Abstract
The electric eel Electrophorus electricus is a fresh water teleost showing an electrogenic tissue that produces electric discharges. This electrogenic tissue is distributed in three well-defined electric organs which may be found symmetrically along both sides of the eel. These electric organs develop from muscle and exhibit several biochemical properties and morphological features of the muscle sarcolema. This review examines the contribution of the cytoskeletal meshwork to the maintenance of the polarized organization of the electrocyte, the cell that contains all electric properties of each electric organ. The cytoskeletal filaments display an important role in the establishment and maintenance of the highly specialized membrane model system of the electrocyte. As a muscular tissue, these electric organs expresses actin and desmin. The studies that characterized these cytoskeletal proteins and their implications on the electrophysiology of the electric tissues are revisited.
Electrophorus electricus; cytoskeleton; desmin; actin; alpha-actinin; vinculin
The cytoskeleton of the electric tissue of Electrophorus electricus, L.* Professor Carlos Chagas Filho was our advisor during his last years. He gave us more than scientific and cultural guidance, he was an example and a friend. * Invited paper ** Member of the Academia Brasileira de Ciências Correspondence to: Vivaldo Moura Neto E-mail: vivaldo@anato.ufrj.br
CLAUDIA DOS SANTOS MERMELSTEIN1, MANOEL LUIS COSTA1
and VIVALDO MOURA NETO2
1Departamento de Histologia e Embriologia
2Departamento de Anatomia, Instituto de Ciências Biomédicas
Universidade Federal do Rio de Janeiro - 21949-590 Rio de Janeiro, RJ, Brazil
Manuscript received on May 3, 2000; accepted for publication on May 9, 2000;
contributed by VIVALDO MOURA NETO** Professor Carlos Chagas Filho was our advisor during his last years. He gave us more than scientific and cultural guidance, he was an example and a friend. * Invited paper ** Member of the Academia Brasileira de Ciências Correspondence to: Vivaldo Moura Neto E-mail: vivaldo@anato.ufrj.br
ABSTRACT
The electric eel Electrophorus electricus is a fresh water teleost showing an electrogenic tissue that produces electric discharges. This electrogenic tissue is distributed in three well-defined electric organs which may be found symmetrically along both sides of the eel. These electric organs develop from muscle and exhibit several biochemical properties and morphological features of the muscle sarcolema. This review examines the contribution of the cytoskeletal meshwork to the maintenance of the polarized organization of the electrocyte, the cell that contains all electric properties of each electric organ. The cytoskeletal filaments display an important role in the establishment and maintenance of the highly specialized membrane model system of the electrocyte. As a muscular tissue, these electric organs expresses actin and desmin. The studies that characterized these cytoskeletal proteins and their implications on the electrophysiology of the electric tissues are revisited.
Key words:Electrophorus electricus, cytoskeleton, desmin, actin, alpha-actinin, vinculin.
INTRODUCTION
The electric eel Electrophorus electricus L. is a fresh water teleost that lives in the basins of the Amazonas and the Orenoco River. It belongs to the family of the gymnotidae, being the only representative of the genus and the species.
Electrophorus electricus L. has an electrogenic tissue that produces electric discharges and which is used in predation and defense. The electrogenic tissue is distributed in three well-defined electric organs that may be found symmetrically along both sides of the animal. The main electric organ extends from behind the peritoneal cavity of the viscera down the tail of the animal where it gives rise to Sach's organ, which occupies the remainder of the caudal portion. Hunter' organ is located subjacent to the other electric organs and dorsal to the long swimming fin on the ventral surface of the animal (Gotter et al. 1998). It is possible that each organ is involved in separate behavioral electric activities (Keynes & Martins-Ferreira 1953).
The morphological and functional cellular unit of the electric organ is a multinucleated syncytium called electroplaque or electrocyte. Each electrocyte is placed in the interior of a insulating septa composed of connective tissue, and disposed by stacks in files superposed one after another (Luft 1957). In an adult animal, there are thousands of electrocytes. The whole discharge is assured by a complex system of coordination that allows the synchronization and distribution of the discharges of the electroplaques (120mV). The electric discharge is produced by nerve excitation and is composed of a certain number of spikes, attaining quite high voltages (400 to 600 volts) in open circuits (Albe-Fessard & Chagas 1955), depending on the eel's size. The electrocytes are flatten cellular structures with six surfaces, two of which are well-defined: the posterior membrane, innervated and flat, and the anterior membrane, non-innervated and with undulations. The papillae of the anterior face are quite prominent while those on the posterior surface are much less pronounced. The anterior surface is rich in  ,
,  - ATPase (Somló et al. 1977, Hassón-Voloch et al. 1993, Araujo et al. 1993) and is rich in acetylcholine receptors (AChR) (Changeux et al. 1969, Meunier et al. 1974) and responsible for the discharge (Keynes & Martins-Ferreira 1953). The electric organ expresses high levels of membrane receptors, ion channels and ATPases, and has been used as a tissue source for the purification and studies of these proteins.
- ATPase (Somló et al. 1977, Hassón-Voloch et al. 1993, Araujo et al. 1993) and is rich in acetylcholine receptors (AChR) (Changeux et al. 1969, Meunier et al. 1974) and responsible for the discharge (Keynes & Martins-Ferreira 1953). The electric organ expresses high levels of membrane receptors, ion channels and ATPases, and has been used as a tissue source for the purification and studies of these proteins.
The electrocyte morphology and the overall cellular distribution in the tissue reflects the specialized physiological function of these cells, which is to produce pronounced hyperpolarizations in membrane potential. This cellular morphology is maintained by a cytoskeletal meshwork (Cartaud et al. 2000).
CYTOSKELETON
All eucaryotic cells possess a three-dimension cytoskeleton composed of a complex network of filaments that provides the cells with shape, rigidity, elasticity, internal spatial organization and motility (Machesky & Schliwa 2000). These filaments are classified, based in their diameter, in three types: microtubules, microfilaments and intermediate filaments (IFs). Each type of filament is formed from a different protein subunit: actin for microfilaments, tubulin for microtubules, and a family of related fibrous proteins for IFs. Actin and tubulin have been highly conserved throughout the evolution of eucaryotes.
Microtubules consist of a dynamic, highly polarized network of microtubule filaments, microtubule-associated proteins (MAPs), microtubule motors and microtubule-organizing proteins (Cassimeris 1999). Microtubules are long and hollow cylinders with an outer diameter of 25 nm. They are made of the protein tubulin, which exists in two closely related globular polypeptides called alpha-tubulin and beta-tubulin. The alpha- and beta-tubulins are present in all eukaryotic cells, but are highly expressed in nervous system with different subtypes distributed in the neural and glial cells (Moura Neto et al. 1983). In fact, more than seven isoelectric variants were described for alpha-tubulin and fourteen beta-tubulin variants were found in the nervous system (Regnard et al. 1996). Since there are few tubulin genes, these variants are provided by post-translational modifications as tyrosylation, acetylation and glycosilation. The tubulin superfamily also comprises gamma-tubulin, which is involved in the nucleation of new microtubules (Oakley et al. 1990) and more recently delta and epsilon-tubulin have been identified as components of centrosomes (Chang & Stearns 2000). Microtubules are implicated in various cellular functions such as: cellular division, secretion and transport of vesicles and organelles (Vale & Milligan 2000). Tubulin molecules have sites that can interact with microtubule-based motor proteins, like dynein and kinesin, and they are implicated in the transport of vesicles in the cell (Allan 1996).
Microfilaments are two-stranded helical polymers with a diameter of 5-9 nm. Its major protein component is actin (43 kd), which is present in all eucaryotic cells. Higher eucaryotes have six different types of actin that are expressed in a tissue-specific way. These isoforms fall into three classes, depending on their isoelectric point. Alpha-actins are found in muscle cells, whereas in non-muscle cells there are two isoactins named beta and gamma-cytoplasmic actins (Vandekerckhove et al. 1986, Otey et al. 1988). Although there are differences in the properties of different forms of actin, the amino-acid sequences have been highly conserved in evolution. Polymerization of monomeric globular actin (G-actin) requires ATP,  and
and  . Like microtubules, microfilaments are polar structures, with two different ends: a slow-growing minus end and a fast-growing plus end. The ability of G-actin to polymerize into filamentous actin (F-actin) gives a dynamic role to this protein in cellular functions such as cytokinesis, secretion and cell locomotion (Singer et al. 1986).
. Like microtubules, microfilaments are polar structures, with two different ends: a slow-growing minus end and a fast-growing plus end. The ability of G-actin to polymerize into filamentous actin (F-actin) gives a dynamic role to this protein in cellular functions such as cytokinesis, secretion and cell locomotion (Singer et al. 1986).
Actin filaments in animal cells are organized into three general types of arrays: in parallel bundles, in contractile bundles and in gel-like networks. An understanding of the functional role of actin in the cell requires detailed knowledge of the expression of several proteins related with the actin network. These proteins are referred to as actin-binding proteins (Hartwig & Kwiatkowski 1991). They control and modulate the length of actin filaments, their stability and the attachment of these filaments with one another and to other components of the cell (like the plasma membrane). Alpha-actinin (100 kd) is an actin-bundling protein that participates in the cross-linking of actin filaments and helps to form the anchorage for the ends of actin filaments where they terminate on the plasma membrane (Viel 1999). Filamin (250 kd) is a gel-forming protein enriched in the cortex of cells, which promotes the formation of a loose network of actin filaments (Matsudaria 1994). Vinculin (130 kd) is an attachment protein present in actin-containing cell junctions, which associates with alpha-actinin helping to anchor actin filament on the plasma membrane (Rudiger 1998).
Intermediate filaments are highly stable protein fibers found in the cytoplasm of most animal cells. They have a diameter of 8-10 nm, between that of microfilaments and microtubules. In most animal cells an extensive network of IFs surrounds the nucleus and extends out to the cell periphery. They are made of intermediate filament proteins, which constitute a large and heterogeneous multigene family (Osborn & Weber 1986). In spite of the ubiquitous characteristics of tubulin and actin, intermediate filament proteins have a cellular specific distribution. Eight major types of IFs have been distinguished by their polypeptide composition: 1) vimentin (54 kd) is present in cells of mesenchymal origin, often expressed transiently during development and normally expressed in tumoral cells; 2) keratins (40-70 kd) are found characteristically in epithelial cells and their derivatives, and are subdivided in type I (acidic) and type II (basic/neutral); 3) neurofilament proteins (60-200 kd) are a triplet of polypeptides (NF-L, NF-M and NF-H) which are present in neurons; 4) GFAP - glial fibrillary acidic protein (50 kd) is expressed in glial cells; 5) desmin (53 kd) is found in muscle cells; 6) nestin is expressed in the central nervous system and muscle precursor cells; 7) peripherin is found in neurons; and 8) nuclear lamins (65-75 kd) are composed of the three polypeptides lamins A, B and C, which are present in the nuclear lamina of all eucaryotic cells (Galou et al. 1997, Gomes et al. 1999). IF proteins show similarities in peptidic composition, as analyzed by amino acid sequence analysis (Geisler et al. 1982) and immunological studies using monoclonal antibodies (Pruss et al. 1981). However, very little is known about their function. The major function attributed to IFs is to provide mechanical stability to animal cells. The structure of IFs is ideally suited for such function, because the fibrous subunit associate side by side in overlapping arrays (Herrmann & Aebi 2000).
Desmin, the IF protein specifically found in muscle cells, is distributed throughout the cytoplasm of smooth muscle cells, and it links together adjacent myofibrils in skeletal and cardiac muscle cells. Desmin is the first muscle structural protein to be expressed during development, and is always expressed in muscle cells, even in muscle de-differentiation or hypertrophy. For instance, TPA-treated chick skeletal muscle cells lose their myofibrils, but still express desmin (Mermelstein et al. 1996). Furthermore, the function of desmin is unclear: some reports suggest that it is involved in regulating the proper size of the sarcomeres and muscle striation, but cells expressing truncated-desmin in culture, which lack the intermediate filament network, have normally spaced striations, and contract normally (Schultheiss et al. 1991). Desmin null transgenic mice show normal overall muscle structure but exhibit minor physiological differences such as less resistance to fatigue (Li et al. 1997). A regulatory role for desmin, as a nuclear binding protein, has also been proposed (Li et al. 1994): it has potential association sites for the muscle regulatory master switch gene MyoD, and unclear nuclear localization.
CYTOSKELETON OF THE ELECTROCYTE OF Electrophorus electricus
The first observation of the cytoskeletal organization of the electrocyte of Electrophorus electricus was obtained by optical and electron microscopy. The presence of myofibrils was observed using young animals (Esquibel et al. 1971). In older eels, the electron microscopy does not show a myofibril organization (Machado et al. 1976). Noteworthy is the fact that microtubules were observed as well as a pattern of filaments (with 7 nm in diameter) which might be characterized as parts of microfilaments (Machado et al. 1976, Benchimol et al. 1979).
Since myofibrils were observed in the electrocytes, we decided to analyze the expression of typical muscle proteins, like desmin and actin, in order to understand the cellular origin and differentiation of these cells. We started our study of the eel's cytoskeleton by the biochemical identification of desmin, which has a muscle-specific cell expression. We purified desmin from the main electric organ of E. electricus, and characterized its molecular weight, peptidic map and immunological identity (Costa et al. 1986). The presence of desmin in the electric organ of Electrophorus electricus described by us (Costa et al. 1986, 1988) supports the concept of a muscular origin of the electrogenic tissue (Mathewson et al. 1961). This result leads to view this electric organ as a study model for muscle dedifferentiation; particularly suitable for biochemical analysis, since one large eel can have a huge mass of electric (muscle-derived) tissue.
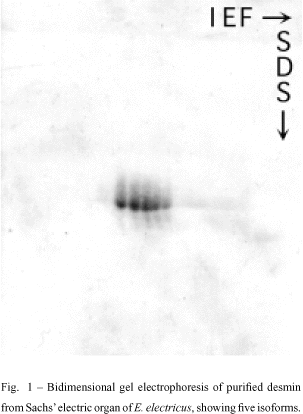
When desmin from the main electric organ of E. electricus was analyzed in an isoelectric focusing gel electrophoresis system (IEF), five isoelectric variants were detected. The same observations were obtained from tissue portions taken from Hunter's or Sach's organs (Costa et al. 1988, Fig. 1). Opposed to the five variants found in preparations obtained from the electric organ, only four desmin isoforms were found in extracts from the dorsal muscle of the eel (Cordeiro et al. 1995).
Desmin is coded by a single gene (Quax et al. 1985) and shows only two or three isoelectric variants in muscle tissues of mammals and birds (Lazarides & Balzer 1978). This contrasts with the five isoelectric desmin variants found in the electric tissue of the E. electricus. It is also interesting that five desmin variants were described in conductive Purkinje fibers of some mammalian hearts. These fibers have a myogenic origin but have nerve-like properties (Thornell et al. 1985). Furthermore, in some human cardiac hypertrophies, the number of isodesmins is increased up to six isovariants (Rappaport et al. 1988). All the above experimental models have in common the loss of myofibrils and changes in the overall shape and electrical properties. Desmin could have only a structural role in the cell, and could be maintained as a mechanical substitute for the myofibrils. The presence of more isoforms suggests that there are some special physiological conditions in these models that promote the generation of the unusual desmin variants. The regulation and physiological differences between desmin isoforms is not known, but we would like to correlate the extra isoforms described in electrocytes with the muscle dedifferentiation of these cells.
At least some of the isoelectric variants of desmin may be produced by post-translational modifications, including phosphorylation (O'Connor et al. 1981, Leão Ferreira et al. 1994). Differences in isodesmins indicate different degrees of desmin phosphorylation in the electric tissue. Some of the five isoelectric variants of desmin found in the electric tissue are phosphorylated (Cordeiro et al. 1995). The mechanism of post-translational modifications could play a role in the organization of desmin filaments network and might be responsible for the interactions of desmin with other components of the cytoskeleton or with other cell constituents like membranes. The same has been suggested for the IF phophorylation in general (Traub 1985).
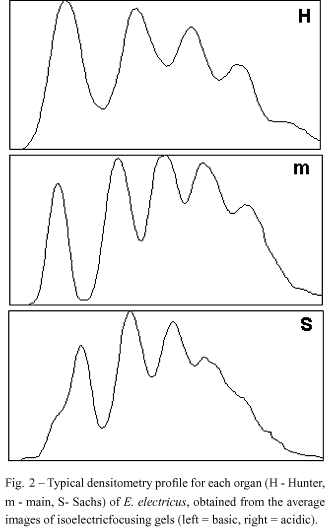
Interestingly, the five isodesmins found in the electric tissue are expressed in a characteristic and different pattern in the three electric organs (main, Hunter and Sachs), as showed by statistical analysis and quantitative densitometry (Figs. 2 and 3) using Coomassie blue-stained bands in IEF (Costa et al. 1998). The organ-specific isodesmin pattern could correlate with differences in each organ's physiology, that would reflect differences in their behavioral electric activities (Keynes & Martins-Ferreira 1953).
Using electron microscopy (Cordeiro et al. 1996) we were able to show a dense network of filaments in the electric tissue of the eel (Fig. 4).
The presence of desmin in fractions of membrane of the main electric organ (Mermelstein et al. 1988, 1997) suggests a possible association between desmin and membranes of electrocytes. In striated muscle, desmin is concentrated in the Z line (and in intercalated disks in cardiac muscle) and on the non-myofibrillar region. In smooth muscle and in young myoblasts, desmin is more abundant around the nucleus, similar to what happen to all intermediate filaments.
Actin also has a specific pattern of isoform expression in different tissues. We also purified actin from the main electric organ of E. electricus (Ayres Sá et al. 1991). Analysis by isoelectric focusing shows two different isoelectric points for actin, as opposed to the unique isoelectric form of actin extracted from the dorsal muscle of Electrophorus electricus. Only one actin variant found in the electric organ has, on the gel system used, the same isoelectric point of the alpha-actin from eel's dorsal muscle and of the alpha-actin from rabbit striated muscle. The other actin variant from the electric organ has the same isoelectric point of gamma-actin from smooth muscle (Ayres Sá et al. 1991). The expression of these two actin isoforms could be related to transient steps of the electrocyte differentiation program.
Actin was found distributed in the cytoplasm of the electrocyte by immunohistochemical methods using an antibody anti-actin and NBD-phallacidin, a probe that binds specifically to F-actin (Taffarel et al. 1985). However, only few and short actin filaments were seen in the electrocyte's cytoplasm. It seems that the major portion of actin is occurring in a globular form in these cells.
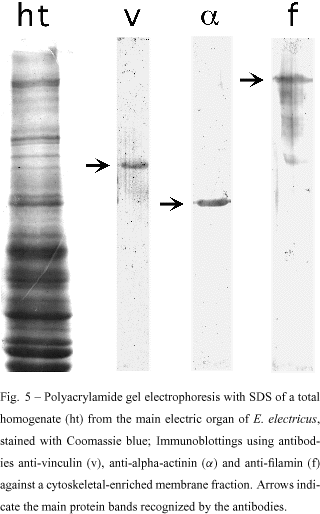
The presence of alpha-actinin, filamin and vinculin was confirmed in cytoskeletal-enriched membrane fractions from the main electric organ of E. electricus (Mermelstein et al. 1988), by SDS-PAGE and immunoblotting (Fig. 5). These fractions were obtained according to Kordeli et al. (1986). It is interesting to keep in mind that microfilaments need the physiological regulation of associated proteins. Proteins like alpha-actinin, filamin and vinculin regulate the overall distribution and architecture of the microfilaments, particularly during myofibrillogenesis, where they nucleate the nascent myofibrils, by aligning microfilaments (stress-fiber like structures). As they are important in myofibrillogenesis, they should be also involved in the breakdown of myofibrils during muscle dedifferentiation. When these cytoskeletal-enriched membrane fractions from the main electric organ were examined by transmission electron microscopy, a network of cytoskeletal filaments associated with membranes could be easily observed (Mermelstein et al. 1997). The analysis of these cytoskeletal-enriched membrane fractions by polyacrylamide gel electrophoresis, showed that actin and desmin could be the cytoskeletal components of these preparations. In fact, a peptidic map produced with these proteins extracted from the gel, confirmed their identity as been actin and desmin (Mermelstein et al. 1988, 1997).
We have not found long actin filaments in the cytoskeletal network of the electrocytes. It is difficult to think that actin filaments cross the whole cytoplasm of the cell. However, actin-binding proteins such as alpha-actinin, filamin and vinculin, are present in the electrocyte tissue. It is possible that actin, organized in short filaments, is linked to the membrane by interactions with alpha-actinin and vinculin. Another possible role for actin is to interact with desmin filaments, allowing this IF protein to interact with membranes. Thus, desmin could indirectly anchor protein receptors in the membrane, participating in the electric stimulation of the electrocytes. In this hypothetical picture, desmin could organize a meshwork with actin and actin-binding proteins with attachment points in the electrocyte membrane, establishing and maintaining the functional polarity of the cell.
CONCLUDING REMARKS
Electrocytes are derived from skeletal muscle, which has a very specialized cytoskeletal organization. The observed changes in the cytoskeleton of electrocytes could be an indirect consequence of the induced modification of the tissue during the processes of acquisition of electric properties and loss of contractile function. On the contrary, the specific morphology suggests an active and important role for the cytoskeleton rearrangement. We studied structural and biochemical changes in two of the most abundant cytoskeletal proteins in muscle cells: actin and desmin (Table I). Microfilaments change from a myofibrillar structure in skeletal muscle cells into a loose network found in electrocytes (Ayres Sá et al. 1991) and desmin IFs change from a Z-band localization into a diffuse network in electrocytes (Cordeiro et al. 1996, Costa et al. 1988). One interesting question about desmin and its isovariants in the electric tissue is to understand if its presence is the cause or consequence of the electrocyte's specialization. Why there are five isoforms of desmin in the electric organ of Electrophorus electricus? Are the different degrees of phosphorylation of these isoforms related to different metabolical signalization in electrocytes?
The study of the cytoskeleton of the electric tissue of Electrophorus electricus may help to understand its role in keeping the polarized organization of the electrocyte. This study may also provide information about a possible role of the cytoskeleton on the bioelectrogenic property of the electric tissue.
ACKNOWLEDGEMENTS
We wish to thank Angela Maria Alves Langer for technical assistance. This work was supported by Conselho de Ensino e Pesquisa para Graduados/Universidade Federal do Rio de Janeiro (CEPG/UFRJ); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ); Financiadora de Estudos e Projetos (FINEP); Fundação Universitária José Bonifácio/Universidade Federal do Rio de Janeiro (FUJB/UFRJ) and PRONEX 052/97, Brasil.
REFERENCES
ALBE-FESSARD D & CHAGAS C. 1955. Études par dérivation intracellulaire des effets sommatifs de deux stimulations successives au niveau d'une électroplaque du gymnote. C R Acad Sci (Paris) 239: 1951-1954.
ALLAN V. 1996. Motor protein: a dynamic duo. Curr Biol 6: 630-633.
ARAUJO GMN, PEDRENHO AR & HASSÓN-VOLOCH A. 1993. The effect of mercury and aluminum on sodium-potassium-Mg2+ dependent-adenosine triphosphatase activity of Electrophorus electricus (L.) electrocyte. Int J Biochem 25: 1729-1735.
AYRES SÁ L, MOURA NETO V, OLIVEIRA M M & CHAGAS C. 1991. Heterogeneity of purified actin in the electric organ of the electric eel Electrophorus electricus. J Exp Zoology 257: 43-50.
BENCHIMOL M, MACHADO RD & SOUZA W. 1979. Staining of microtubules of the electrocyte of Electrophorus electricus L. by alcian blue and lanthanum. Experientia 35: 670-671.
CARTAUD J, CARTAUD A, KORDELI E, LUDOSKY MA, MARCHAND S & STETZKOWSKI-MARDEN F. 2000. The Torpedo electrocyte: a model to study membrane-cytoskeleton interactions at the post-synaptic membrane. Microsc Res Tech 49: 73-83.
CASSIMERIS L. 1999. Accessory protein regulation of microtubule dynamics throughout the cell cycle. Curr Opin Cell Biol 11: 134-141.
CHANG P & STEARNS T. 2000. Gamma-tubulin and epsilon-tubulin: two new human centrosomal tubulins reveal new aspects of centrosome structure and function. Nature Cell Biol 2: 30-35.
CHANGEUX JP, GAUTRON J, ISRAËL M & PODLESKI T. 1969. Séparation de membranes excitables à partir de l'organe électrique de l' Electrophorus electricus. C R Acad Sci (Paris) 269: 1788-1791.
CORDEIRO MCR, MOURA NETO V, BENCHIMOL M, FARIA MVC & CHAGAS C. 1995. Microheterogeneity of desmin in the electric organ and dorsal muscle of the Electrophorus electricus. Comp Biochem Physiol 111A: 345-350.
CORDEIRO MCR, BENCHIMOL M, MERMELSTEIN CS, SÁ LA, FARIA MVC, CHAGAS C & MOURA NETO V. 1996. Desmin filaments in the electrocytes of the electric organ of the Electrophorus electricus. Cell Tissue Res 285: 387-393.
COSTA ML, OLIVEIRA MM, ALBERTI JR O, NETO VM & CHAGAS C. 1986. Caractérisation de la desmine dans l'organe électrique de l' Electrophorus electricus L. C R Acad Sci (Paris) 303: 547-550.
COSTA ML, NETO VM & CHAGAS C. 1988. Desmin heterogeneity in the main electric organ of Electrophorus electricus (Linnaeus). Biochimie 70: 783-789.
COSTA ML, MERMELSTEIN CS, FRÓES MM, CHAGAS C & NETO VM. 1998. Differences in the isodesmin pattern between the electric organs of Electrophorus electricus L. Comp Biochem Physiol 119: 715-719.
ESQUIBEL MA, ALONSO I, MEYER H, CASTRO GO & CHAGAS C. 1971. Quelques aspects de l'histogenese et de l'ontogenese des organes électriques chez l' Electrophorus electricus L. C R Acad Sci (Paris) 273: 196-199.
GALOU M, GAO J, HUMBERT J, MERICSKAY M, LI Z, PAULIN D & VICART P. 1997. The importance of intermediate filaments in the adaptation of tissues to mechanical stress: evidence from gene knockout studies. Biol cell 89: 85-97.
GEISLER N, PLESSMAN U & WEBER K. 1982. Related amino acid sequences in neurofilaments and non-neuronal intermediate filaments. Nature 296: 448-450.
GOMES F, PAULIN D & MOURA NETO V. 1999. Glial fibrillary acidic protein (GFAP): modulation by growth factors and its implication in astrocyte differentiation. Braz J Med Biol Res 32: 619-631.
GOTTER AL, KAETZEL MA & DEDMAN JR. 1998. Electrophorus electricus as a model system for the study of membrane excitability. Comp Biochem Physiol 119: 225-241.
HARTWIG JH & KWIATKOWSKI DJ. 1991. Actin-binding proteins. Curr Opin Cell Biol 3: 87-97.
HASSÓN-VOLOCH A, SOMLÓ C, NUNES-DE-ARAUJO GM & GOMES-QUINTANA E. 1993. Mg2+-dependent ATPases in the electrocyte of Electrophorus electricus (L.). Brazilian J Med Biol Res 26: 351-354.
HERRMANN H & AEBI U. 2000. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opin Cell Biol 12: 79-90.
KEYNES RD & MARTINS-FERREIRA H. 1953. Membrane potentials in the electroplates of the electric eel. J Physiol 119: 315-351.
KORDELI E, CARTAUD J, NGHIÊM HD, PRADEL LA, DUBREUIL C, PAULIN D & CHANGEUX JP. 1986. Evidence for a polarity in the distribution of proteins from the cytoskeleton in Torpedo marmorata electrocytes. J Biol Chem 102: 748-761.
LAZARIDES E & BALZER DR. 1978. Specificity of desmin to avian and mammalian muscle cells. Cell 14: 429-438.
LEÃO FERREIRA LR, MOUSSATCHÉ N & MOURA NETO V. 1994. Rearrangement of intermediate filament network of BHK-21 cells infected with vaccinia virus. Arch Virol 138: 273-285.
LI H, CHOUDHARY SK, MILNER DJ, MUNIR MI, KUISK, IR & CAPETANAKI Y. 1994. Inhibition of desmin expression blocks myoblast fusion and interferes with the myogenic regulators MyoD and Myogenin. J Cell Biol 124: 827-841.
LI Z, MERICSKAY M, AGBULUT O, BUTLER-BROWNE G, CARLSSON L, THORNELL L-E, BABINET C & PAULIN, D. 1997. Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. J Cell Biol 139: 129-144.
LUFT JH. 1957. The histology and cytology of the electric organs of electric eel ( Electrophorus electricus L.). J Morph 100: 113-139.
MACHADO RD, DE SOUZA W, COTTA PEREIRA G & DE OLIVEIRA G. 1976. On the fine structure of the electrocyte of Electrophorus electricus L. Cell Tissue Res 174: 355-366.
MACHESKY LM & SCHLIWA M. 2000. Cell dynamics: a new look at the cytoskeleton. Nature Cell Biol 2: E17-E18.
MATHEWSON R, WACHTEL A & GRUNDFEST H. 1961. Fine structure of electroplaques. In: CHAGAS C & PAES CARVALHO A (Ed.); Bioelectrogenesis. Amsterdam: Elsevier Publishing Co., p. 25-53.
MATSUDARIA P. 1994. Actin crosslinking proteins at the leading edge. Semin Cell Biol 5: 165-174.
MERMELSTEIN CS, MOURA NETO V & CHAGAS C. 1988. C. Alpha-actinine dans l'organe electrique de l' Electrophorus electricus, L. C R Acad Sci (Paris) 307: 717-721.
MERMELSTEIN CS, COSTA ML, CHAGAS C & MOURA NETO V. 1996. Intermediate filament proteins in TPA-treated skeletal muscle cells in culture. J Muscle Res Cell Mot 17: 199-296.
MERMELSTEIN CS, BENCHIMOL M, TAFFAREL M, CORDEIRO MCR, CHAGAS C & MOURA NETO V. 1997. Desmin and actin filaments in membrane-cytoskeletal preparations of the electric tissue of Electrophorus electricus, L. Arch Histol Cytol 60: 445-452.
MEUNIER JC, SEALOCK R, OLSEN R & CHANGEUX JP. 1974. Purification and properties of the choline receptor protein from Electrophorus electricus electric tissue. Eur J Biochem 45: 371-394.
MOURA NETO V, MALLAT M, JEANTET C & PROCHIANTZ A. 1983. Microheterogeneity of tubulin proteins in neuronal and glial cells from the mouse brain in culture. EMBO J 2: 1243-1248.
OAKLEY BR, OAKLEY CE, YOON Y & JUNG MK. 1990. Gamma-tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergilla nidulans. Cell 61: 1289-1301.
O'CONNOR M, GARD DL & LAZARIDES E. 1981. Phosphorylation of intermediate filament protein by cAMP-dependent protein quinases. Cell 23: 135-143.
OSBORN M & WEBER K. 1986. Intermediate filament proteins: a multigene family distinguishing major cell lineages. TIBS 11: 469-472.
OTEY CA, KALNOSKI MH & BULINSKI JC. 1988. Immunolocalization of muscle and nonmuscle isoforms of actin in myogenic cells and skeletal muscle. Cell Mot Cytoskeleton 9: 337-348.
PRUSS RM, MIRSKY R & RAFF MC. 1981. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell 27: 419-428.
QUAX W, VAN DEN BROCK L, VREE EGHERTS W, RAMAEKERS F & BLOEMENDAL H. 1985. Characterization of the hamster desmin gene: expression and formation of desmin filaments in non-muscle cells after gene transfer. Cell 43: 327-328.
RAPPAPORT L, CONTARD F, SAMUEL JL, DELCAYRE C, MAROTTE F, TOME F & FARDEAU M. 1988. Storage of phosphorylated desmin in a familial myopathy. FEBS Lett 231: 421-425.
REGNARD C, AUDEBERT S, BOUCHER D, LARCHER JC, EDDE B & DENOULET P. 1996. Les microtubules: polymorphismes fonctionnels de la tubuline et des protéines associées (MAPs structurales et motrices). Compt Rend Soc Biol 190: 255-268.
RUDIGER M. 1998. Vinculin and alpha-catenin: shared and unique functions in adherens junctions. Bioessays 20: 733-740.
SCHULTHEISS T, LIN Z, ISHIKAWA H, ZAMIR I, STOECKERT CJ & HOLTZER H. 1991. Desmin/vimentin intermediate filaments are dispensable for many aspects of myogenesis. J Cell Biol 114: 953-966.
SINGER SJ, MALHER PA, ROGALSKI AA, KUPPER A & COX GF. 1986. Progress in the study of membrane cytoskeletal associations. In: BENETT V et al. (Ed.); Membrane skeletons and cytoskeletal-membrane associations. Alan R Lins Inc, p. 261-268.
SOMLÓ C, DE SOUZA W, MACHADO RD, HASSÓN-VOLOCH A. 1977. Biochemical and cytochemical localization of ATPases on the membranes of the electrocyte of Electrophorus electricus. Cell Tissue Res 185: 115-128.
TAFFAREL M, DE SOUZA MF, MACHADO RD & DE SOUZA W. 1985. Localization of actin in the electrocyte of Electrophorus electricus L. Cell Tissue Res 242: 453-455.
THORNELL L-E, ERIKSSON A, JOHANSSON B, KJÖRELL U, FRANKE WW, VIRTANEN I & LEHTO V-P. 1985. Intermediate filament and associated proteins in heart Purkinje fibers: a membrane-myofibril anchored cytoskeletal system. Ann N Y Acad Sci 455: 213-239.
TRAUB P. 1985. Intermediate filaments: a review. North Holland: Elsevier.
VALE RD & MILLIGAN RA. 2000. The way things move: looking under the hood of molecular motor proteins. Science 288: 88-95.
VANDEKERCKHOVE J, BUGAISKY G & BUCKINGHAM M. 1986. Simultaneous expression of skeletal muscle and heart actin proteins in various striated muscle tissues and cells. J Biol Chem 261: 1838-1843.
VIEL A. 1999. Alpha-actinin and spectrin structures: an unfolding family story. FEBS Lett 5: 391-394.
- ALBE-FESSARD D & CHAGAS C. 1955. Études par dérivation intracellulaire des effets sommatifs de deux stimulations successives au niveau d'une électroplaque du gymnote. C R Acad Sci (Paris) 239: 1951-1954.
- ALLAN V. 1996. Motor protein: a dynamic duo. Curr Biol 6: 630-633.
- ARAUJO GMN, PEDRENHO AR & HASSÓN-VOLOCH A. 1993. The effect of mercury and aluminum on sodium-potassium-Mg2+ dependent-adenosine triphosphatase activity of Electrophorus electricus (L.) electrocyte. Int J Biochem 25: 1729-1735.
- AYRES SÁ L, MOURA NETO V, OLIVEIRA M M & CHAGAS C. 1991. Heterogeneity of purified actin in the electric organ of the electric eel Electrophorus electricus. J Exp Zoology 257: 43-50.
- BENCHIMOL M, MACHADO RD & SOUZA W. 1979. Staining of microtubules of the electrocyte of Electrophorus electricus L. by alcian blue and lanthanum. Experientia 35: 670-671.
- CARTAUD J, CARTAUD A, KORDELI E, LUDOSKY MA, MARCHAND S & STETZKOWSKI-MARDEN F. 2000. The Torpedo electrocyte: a model to study membrane-cytoskeleton interactions at the post-synaptic membrane. Microsc Res Tech 49: 73-83.
- CASSIMERIS L. 1999. Accessory protein regulation of microtubule dynamics throughout the cell cycle. Curr Opin Cell Biol 11: 134-141.
- CHANG P & STEARNS T. 2000. Gamma-tubulin and epsilon-tubulin: two new human centrosomal tubulins reveal new aspects of centrosome structure and function. Nature Cell Biol 2: 30-35.
- CHANGEUX JP, GAUTRON J, ISRAËL M & PODLESKI T. 1969. Séparation de membranes excitables ŕ partir de l'organe électrique de l' Electrophorus electricus. C R Acad Sci (Paris) 269: 1788-1791.
- CORDEIRO MCR, MOURA NETO V, BENCHIMOL M, FARIA MVC & CHAGAS C. 1995. Microheterogeneity of desmin in the electric organ and dorsal muscle of the Electrophorus electricus. Comp Biochem Physiol 111A: 345-350.
- CORDEIRO MCR, BENCHIMOL M, MERMELSTEIN CS, SÁ LA, FARIA MVC, CHAGAS C & MOURA NETO V. 1996. Desmin filaments in the electrocytes of the electric organ of the Electrophorus electricus. Cell Tissue Res 285: 387-393.
- COSTA ML, OLIVEIRA MM, ALBERTI JR O, NETO VM & CHAGAS C. 1986. Caractérisation de la desmine dans l'organe électrique de l' Electrophorus electricus L. C R Acad Sci (Paris) 303: 547-550.
- COSTA ML, NETO VM & CHAGAS C. 1988. Desmin heterogeneity in the main electric organ of Electrophorus electricus (Linnaeus). Biochimie 70: 783-789.
- COSTA ML, MERMELSTEIN CS, FRÓES MM, CHAGAS C & NETO VM. 1998. Differences in the isodesmin pattern between the electric organs of Electrophorus electricus L. Comp Biochem Physiol 119: 715-719.
- ESQUIBEL MA, ALONSO I, MEYER H, CASTRO GO & CHAGAS C. 1971. Quelques aspects de l'histogenese et de l'ontogenese des organes électriques chez l' Electrophorus electricus L. C R Acad Sci (Paris) 273: 196-199.
- GALOU M, GAO J, HUMBERT J, MERICSKAY M, LI Z, PAULIN D & VICART P. 1997. The importance of intermediate filaments in the adaptation of tissues to mechanical stress: evidence from gene knockout studies. Biol cell 89: 85-97.
- GEISLER N, PLESSMAN U & WEBER K. 1982. Related amino acid sequences in neurofilaments and non-neuronal intermediate filaments. Nature 296: 448-450.
- GOMES F, PAULIN D & MOURA NETO V. 1999. Glial fibrillary acidic protein (GFAP): modulation by growth factors and its implication in astrocyte differentiation. Braz J Med Biol Res 32: 619-631.
- GOTTER AL, KAETZEL MA & DEDMAN JR. 1998. Electrophorus electricus as a model system for the study of membrane excitability. Comp Biochem Physiol 119: 225-241.
- HARTWIG JH & KWIATKOWSKI DJ. 1991. Actin-binding proteins. Curr Opin Cell Biol 3: 87-97.
- HASSÓN-VOLOCH A, SOMLÓ C, NUNES-DE-ARAUJO GM & GOMES-QUINTANA E. 1993. Mg2+-dependent ATPases in the electrocyte of Electrophorus electricus (L.). Brazilian J Med Biol Res 26: 351-354.
- HERRMANN H & AEBI U. 2000. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opin Cell Biol 12: 79-90.
- KEYNES RD & MARTINS-FERREIRA H. 1953. Membrane potentials in the electroplates of the electric eel. J Physiol 119: 315-351.
- KORDELI E, CARTAUD J, NGHIĘM HD, PRADEL LA, DUBREUIL C, PAULIN D & CHANGEUX JP. 1986. Evidence for a polarity in the distribution of proteins from the cytoskeleton in Torpedo marmorata electrocytes. J Biol Chem 102: 748-761.
- LAZARIDES E & BALZER DR. 1978. Specificity of desmin to avian and mammalian muscle cells. Cell 14: 429-438.
- LEĂO FERREIRA LR, MOUSSATCHÉ N & MOURA NETO V. 1994. Rearrangement of intermediate filament network of BHK-21 cells infected with vaccinia virus. Arch Virol 138: 273-285.
- LI H, CHOUDHARY SK, MILNER DJ, MUNIR MI, KUISK, IR & CAPETANAKI Y. 1994. Inhibition of desmin expression blocks myoblast fusion and interferes with the myogenic regulators MyoD and Myogenin. J Cell Biol 124: 827-841.
- LI Z, MERICSKAY M, AGBULUT O, BUTLER-BROWNE G, CARLSSON L, THORNELL L-E, BABINET C & PAULIN, D. 1997. Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. J Cell Biol 139: 129-144.
- LUFT JH. 1957. The histology and cytology of the electric organs of electric eel ( Electrophorus electricus L.). J Morph 100: 113-139.
- MACHADO RD, DE SOUZA W, COTTA PEREIRA G & DE OLIVEIRA G. 1976. On the fine structure of the electrocyte of Electrophorus electricus L. Cell Tissue Res 174: 355-366.
- MACHESKY LM & SCHLIWA M. 2000. Cell dynamics: a new look at the cytoskeleton. Nature Cell Biol 2: E17-E18.
- MATHEWSON R, WACHTEL A & GRUNDFEST H. 1961. Fine structure of electroplaques. In: CHAGAS C & PAES CARVALHO A (Ed.); Bioelectrogenesis Amsterdam: Elsevier Publishing Co., p. 25-53.
- MATSUDARIA P. 1994. Actin crosslinking proteins at the leading edge. Semin Cell Biol 5: 165-174.
- MERMELSTEIN CS, COSTA ML, CHAGAS C & MOURA NETO V. 1996. Intermediate filament proteins in TPA-treated skeletal muscle cells in culture. J Muscle Res Cell Mot 17: 199-296.
- MERMELSTEIN CS, BENCHIMOL M, TAFFAREL M, CORDEIRO MCR, CHAGAS C & MOURA NETO V. 1997. Desmin and actin filaments in membrane-cytoskeletal preparations of the electric tissue of Electrophorus electricus, L. Arch Histol Cytol 60: 445-452.
- MEUNIER JC, SEALOCK R, OLSEN R & CHANGEUX JP. 1974. Purification and properties of the choline receptor protein from Electrophorus electricus electric tissue. Eur J Biochem 45: 371-394.
- MOURA NETO V, MALLAT M, JEANTET C & PROCHIANTZ A. 1983. Microheterogeneity of tubulin proteins in neuronal and glial cells from the mouse brain in culture. EMBO J 2: 1243-1248.
- OAKLEY BR, OAKLEY CE, YOON Y & JUNG MK. 1990. Gamma-tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergilla nidulans. Cell 61: 1289-1301.
- O'CONNOR M, GARD DL & LAZARIDES E. 1981. Phosphorylation of intermediate filament protein by cAMP-dependent protein quinases. Cell 23: 135-143.
- OSBORN M & WEBER K. 1986. Intermediate filament proteins: a multigene family distinguishing major cell lineages. TIBS 11: 469-472.
- OTEY CA, KALNOSKI MH & BULINSKI JC. 1988. Immunolocalization of muscle and nonmuscle isoforms of actin in myogenic cells and skeletal muscle. Cell Mot Cytoskeleton 9: 337-348.
- PRUSS RM, MIRSKY R & RAFF MC. 1981. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell 27: 419-428.
- QUAX W, VAN DEN BROCK L, VREE EGHERTS W, RAMAEKERS F & BLOEMENDAL H. 1985. Characterization of the hamster desmin gene: expression and formation of desmin filaments in non-muscle cells after gene transfer. Cell 43: 327-328.
- RAPPAPORT L, CONTARD F, SAMUEL JL, DELCAYRE C, MAROTTE F, TOME F & FARDEAU M. 1988. Storage of phosphorylated desmin in a familial myopathy. FEBS Lett 231: 421-425.
- REGNARD C, AUDEBERT S, BOUCHER D, LARCHER JC, EDDE B & DENOULET P. 1996. Les microtubules: polymorphismes fonctionnels de la tubuline et des protéines associées (MAPs structurales et motrices). Compt Rend Soc Biol 190: 255-268.
- RUDIGER M. 1998. Vinculin and alpha-catenin: shared and unique functions in adherens junctions. Bioessays 20: 733-740.
- SCHULTHEISS T, LIN Z, ISHIKAWA H, ZAMIR I, STOECKERT CJ & HOLTZER H. 1991. Desmin/vimentin intermediate filaments are dispensable for many aspects of myogenesis. J Cell Biol 114: 953-966.
- SINGER SJ, MALHER PA, ROGALSKI AA, KUPPER A & COX GF. 1986. Progress in the study of membrane cytoskeletal associations. In: BENETT V et al. (Ed.); Membrane skeletons and cytoskeletal-membrane associations Alan R Lins Inc, p. 261-268.
- SOMLÓ C, DE SOUZA W, MACHADO RD, HASSÓN-VOLOCH A. 1977. Biochemical and cytochemical localization of ATPases on the membranes of the electrocyte of Electrophorus electricus. Cell Tissue Res 185: 115-128.
- TAFFAREL M, DE SOUZA MF, MACHADO RD & DE SOUZA W. 1985. Localization of actin in the electrocyte of Electrophorus electricus L. Cell Tissue Res 242: 453-455.
- THORNELL L-E, ERIKSSON A, JOHANSSON B, KJÖRELL U, FRANKE WW, VIRTANEN I & LEHTO V-P. 1985. Intermediate filament and associated proteins in heart Purkinje fibers: a membrane-myofibril anchored cytoskeletal system. Ann N Y Acad Sci 455: 213-239.
- TRAUB P. 1985. Intermediate filaments: a review. North Holland: Elsevier.
- VALE RD & MILLIGAN RA. 2000. The way things move: looking under the hood of molecular motor proteins. Science 288: 88-95.
- VANDEKERCKHOVE J, BUGAISKY G & BUCKINGHAM M. 1986. Simultaneous expression of skeletal muscle and heart actin proteins in various striated muscle tissues and cells. J Biol Chem 261: 1838-1843.
- VIEL A. 1999. Alpha-actinin and spectrin structures: an unfolding family story. FEBS Lett 5: 391-394.
Publication Dates
-
Publication in this collection
05 Oct 2000 -
Date of issue
Sept 2000
History
-
Accepted
09 May 2000 -
Received
03 May 2000