Abstract
Chagas' disease is a debilitating and often fatal disease caused by the protozoan parasite Trypanosoma cruzi. The great majority of surface molecules in trypanosomes are either inositol-containing phospholipids or glycoproteins that are anchored into the plasma membrane by glycosylphosphatidylinositol anchors. The polyalcohol myo-inositol is the precursor for the biosynthesis of these molecules. In this brief review, recent findings on some aspects of the molecular and cellular fate of inositol in T. cruzi life cycle are discussed and identified some points that could be targets for the development of parasite-specific therapeutic agents.
inositol; Trypanosoma cruzi; Chagas' disease; chemotherapy
Inositol Metabolism in Trypanosoma cruzi: Potential Target for Chemotherapy Against Chagas' Disease* Dedicated to the memory of Prof. Carlos Chagas Filho * Invited paper ** Member of the Academia Brasileira de Ciências Correspondence to: Mecia M. Oliveira E-mail: mecia@biof.ufrj.br
MECIA M. OLIVEIRA1 and MARCELO EINICKER-LAMAS1,2
1Instituto de Biofísica Carlos Chagas Filho
2Departamento de Bioquímica Médica, ICB
Universidade Federal do Rio de Janeiro, Ilha do Fundão - 21941-590 Rio de Janeiro, Brasil.
Manuscript received on May 18, 2000; accepted for publication on May 22, 2000;
contributed by MECIA M. OLIVEIRA** Dedicated to the memory of Prof. Carlos Chagas Filho * Invited paper ** Member of the Academia Brasileira de Ciências Correspondence to: Mecia M. Oliveira E-mail: mecia@biof.ufrj.br
ABSTRACT
Chagas' disease is a debilitating and often fatal disease caused by the protozoan parasite Trypanosoma cruzi. The great majority of surface molecules in trypanosomes are either inositol-containing phospholipids or glycoproteins that are anchored into the plasma membrane by glycosylphosphatidylinositol anchors. The polyalcohol myo-inositol is the precursor for the biosynthesis of these molecules. In this brief review, recent findings on some aspects of the molecular and cellular fate of inositol in T. cruzi life cycle are discussed and identified some points that could be targets for the development of parasite-specific therapeutic agents.
Key words: inositol, Trypanosoma cruzi, Chagas' disease, chemotherapy.
INTRODUCTION
CHAGAS' DISEASE
American trypanosomiasis or Chagas'disease, whose etiologic agent is
Trypanosoma cruzi, was discovered by Carlos Chagas who characterized the parasite, its life cycle and its vector as well as the transmission process (Chagas 1909). Chagas disease ranks high in the group of infectious and parasitic diseases in terms of disability-adjusted years of life lost in Latin America. Only acute respiratory infections, diarrhoeal diseases and HIV / AIDS account for a greater burden (Schmunis
et al. 1996). World Health Organization estimates that 15 to 20 million people are infected with
T. cruzi. The disease is acquired by invasive trypomastigotes which are transmitted by insect vectors, blood transfusion, transplacental and organ transplantation (Brener & Gazzinelli 1997).
T. cruzi is now viewed as an emerging human pathogen of HIV-infected individuals, since it induces dramatic brain pathology and faster death in the affected patients (Del Castillo
et al. 1990).
The progression of Chagas'disease even today defies the attempts at efficient and safe chemotherapy. There are no vaccines and the drug presently in use to control the acute stage of the disease shows restrict applicability in chronic patients, besides presenting severe side effects (De Castro 1993). The best hope of finding new therapies rests in the development research programs that explore the differences between the parasite and its host, as it has been done by several research groups (Rodriguez & Gross 1995). This article focuses on segments of the known and suggested differences between Trypanosoma cruzi and mammalian inositol metabolism.
INOSITOL LIPIDS
Inositol, found in animals and plants, has been cited as essential in the growth of animals and microorganisms, including T. cruzi (Eagle et al. 1957, Holub 1982, Einicker-Lamas et al. 1999). Its action rests in the formation of a complex set of inositol-containing lipids, whose parent compound is the phospholipid phosphatidylinositol (PI), which can be further phosphorylated or glycosylated forming new classes of bioactive molecules, as depicted in Figure 1.
The glycosylated inositol derivatives are found in the outer leaflet of the plasma membrane facing the external medium, whereas the phosphorylated phosphatidylinositols are found on the inner leaflet of the lipid bilayer and play an important role on signaling pathways, modulating vital functions of the parasite. Glycosylphosphatidylinositol (GPI) -anchored or GPI-related molecules are present in the cell surface of the trypanosomatids at all stages of their life cycles. The detailed structure and the biosynthesis of the GPI anchors in African trypanosomes led the way for the development of this area of study. The cell coat of Trypanosoma brucei comprises approximately  copies/cell of variant specific glycoproteins anchored to the membrane by a GPI moiety (Ferguson 1999). Although GPI-anchors are present in many membrane proteins of mammalian cells, the biosynthetic pathways and particularities of this anchor in African trypanosomes is now being considered as a therapeutic target for African trypanosomiasis (Ferguson et al. 1999). In T. cruzi, most of the GPI structures are not attached to proteins, but form glycoinositol phospholipids (GIPLs) (Previato et al. 1990, Lederkremer et al. 1991, Carreira et al. 1996). The cell coat of T. cruzi is made up of a layer of GIPLs (
copies/cell of variant specific glycoproteins anchored to the membrane by a GPI moiety (Ferguson 1999). Although GPI-anchors are present in many membrane proteins of mammalian cells, the biosynthetic pathways and particularities of this anchor in African trypanosomes is now being considered as a therapeutic target for African trypanosomiasis (Ferguson et al. 1999). In T. cruzi, most of the GPI structures are not attached to proteins, but form glycoinositol phospholipids (GIPLs) (Previato et al. 1990, Lederkremer et al. 1991, Carreira et al. 1996). The cell coat of T. cruzi is made up of a layer of GIPLs ( copies/cell) and small mucin-like GPI-anchored glycoproteins (
copies/cell) and small mucin-like GPI-anchored glycoproteins ( copies/cell) that project above this layer (Almeida et al. 1994, Previato et al. 1995, Serrano et al. 1995). The mucins from the bloodstream trypomastigote stage of the parasite are extremelly potent inducers of proinflammatory cytokines, and this activity resides in GPI-anchor component. Thus, some parasite GPI-anchors appear to be bioactive and to modulate de host immune system (Brener & Gazzinelli 1997).
copies/cell) that project above this layer (Almeida et al. 1994, Previato et al. 1995, Serrano et al. 1995). The mucins from the bloodstream trypomastigote stage of the parasite are extremelly potent inducers of proinflammatory cytokines, and this activity resides in GPI-anchor component. Thus, some parasite GPI-anchors appear to be bioactive and to modulate de host immune system (Brener & Gazzinelli 1997).
The phosphorylated inositol derivatives in T. cruzi are found in fewer copies per cell than the GIPLs, amounting in approximately 12% of all phospholipids (Antunes & Oliveira 1981, Kaneda et al. 1986). Phosphoinositides in addition to their structural function, play active roles in cell signaling pathways as precursors of the potent second messengers inositol trisphosphate (IP3) and diacylglycerol (DAG) (Nishizuka 1984, Berridge 1984) or directly mediating cell functions through the products of the phosphoinositide-3-OH-kinase (PI-3-K) (Toker & Cantley 1997). These signaling pathways were found to modulate different steps of T. cruzi life cycle.
INOSITOL-LIPIDS SIGNALING PATHWAYS
Activation of IP
3/DAG system by exogenous CaCl
2 in digitonin-permeabilised epimastigotes was observed by Docampo and Pignataro (1991), and it was also reported an alteration of the phosphoinositides metabolism after cholinergic stimulation (Machado de Domenech
et al. 1992). Earlier work from our laboratory suggested a phosphoinositide role in
T. cruzi proliferation (Antunes & Oliveira 1981). Further investigation cleared the role of phosphoinositide signaling pathway, as we have shown that the exposure of
T. cruzi to mitogenic factors in foetal calf serum (FCS) stimulate a PI-specific phospholipase C (PI-PLC) leading to the accumulation of IP
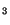
and DAG (Oliveira
et al. 1993). Among all the phospholipids only the phosphatidylinositol(4, 5)
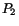
(PIP
) was mobilised by serum, and the PIP
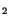
hydrolysis as well as epimastigotes proliferation were hindered by specific PI-PLC inhibitors. The other phosphoinositides had no significant modification, as well as the major phospholipids such as phosphatidylcholine and phosphatidylethanolamine. Lysophosphatidic acid (LPA), reported to be the active agent in some serum effects, had no mitogenic activity when added to epimastigotes cultures. The intracellular signaling downstream of PI-PLC activation was mediated by Ca
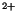
/phospholipid -dependent protein kinase and Ca
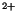
/Calmodulin dependent kinase II (Malaquias & Oliveira 1999).
A subversion of the host cell phosphoinositide signaling pathway was reported. In the invasion of T. cruzi into fibroblasts. Andrews and colleagues observed activation of the PI-PLC leading to IP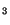 formation, followed by calcium mobilization into the host cell cytosol (Rodriguez et al. 1995). Results from our laboratory showed that a different phosphatidylinositol signaling pathway is activated during the infection of T. cruzi in macrophages, the first step in the naturally occurring Chagas disease. The entry of trypomastigotes strongly stimulated the formation of the lipid products of the PI-3-OH-kinases: phosphatidylinositol-3-phosphate (PI-3-P), phosphatidylinositol-3,4-bisphosphate (PI-3,4-
formation, followed by calcium mobilization into the host cell cytosol (Rodriguez et al. 1995). Results from our laboratory showed that a different phosphatidylinositol signaling pathway is activated during the infection of T. cruzi in macrophages, the first step in the naturally occurring Chagas disease. The entry of trypomastigotes strongly stimulated the formation of the lipid products of the PI-3-OH-kinases: phosphatidylinositol-3-phosphate (PI-3-P), phosphatidylinositol-3,4-bisphosphate (PI-3,4-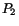 ) and phosphatidylinositol-3,4,5-trisphosphate (PI-3,4,5-
) and phosphatidylinositol-3,4,5-trisphosphate (PI-3,4,5- ), but not the other phosphoinositides. Pre-treatment of the macrophages with the PI-3-K inhibitor, Wortmannin, markedly arrested T. cruzi infection. Using specific antibodies for each of the three classes of PI-3-K, and assaying the immunoprecipitates for enzymatic activity, we found different levels of stimulation in each PI-3-K class, thus suggesting differential roles for these inositol lipids in the process of parasite/host-cell interaction (Todorov et al. 2000). Further studies are necessary to clarify these points.
), but not the other phosphoinositides. Pre-treatment of the macrophages with the PI-3-K inhibitor, Wortmannin, markedly arrested T. cruzi infection. Using specific antibodies for each of the three classes of PI-3-K, and assaying the immunoprecipitates for enzymatic activity, we found different levels of stimulation in each PI-3-K class, thus suggesting differential roles for these inositol lipids in the process of parasite/host-cell interaction (Todorov et al. 2000). Further studies are necessary to clarify these points.
FLUORINATED INOSITOL ANALOGUES
To further evaluate the functional responses coupled to the inositol metabolism in
T. cruzi, we have used the six isomers of
myo-inositol in which a single hydroxyl group was replaced by a fluorine and evaluated their role on
T. cruzi cell biology (Einicker-Lamas
et al. 1999). The inclusion of fluorine in enzyme substrates has been recognised as a useful technique for the generation of enzyme inhibitors. Fluorinated analogues of
myo-inositol were used as potential inhibitors of the phosphoinositide metabolism in different cells (Moyer
et al. 1988, Kozikowski
et al. 1990, McPhee
et al. 1991, Offer
et al. 1993, Cosulich
et al. 1993). The rationale was that these monodeoxyfluoro-
myo-inositols (nFIns) might be taken up into cells by the same uptake process as
myo-inositol, and either be incorporated into phosphoinositides or prevent their formation, depending on the position of the fluorine substituent. We found differences between
T. cruzi and mammalian systems in response to the fluorinated inositols. The inositol transport system in
T. cruzi has different features than the one operating in thymocytes (Offer
et al. 1993). All the
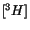
-labeled fluoroinositols were taken up by thymocytes whereas in epimastigotes only the analogues with fluor in 1, 2 and 4 position on the inositol ring, entered the parasite and were weak substrates for the PI synthases. The
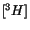
-3Fins,
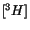
-5Fins and
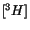
-6Fins were not internalised, indicating that they were not recognized by the parasite inositol transport system. This was further confirmed in competitive assays for the uptake of
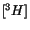
-inositol and its incorporation into phosphoinositides (Einicker-Lamas
et al. 1999).
One major difference between the parasite and the mammalian pathways was the action of the 6-deoxy-6-fluoro-inositol, that although fully permeable to fibroblasts was innocuos to these cells, while having the strongest inhibitory effect on amastigotes and epimastigotes proliferation (Einicker-Lamas et al. 1999). The other cell-impermeable 3Fins and 5Fins analogues also hampered T. cruzi cell division, as they did in fibroblasts (Cosulich et al. 1993). The inhibitory mechanism displayed by the 3Fins and 5Fins were different in the parasite and in fibroblasts, where they interfered directly with the phosphoinositide metabolism (Cosulich et al. 1993).
The selective action of these inositol analogues between T. cruzi and mammalian system opens a line of research leading to a chemical formulation of a parasite specific inhibitor with therapeutic potential.
INOSITOL TRANSPORT IN TRYPANOSOMA CRUZI
Adaptation of protozoan parasites to hostile environments within their vectors and hosts depends on their ability to maintain intracellular homeostasis of ions and nutrients. The level of expression and function of membrane transporters is therefore critical for parasite survival inside their hosts. Transport processes through the parasite plasma membrane are potential targets for new chemotherapeutic drugs.
The results with fluoro-inositols led us to investigate the inositol transport system in T. cruzi. Inositol can be synthesized from glucose, as depicted in Figure 1, but most cells possess a specific transport system for the uptake of inositol from the medium. In T. cruzi it is not known whether the biosynthesis of inositol is occurring, but its absence from the culture medium impairs epimastigotes growth (Einicker-Lamas et al. 1999). Inositol transport in T. cruzi is an active transport process, as it is almost completed ablated by inhibitors of the energy metabolism. Also there is a partial dependence on extracellular sodium for this transport, implying the operation of a Na  /inositol symport (Einicker-Lamas et al. 2000). In view of the Na
/inositol symport (Einicker-Lamas et al. 2000). In view of the Na  -requirement for the inositol transport we investigated the Na
-requirement for the inositol transport we investigated the Na  homeostasis in T. cruzi. We found two different Na
homeostasis in T. cruzi. We found two different Na  pumps in the parasite: a classical ouabain-sensitive (Na
pumps in the parasite: a classical ouabain-sensitive (Na  +K
+K  )-ATPase (Caruso-Neves et al. 1998) and a Na
)-ATPase (Caruso-Neves et al. 1998) and a Na  -ATPase (ouabain-insensitive) furosemide-inhibitable (Caruso-Neves et al. 1999). The inhibition of the inositol transport by furosemide, but not by ouabain suggested that the Na
-ATPase (ouabain-insensitive) furosemide-inhibitable (Caruso-Neves et al. 1999). The inhibition of the inositol transport by furosemide, but not by ouabain suggested that the Na  -ATPase may have a more important role in the creation and maintenance of the Na
-ATPase may have a more important role in the creation and maintenance of the Na  gradient across the T. cruzi cell membrane, thus enabling an Na
gradient across the T. cruzi cell membrane, thus enabling an Na  /inositol symport.
/inositol symport.
The characteristics of inositol transport system in T. cruzi are different from the ones found in the closely related trypanosomatid Leishmania donovani, as this organism features an  /inositol co-transport (Drew et al. 1995) and from the well studied trypanosome glucose carriers (Barrett et al. 1998). The inositol transported into epimastigotes is used for phosphoinositides and inositol-phosphates synthesis. These are not products of the PI-PLC action since we found inositol-phosphates with different degrees of phosphorylation (Einicker-Lamas et al. 1999, 2000). The function of these inositol-polyphosphates in T. cruzi cell biology is unknown at present, and further studies are necessary to elucidate this point. It is possible that the T.cruzi inositol carrier may represent either a chemoterapeutic target or a gateway, which allow the targeting of other toxic molecules to these parasites.
/inositol co-transport (Drew et al. 1995) and from the well studied trypanosome glucose carriers (Barrett et al. 1998). The inositol transported into epimastigotes is used for phosphoinositides and inositol-phosphates synthesis. These are not products of the PI-PLC action since we found inositol-phosphates with different degrees of phosphorylation (Einicker-Lamas et al. 1999, 2000). The function of these inositol-polyphosphates in T. cruzi cell biology is unknown at present, and further studies are necessary to elucidate this point. It is possible that the T.cruzi inositol carrier may represent either a chemoterapeutic target or a gateway, which allow the targeting of other toxic molecules to these parasites.
PERSPECTIVES
Some progress has been made on the biochemistry of the inositol metabolism in
T. cruzi. Despite the overall conservation of the GPI anchors and the phosphoinositide signaling pathways, significant differences in the specificities of trypanosome and mammalian inositol metabolism have been demonstrated. The effects of 6-deoxy-6-fluoro-inositol constitute a promising research avenue to the design of specific drugs anti-
T. cruzi. The biosynthetic routes involving inositol and principally the inositol transporter as the primary target, could be specific and potential therapeutic points to be explored in order to develop new drugs against Chagas disease. The clonning of
T. cruzi inositol transport genes and structural information on this protein remain important goals for the future.
ACKNOWLEDGMENTS
The authors cited work was supported by CNPq, FINEP, FAPERJ, FUJB-UFRJ and a donation from GlaxoWellcome.
- REFERENCES ALMEIDA IC, FERGUSON MAJ, SCHENKMAN S & TRAVASSOS LR. 1994. Lytic anti a-galactosyl antibodies from patients with chronic Chagas' disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem J 304: 793-802.
- ANTUNES A & OLIVEIRA MM. 1981. Phospholipid metabolism in Trypanosoma cruzi-I. phosphate moiety turnover. Comp Biochem Physiol 70: 327-330.
- BARRETT MP, TETAUD E, SEYFANG A, BRINGAUD F & BALTZ T. 1998. Trypanosome glucose transporters. Mol Biochem Parasitol 91: 195-205.
- BERRIDGE MJ. 1984. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J 220: 345-360.
- BRENER Z & GAZZINELLI RT. 1997. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas'disease. Int Arch Allergy Immunol 114: 103-110.
- CARREIRA JC, JONES C, WAIT R, PREVIATO JO & MENDONÇA-PREVIATO L. 1996. Structural variations in the glycoinositolphospholipids of different strains of Trypanosoma cruzi. Glycoconj J 13: 955-966.
- CARUSO-NEVES C, EINICKER-LAMAS M, CHAGAS C, OLIVEIRA MM, VIEYRA A & LOPES AG. 1998. Trypanosoma cruzi epimastigotes express the ouabain- and vanadate-sensitive (Na+ + K+)ATPase activity. Z Naturf 53C: 1049-1054.
- CARUSO-NEVES C, EINICKER-LAMAS M, CHAGAS C, OLIVEIRA MM, VIEYRA A & LOPES AG. 1999. Ouabain-insensitive Na+-ATPase activity in Trypanosoma cruzi epimastigotes. Z Naturf 54C: 100-104.
- CHAGAS, C. 1909. Nova tripanosomíase humana. Estudos sobre a morfologia e o ciclo evolutivo de Schizotrypanum cruzi n. gen., n. sp., agente etiológico de nova entidade mórbida do homem. Mem Inst Oswaldo Cruz 1: 159-219.
- COSULICH SC, OFFER JL, SMITH GA, HESKETH R & METCALFE JC. 1993. Effects of fluorinated inositols on the proliferation of Swiss 3T3 fibroblasts. Biochem J 292: 719-724.
- DE CASTRO SL. 1993. The challenge of Chagas Disease chemotherapy: an update of drugs assayed against Trypanosoma cruzi. Acta Trop 53: 83-98.
- DEL CASTILLO M, MENDOZA G, OVIEDO J, PEREZ BIANCO RP, ANSELMO AE & SILVA M. 1990. AIDS and Chagas' disease with central nervous system tumor-like lesion. Am J Med 88(6): 693-694.
- DOCAMPO R & PIGNATARO OP. 1991. The inositol phosphate/diacylglycerol signalling pathway in Trypanosoma cruzi. Biochem J 194: 2-7.
- DREW ME, LANGFORD CK, KLAMO EM, RUSSEL DG, KAVANAUGH MP & LANDFEAR SM. 1995. Functional expression of a myo-inositol/H+ symporter from Leishmania donovani. Mol Cell Biol 15: 5508-5515.
- EAGLE H, OYAMA VI, LEVY M & FREEMAN AE. 1957. myo-inositol as an essential growth factor for normal and malignant human cells in tissue culture. J Biol Chem 248: 191-205.
- EINICKER-LAMAS M, MALAQUIAS AT, DE CASTRO SL, SMITH GA & OLIVEIRA MM. 1999. Effects of fluorinated inositols on the phosphoinositides metabolism and the proliferation of Trypanosoma cruzi. Parasitol Res 85: 232-238.
- EINICKER-LAMAS M, ALMEIDA AC, TODOROV AG, DE CASTRO SL, CARUSO-NEVES C & OLIVEIRA MM. 2000. Characterization of myo-inositol transport system in Trypanosoma cruzi. Eur J Biochem 267: 1-6.
- FERGUSON MAJ. 1999. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Science 112: 2799-2809.
- FERGUSON MAJ, BRIMACOMBE JS, BROWN JS, CROSSMAN A, DIX A, FIELD RA, GUTHER MLS, MILNE KG, SHARMA DK & SMITH TK. 1999. The GPI biosynthetic pathway as a therapeutic target for African sleeping sickness. Biochim Biophys Acta 1455: 327-340.
- HOLUB BJ. 1982. Advances in nutritional research. In: DRAPER HH ed., p.107, Plenum Press NY.
- KANEDA Y, NAGAKURA K & GOUTSU T. 1986. Lipid composition of three morphological stages of Trypanosoma cruzi. Comp Biochem Physiol 83B: 533-536.
- KOZIKOWSKI AP, FAUQ AH, POWIS G & MELDER DC. 1990. Efficient synthetic routes to fluorinated isosteres of inositol and their effects on cellular growth. J Am Chem Soc 112: 4528-4531.
- LEDERKREMER RM, LIMA C, RAMIREZ ML, FERGUSON MAJ, HOMANS SW & THOMAS-OATES JE. 1991. Complete structure of the glycan of lipopeptidophosphoglycan from Trypanosoma cruzi epimastigotes. J Biol Chem 266: 23670-23675.
- MACHADO DE DOMENECH EE, GARCIA M, GARRIDO MN & RACAGNI G. 1992. Phospholipids of Trypanosoma cruzi: increase of polyphosphoinositides and phosphatidic acid after cholinergic stimulation. FEMS Microbiol Lett 74: 267-270.
- MALAQUIAS AT & OLIVEIRA MM. 1999. Phospholipid signalling in Trypanosoma cruzi growth control. Acta Trop 73: 93-108.
- MCPHEE F, DOWNES CP & LOWE G. 1991. Studies of inositol analogues as inhibitors of the phosphoinositide pathway, and incorporation of 2-deoxy-2-fluoro- myo-inositol to give analogues of phosphatidylinositol intermediates. Biochem J 277: 407-412.
- MOYER JD, REIZES O, AHIR S, JIANG C, MALINOWISKY N & BAKER DC. 1988. Substrate properties of analogs of myo-inositol. Mol Pharmacol 33: 683-689.
- NISHIZUKA Y. 1984. Turnover of inositol phospholipids and signal transduction. Science 225: 1365-1370.
- OFFER J, METCALFE JC & SMITH GA. 1993. The uptake of 3H-labelled monodeoxyfluoro- myo-inositols into thymocytes and their incorporation into phospholipid in permeabilized cells. Biochem J 291: 553-560.
- OLIVEIRA MM, ROCHA ED, RONDINELLI E, ARNHOLDT AV & SCHARFSTEIN J. 1993. Signal transduction in Trypanosoma cruzi: opposite effects of adenylcyclase and phospholipase C systems in growth control. Mol Cell Biochem 124: 91-99.
- PREVIATO JO, GORIN PAJ, MAZUREK MT, FOURNET B, WIERUSZESK JM & MENDONÇA-PREVIATO L. 1990. Primary structure of the oligosaccharide chain of lipopeptidophosphoglycan of epimastigote forms of Trypanosoma cruzi. J Biol Chem 265: 2528-2526.
- PREVIATO JO, JONES C, XAVIER MT, WAIT R, TRAVASSOS LR & MENDONÇA-PREVIATO L. 1995. Structural characterisation of the major glycosylphosphatidylinositol membrane anchored glycoprotein from epimastigote forms of Trypanosoma cruzi. J Biol Chem 270: 7241-7250.
- RODRIGUEZ A, RIOULT MG, ORA A & ANDREWS NW. 1995. A trypanosome-soluble factor induces IP3 formation, intracellular Ca2+ mobilization and microfilament rearrangement in host cells. J Cell Biol 129: 1263-1273.
- RODRIGUEZ JB & GROSS EG. 1995. Recent developments in the control of Trypanosoma cruzi, the causative agent for Chagas' disease. Curr Med Chem 2: 723-742.
- SCHMUNIS GA, ZICKER F & MONCAYO A. 1996. Interruption of Chagas disease transmission through vector elimination. The Lancet 348: 1171.
- SERRANO AA, SCHENKMAN S, YOSHIDA N, MEHLERT A, RICHARDSON JM & FERGUSON MAJ. 1995. The lipid structure of the GPI-anchored mucin-like sialic acid acceptors of Trypanosoma cruzi changes during parasite differentiation from epimastigotes to infective metacyclic trypomastigote forms. J Biol Chem 270: 27244-27253.
- TODOROV AG, EINICKER-LAMAS M, DE CASTRO SL, OLIVEIRA MM & GUILHERME AL. 2000. Activation of host cell class I p85/p110 and class II C2a phosphatidylinositol-3-kinase by Trypanosoma cruzi infection. J Biol Chem in press.
- TOKER A & CANTLEY LC. 1997. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 387: 673-676.
Publication Dates
-
Publication in this collection
05 Oct 2000 -
Date of issue
Sept 2000
History
-
Accepted
22 May 2000 -
Received
18 May 2000


