 2004;80(2):125-41. doi: 10.1016/j.earlhumdev.2004.06.003.
» https://doi.org/10.1016/j.earlhumdev.2004.06.003 -
3Bratina P, Greenberg L, Pasteur W, Grotta JC. Cruenta emergency department management of stroke in Houston, Texas. Stroke. 1995;26(3):409-14. PMID: 7886715.
-
4Colli BO, Silva MN, Carlotti Jr Cg. Isquemia cerebral experimental. In: Silva Jr OC, Zucoloto S, Beer Jr A. Modelos experimentais de pesquisa em cirurgia. São Paulo: Editora Robe; 1998. p.643-62.
-
5Carlotti Jr CG, Colli BO, Kazuo JY. Avaliação da isquemia cerebral pela respiração mitocondrial. Arq Neuropsiquiatr. 2001;59(2-B):365-71. PMID: 11460181.
-
6Clair HR St. Recognizing alcoholism and its effects: a mine-guide. Basel: S. Ed. Karger; 1991. p.105-21.
-
7Kalant H. Absorption, diffusion, distribution and elimination of ethanol: effects on biological membranes. In: Kissin B, Begleiter H. The biology of alcoholism. Switzerland: Springer Nature; 1971; p.1-62.
-
8Strong R, Grotta JC, Aronowski J. Combination of low dose ethanol and caffeine protects brain from damage produced by focal ischemia in rats. Neuropharmacology. 2000;39(3):515-22. PMID: 10698017.
-
9Kriz J. Inflammation in ischemia brain injury: timing is important. Crit Rev Neurobiol. 2006;18(1-2):145-57. PMID: 17725517.
-
10Chaitanya GV, Schwaninger M, Alexander JS, Babu PP. Granzyme-B is involved in mediating post-ischemic neuronal death during focal cerebral ischemia in rat model. Neuroscience. 2010;165(4):1203-16. doi: 10.1016/j.neuroscience.2009.10.067.
» https://doi.org/10.1016/j.neuroscience.2009.10.067 -
11Kamestu Y, Osuga S, Hakim AM. Apoptosis occurs in the penumbra zone during short-duration focal ischemia in the rat. J Cereb Blood Flow Metab. 2003;23:416-22. doi: 10.1097/01.WCB.0000052281.23292.7C.
» https://doi.org/10.1097/01.WCB.0000052281.23292.7C -
12Renolleau S, Aggoun-Zouaoui D, Ben-Ari Y, Charriaut-Marlan-Gue C. A model of transient unilateral focal ischemia with reperfusion in the P7 neonatal rat: morphological changes indicative of apoptosis. Stroke. 1998;29(7):1454-60. PMID: 9660403.
-
13Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli Kj, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659-68. PMID: 9570797.
-
14Shibata M, Hattori H, Sasaki T, Gotoh J, Hamada J, Fukuuchi Y. Subcellular localization of a promoter and an inhibitor of apoptosis (Smac/DIABLO and XIAP) during brain ischemia/reperfusion. Neuroreport. 2002;13:1885-88. PMID: 12395105.
-
15Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke. 1989;20(12):1627-42. PMID:2688195
-
16Kluver H, Barrera E. A method for the combined staining of cells and fibers in the central nervous system. J Neuropath Exp Neurol. 1953;12:400-3. PMID: 13097193.
-
17Zhang LAN, Chopp M, Zheng G, Jiang Q, James R. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;22(1-2):83-92. PMID: 9359590.
-
18Chopp M, Li Y. Apoptosis in focal cerebral ischemia. Acta Neurochir Suppl. 1996;66:21-6. PMID: 8780792.
-
19Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke. 1999;20(12):1627-42. PMID: 2688195.
-
20Sun H, Zhao H, Sharpe GM, Arrick DM, Mayhan WG. Effect of chronic alcohol consumption on brain damage following transient focal ischemia. Brain Res. 2008;15:1173-80. doi: 10.1016/j.brainres.2007.11.061.
» https://doi.org/10.1016/j.brainres.2007.11.061 -
21Huang Y, Park YC, Rich RL, Segal D, Myszka DG, Wu H. Structural basis of caspase inhibition by XIAP: differencial roles of the linker versus the BIR domain. Cell. 2001;104(5):781-90. doi: 10.1016/S0092-8674(01)00273-2.
» https://doi.org/10.1016/S0092-8674(01)00273-2 -
22Askalan R, Salweski R, Tuor UI, Hutchison J, Hawkins C. X-linked inhibitor of apoptosis protein expression after ischemic injury in the human and rat developing brain. Pediatric Res. 2009;1:21-6. doi: 10.1203/PDR.0b013e3181894a25.
» https://doi.org/10.1203/PDR.0b013e3181894a25 -
23Dhodda VK, Saiolor KA, Bowen KK, Vemuganti R. Putative endogenous mediators of preconditioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. J Neurochem. 2004;89(1):73-89. PMID: 15030391.
-
24Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54:34-66. doi: 10.1016/j.brainresrev.2006.11.003.
» https://doi.org/10.1016/j.brainresrev.2006.11.003 -
25Charriaut-Marlangue C, Margaill I, Represa A, Popovici T, Plotkine M, Ben-Ari Y. Apoptosis and necrosis after reversible focal ischemia: an in situ DNA fragmentation analysis. J Cereb Blood Flow Metab. 1996;16(2):186-94. PMID: 8594049. doi: 10.1097/00004647-199603000-00002.
» https://doi.org/10.1097/00004647-199603000-00002 -
26Chen J, GrahamSH, Zhu RL, Simon RP. Stress proteins and tolerance to focal cerebral ischemia. J Cereb Blood Flow Metab. 1996;16(4):566-77. doi: 10.1097/00004647-199607000-00006.
» https://doi.org/10.1097/00004647-199607000-00006 -
27Zhu HC, Gao XQ, Xing Y, Sun SG, Li HG, Wang YF. Inhibition of caspase-3 activation and apoptosis is involved in 3-nitropropionic acid-induced ischemic tolerance to transient focal cerebral ischemia in rats. J Mol Neurosci. 2004;24(2):299-305. doi: 10.1385/JMN:24:2:299.
» https://doi.org/10.1385/JMN:24:2:299 -
28Wang F1, Wang Y, Geng X, Asmaro K, Peng C, Sullivan JM, Ding JY, Ji X, Ding Y. Neuroprotective effect of acute ethanol administration in a rat with transient cerebral Ischemia. Stroke. 2012;43(1):205-10. doi: 10.1161/STROKEAHA.111.629576.
» https://doi.org/10.1161/STROKEAHA.111.629576 -
29Carvalho CAM, Tirapelli DPC, Rodrigues AR, Lizarte Neto FS, Novais PC, Silva, JP, Carlotti CG Júnior, Colli BO, Tirapelli LF. Morphological and immunohistochemical analysis of apoptosis in the cerebellum of rats subjected to focal cerebral ischemia with or without alcoholism model. Acta Cir Bras. 2016;31:629-37. doi: 10.1590/S0102-865020160090000009.
» https://doi.org/10.1590/S0102-865020160090000009 -
30Sun M, Zhao Y, Gu Y, Xu C. Neuroprotective actions of aminoguanidine involve reduced the activation of calpain and caspase-3 in a rat model of stroke. Neurochem Int. 2010;56:634-41. doi: 10.1016/j.neuint.2010.01.009.
» https://doi.org/10.1016/j.neuint.2010.01.009
-
Financial source:
none
-
1
Research performed at Biomolecular Laboratory, Department of Anatomy and Surgery, Medical School, Universidade de São Paulo (USP), Ribeirao Preto-SP, Brazil. Part of Master degree thesis, Postgraduate Program in Clinical Surgery. Tutor: Prof. Dr. Luís Fernando Tirapelli.
Publication Dates
-
Publication in this collection
Aug 2018
History
-
Received
18 Apr 2018 -
Reviewed
15 June 2018 -
Accepted
14 July 2018