Abstracts
In this work, we report evidences that the association of phosphofructokinase and F-actin can be affected by insulin stimulation in rabbit skeletal muscle homogenates and that this association can be a mechanism of phos-phofructokinase regulation. Through co-sedimentation techniques, we observed that on insulin-stimulated tissues, approximately 70% of phosphofructokinase activity is co-located in an actin-enriched fraction, against 28% in control. This phenomenon is accompanied by a 100% increase in specific phosphofructokinase activity in stimulated homogenates. Purified F-actin causes an increase of 230% in phosphofructokinase activity and alters its kinetic parameters. The presence of F-actin increases the affinity of phosphofructokinase for fructose 6-phosphate nevertheless, with no changes in maximum velocity (Vmax). Here we propose that the modulation of cellular distribution of phosphofructokinase may be one of the mechanisms of control of glycolytic flux in mammalian muscle by insulin.
phosphofructokinase; hormone; rabbit; actin; metabolism; glycolysis
Neste trabalho, nós relatamos evidencias de que a associação entre fosfofrutocinase e F-actina pode ser afetada por estímulos pela insulina em homogeneizados de músculo esquelético de coelho e que esta associação pode ser um mecanismo de regulação da fosfofrutocinase. Através de tecnicas de co-sedimentação, nós observamos que em tecidos estimulados pela insulina, aproximadamente 70% da atividade fosfofrutocinásica esta co-localizada a fração enriquecida com actina, contra 28% no controle. Este fenômeno é acompanhado por um aumento de 100% na atividade específica da fosfofrutocinase em homogeneizados estimulados. F-actina purificada causou um aumento de 230% na atividade da fosfofrutocinase e alterou seus parâmetros cinéticos. A presença de F-actina aumentou a afinidade da fosfofrutocinase pela frutose 6-fosfato, sem alterar, no entanto, a velocidade máxima (Vmax). Nós propomos que a modulação da distribuição celular da fosfofrutocinase pode ser um dos mecanismos de controle do fluxo glicolítico em músculos de mamíferos pela insulina.
fosfofrutocinase; hormônio; coelho; actina; metabolismo; glicólise
BIOLOGICAL SCIENCES
Effects of insulin and actin on phosphofructokinase activity and cellular distribution in skeletal muscle
Ana Paula P. SilvaI, II; Gutemberg G. AlvesI, III; Alexandre H.B. AraújoII; Mauro Sola-PennaI
ILaboratório de Enzimologia e Controle do Metabolismo (LabECoM), Departamento de Fármacos, Faculdade de Farmácia, Universidade Federal do Rio de Janeiro, 21941-590 Rio de Janeiro, Brasil
IIDepartamento de Nutrição Animal, Instituto de Zootecnia, Universidade Federal Rural do Rio de Janeiro, BR-465, Km 7, 23851-000 Seropédica, RJ, Brasil
IIIDepartamento de Bioquímica Médica, Instituto de Ciências Biomédicas, Universidade Federal do Rio de Janeiro, 21941-590 Rio de Janeiro, Brasil
Correspondence Correspondence to Mauro Sola-Penna E-mail: maurosp@ufrj.br
ABSTRACT
In this work, we report evidences that the association of phosphofructokinase and F-actin can be affected by insulin stimulation in rabbit skeletal muscle homogenates and that this association can be a mechanism of phos-phofructokinase regulation. Through co-sedimentation techniques, we observed that on insulin-stimulated tissues, approximately 70% of phosphofructokinase activity is co-located in an actin-enriched fraction, against 28% in control. This phenomenon is accompanied by a 100% increase in specific phosphofructokinase activity in stimulated homogenates. Purified F-actin causes an increase of 230% in phosphofructokinase activity and alters its kinetic parameters. The presence of F-actin increases the affinity of phosphofructokinase for fructose 6-phosphate nevertheless, with no changes in maximum velocity (Vmax). Here we propose that the modulation of cellular distribution of phosphofructokinase may be one of the mechanisms of control of glycolytic flux in mammalian muscle by insulin.
Key words: phosphofructokinase, hormone, rabbit, actin, metabolism, glycolysis.
RESUMO
Neste trabalho, nós relatamos evidencias de que a associação entre fosfofrutocinase e F-actina pode ser afetada por estímulos pela insulina em homogeneizados de músculo esquelético de coelho e que esta associação pode ser um mecanismo de regulação da fosfofrutocinase. Através de tecnicas de co-sedimentação, nós observamos que em tecidos estimulados pela insulina, aproximadamente 70% da atividade fosfofrutocinásica esta co-localizada a fração enriquecida com actina, contra 28% no controle. Este fenômeno é acompanhado por um aumento de 100% na atividade específica da fosfofrutocinase em homogeneizados estimulados. F-actina purificada causou um aumento de 230% na atividade da fosfofrutocinase e alterou seus parâmetros cinéticos. A presença de F-actina aumentou a afinidade da fosfofrutocinase pela frutose 6-fosfato, sem alterar, no entanto, a velocidade máxima (Vmax). Nós propomos que a modulação da distribuição celular da fosfofrutocinase pode ser um dos mecanismos de controle do fluxo glicolítico em músculos de mamíferos pela insulina.
Palavras-chave: fosfofrutocinase, hormônio, coelho, actina, metabolismo, glicólise.
INTRODUCTION
Glycolysis is, in most organisms, the main pathway of carbohydrate metabolism (Fruton 1972, Philips et al. 1981, Stanley and Connett 1991). Phosphofructokinase (PFK; ATP:D-fructose-6-phosphate 1-phosphotransferase, EC 2.7.1.11) which catalyzes the phosphorylation of fructose-6-phosphate to fructose 1,6-bisphosphate by Mg-ATP is a key enzyme on the regulation of this pathway. As such, this enzyme is subject to tight regulation by several effectors and hormones, allowing glycolytic flux control according to cellular needs for energy or glycolytic intermediaries (Uyeda 1979, Paetkau and Lardy 1967, Schirmer and Evans 1990).
Several studies in the distribution and cellular localization of muscle PFK have shown that this enzyme associates with high affinity to filamentous actin (F-actin), the major constituent of thin filaments in skeletal muscle (Arnold and Pette 1968, Arnold et al. 1971, Clarke and Masters, 1975). It was also shown that its kinetics properties may be influenced by this association (Liou and Anderson 1980, Andrés et al. 1996). Possibly this process represents an important mechanism of glycolytic flux control, since associated enzymes are known to be more active than their soluble (non-associated) form (Liou and Anderson 1980, Roberts and Somero 1987, Andrés et al. 1996). Hormones such as adrenaline and insulin may be involved in the induction of a rapid association of PFK with cellular structure (Chen-Zion et al. 1992, Ashkenazy-Shahar et al. 1998, Alves and Sola-Penna 2003). In muscle, insulin leads to a series of effects that includes an increase in the rate of glucose transport into the cell and activation of enzymes that participates in glucose metabolism, although the specific mechanisms involved in this process are not completely understood (Newsholme and Dimitriadis 2001).
In this work we aim to elucidate insulin participation in the regulation of rabbit skeletal muscle PFK through the study of the action of this hormone on the association of PFK and F-actin, and the effects of this association on PFK activity.
MATERIALS AND METHODS
Rabbit skeletal muscle homogenate: Rabbits killed by cervical dislocation were bled by cutting the blood vessels of the neck and the muscles of hind legs and back were quickly removed and cleaned to remove fat and connective tissue. 1 ml of 0.01 U × mlinsulin or 0.9% NaCl (saline) as control was injected into muscle slices weighting 10g. After 15 minutes at room temperature, each portion was homogenized for 30s in Polytron (Brinkmann Instruments, Westbury, NY, USA) in the presence of 30 ml of a solution containing 50 mM Tris, 30 mM NaF, 4 mM EDTA and 1 mM dithiothreitol, pH 7.5 (homogenizing buffer). Homogenized tissues were centrifuged for 5 min at 100g, 4°C, for separation of cellular debris. The resultant supernatant, called total homogenate (TH), represents total enzyme activity.
Tissue fractionation: Tissue fractionation was performed after a modification of the protocol proposed by Lilling and Beitner (1990), based on actin-bound enzyme extraction protocols (Kuo et al. 1986, Luther and Lee 1986). Total homogenate (TH) was centrifuged for 15 min at 27,000g (4°C). The pellet was resuspended in 200ml of homogenizing buffer, and called P1. The supernatant (S1) was centrifuged for 30 min at 110,000g (4°C). The supernatant (S2) was collected and the pellet resuspended in 100ml of homogenizing buffer (P2). This pellet was previously characterized as an actin-enriched fraction (Alves and Sola-Penna 2003, El-Bacha et al. 2003). All fractions were assayed for PFK activity and protein concentration. Protein quantifications were performed as described by Lowry et al. (1951).
Rabbit skeletal muscle phosphofructokinase purification: This purification was realized according to the method developed by Kemp (1975), with modifications introduced by Kuo et al. (1986).
Chicken breast muscle F-actin purification and polimerization: F-actin was prepared according to the protocol proposed by Pardee and Spudich (1982). Before the experiments F-actin was clarified according to these authors.
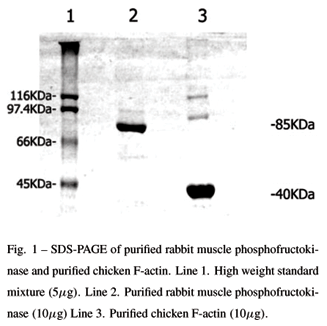
Gel electrophoresis: PFK purified from rabbit skeletal muscle and F-actin purified from chicken breast muscle samples were run on 10% SDS-PAGE gels (Figure 1) realized according Laemmli (1970), using modifications introduced by Giulian et al.(1983). The results were compared with a high molecular weight standard mixture (Figure 1, line 1). Linear regression of the values corresponding to the eletrophoretic distance of the PFK sample revealed a molecular weight of approximately 85 KDa (Figure 1, line 2), which is in accordance with the results of Uyeda (1979) for PFK muscle monomer. Analysis of F-actin band shows a molecular weigth of approximately 40 KDa (Figure 1, line 3).
Radioassay for phosphofructokinase activity: Enzyme activity was measured according to Sola-Penna et al. (2002). The assays were performed at 37°C in 0.4 ml of reaction medium, unless indicated, contained 50 mM Tris-HCl (pH 7.4), 5 mM (NH)SO, 5 mM MgCl, 1 mM [g-32P] ATP (4mCi × mmol-1), 1 mM fructose 6-phosphate and purified PFK (10mg × ml-1) or muscle homogenate (50mg × ml-1). Duplicates were performed for all assays and blanks were obtained in the absence of fructose 6-phosphate. We defined one enzymatic unit (U) as the production of 1 nmol fructose 1,6-bisphosphate per minute.
Statistics: Kinetic parameters for PFK activation in the presence of fructose 6-phosphate were obtained with a non linear regression using Sigma Plot 2001 (Jandell Scientific, USA) adjusting the equation parameters: V = (Vmax × Sn) (Km + Sn) to the experimental results, where Vmax: maximal catalytic velocity, S: fructose 6-phosphate concentration, Km: Michaelis-Menten constant, n: cooperativity index and K0.5 = nKm.
Unpaired Student's t test was realized, considering significant values of P < 0.05.
Material: ATP, fructose-6-phosphate, and high molecular weight standard mixture were purchased from Sigma Chemical (St. Louis, MO, USA). Pi was purchased from Instituto de Pesquisas Energéticas e Nucleares (São Paulo, SP, Brazil). [g-32P] ATP was prepared according to Maia et al. (1983). Other reagents were of the highest purity available.
RESULTS AND DISCUSSION
Activation of phosphofuctokinase on skeletal muscle homogenates induced by insulin: PFK is an important site for hormonal regulation of carbohydrate metabolism on skeletal muscle (Bertrand et al. 1999, Hue et al. 2002). Several works have suggested that, aside from the classical allosteric regulation of PFK by effectors such as fructose-2,6 bisphosphate, the intracellular distribution of this enzyme could also play a role in the rapid cell response external stimuli such as hormonal signaling, including adrenaline and insulin (Lilling and Beitner 1990, Chen-Zion et al. 1992, Alves and Sola-Penna 2003, El-Bacha et al. 2003).
The effects of insulin on PFK activity and the possible association of this enzyme with cellular structure were investigated. Treatment of rabbit skeletal muscle with insulin results in a two fold increase of total homogenate PFK activity (Figure 2). Although these stimulatory effects have already been described (Beitner and Kalant 1971), the precise mechanisms involved are not yet completely understood on skeletal muscle.
Insulin effects on intracellular distribution of phosphofructokinase activity from muscle homogenates: Analysis of PFK activity among subcellular fractions revealed that insulin promoted a significant change in the distribution pattern of this activity (Figure 3). Whereas in control samples much of the activity recovered after high-speed centrifugation of S1 is found in S2 (50%), this values were significantly decreased on insulin stimulated samples (30%). Additionally, insulin promoted a 2.5-fold increase in PFK activity in the actin-enriched fraction (P2), 28% against 70% respectively. These phenomena of decrease of PFK activity in fraction S2 and increase of PFK activity of fraction P2 strongly suggests that insulin stimulates the association of PFK and F-actin. We have previously characterized that fraction P2 is enriched in F-actin and that the increase of PFK activity in this fraction is due to the association of these two proteins (Alves and Sola-Penna 2003). Curiously, we also found that fraction P1 increased on insulin-treated samples, as previously described by other authors (Chen-Zion et al. 1992, Ashkenazy-Shahar et al. 1998), but in a much smaller degree as that observed in P2.
Activation of phosphofructokinase induced by F-actin: Since PFK activity seems to increase on an actin-enriched fraction by insulin stimulation, we decided to investigate the effects of F-actin on this activity. Purified enzyme was incubated with different concentrations of F-actin, as described in material and methods. Figure 4 shows that the activation promoted by F-actin depends on ATP and F-actin concentration. Maximal activation of PFK was obtained with 0.07 mg.ml F-actin, achieving an increase of about 230% of control activity. Higher concentrations of F-actin, however, induced no alteration on enzyme activity.
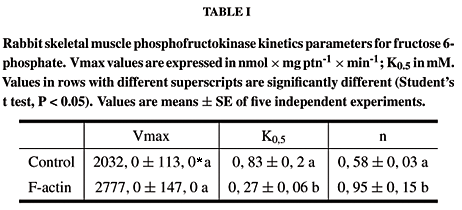
The dependence of PFK activity for its substrate fructose 6-phosphate is shown in Figure 5. Incubation with F-actin induced changes in the kinetic parameters as presented in Table I. The affinity of PFK for fructose 6-phosphate was increased in the presence of 0.05 mg × ml-1 of F-actin, as observed by the decreased K0.5 of the enzyme, nevertheless, with no changes in maximum velocity (Vmax). Cooperativity index (n) was higher in the presence of F-actin, revealing a decrease in negative cooperativity between enzyme substrate sites. The activation of PFK induced by incubation with F-actin seems to depend on the concentration of ATP (Figure 4). 0.5 mM ATP induced no change in enzyme activity, despite of actin concentration. However, when 2 mM ATP was utilized, there was a significant increase in PFK activity depending on F-actin concentration. Since this enzyme-protein interaction alters the K0.5 for fructose-6-phosphate, this dependence on ATP may be related with the capacity of ATP to affect this same parameter, especially at higher concentrations (Kemp and Foe 1983). Curiously, a similar dependence for ATP was described previously for the stimulatory action of insulin on muscle PFK (Livnat et al. 1993).
Our results supply the idea that association of PFK with F-actin can be one of the results of hormone signaling on skeletal muscle. In the case of insulin, inducing a change on intracellular distribution of PFK may increase glycolytic flux, and therefore, glucose uptake. The physiological role of this activation must be in accordance with individual nutritional state. In prolonged starvation, with the decrease on serum insulin, the major proportion of the enzyme would be expected in the cytosolic phase, with a probable decrease on enzyme activity. In accordance with this are the observations of Lowery et al. 1987, which described a decrease of associated enzyme on fish skeletal muscle in prolonged starvation, in a situation that could be reversed after re-feeding. Although the physiological relevance of these rapid changes is not determined, our data suggests that in addition to the raise on allosteric regulation, the modulation of PFK intracellular distribution could be relevant in skeletal muscle, as this tissue plays a significant role on glucose uptake at post-prandial state (Newsholme and Dimitriadis 2001). The mechanism of insulin-induced association also remains to be determined; however, as it was shown that insulin receptor kinase is able to directly phosphorylate PFK (Sale et al. 1987) and a phosphorylated form of this enzyme was previously described as preferentially associated to F-actin (Luther and Lee 1986), insulin-induced phosphorylation may represent a prominent target for future studies on PFK/F-actin association.
ACKNOWLEDGMENTS
We thank Dr. Tatiana El-Bacha for the critical reading of the manuscript. This project was supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Programa de Apoio a Núcleos de Excelência (Pronex) and Fundação Universitária José Bonifácio.
Manuscript received on October 8, 2003; accepted for publication on May 26, 2004;
presented by LUCIA MENDONçA PREVIATO
- ALVES GG AND SOLA-PENNA M. 2003. Epinephrine modulates cellular distribution of muscle phosphofructokinase. Mol Gen Met 78: 302-306.
- ANDRÉS V, CARRERAS J AND CUSSO R. 1996. Myofibril-bound muscle phosphofructokinase is less sensitive to inhibition by ATP than the free enzyme, but retains its sensitivity to stimulation by bisphosphorylated hexoses. Int J Biochem 28: 1179-1184.
- ARNOLD H AND PETTE D. 1968. Binding of glycolytic enzymes to structure proteins of the muscle. Eur J Biochem 6: 163-177.
- ARNOLD H, HENNING R AND PETTE D. 1971. Quantitative comparision of the binding of various glycolytic enzimes to F-actin and the interaction of aldolase with G-actin. Eur J Biochem 22: 121-126.
- ASHKENAZY-SHAHAR M, BEN-PORAT H AND BEITNER R. 1998. Insulin stimulates binding of phosphofructokinase to cytoskeleton and increases glucose 1,6-bisphosphate levels in NIH-3T3 fibroblasts, which is prevented by calmodulin antagonists. Mol Gen Met 65: 213-219.
- BEITNER R AND KALANT N. 1971. Stimulation of glycolysis by insulin. J Biol Chem 246: 500-503.
- BERTRAND L, ALESSI DR, DEPREZ J, DEAK M, VIAENE E, RIDER MH AND HUE L. 1999. Heart 6-phosphofructo-2-kinase activation by insulin results from Ser-466 and Ser-483 phosphorylation and requires 3-phosphoinositide-dependent kinase-1, but not protein kinase B. J Biol Chem 274: 30927-30933.
- CHEN-ZION M, BASSUKEVITZ Y AND BEITNER R. 1992. Sequence of insulin effects on cytoskeletal and cytosolic phosphofructokinase, mitochondrial hexokinase, glucose 1,6-bisphosphate and fructose 2,6-bisphosphate levels, and the antagonistic action of calmodulin inhibitors, in diaphragm muscle. Int J Biochem 24: 1661-1667.
- CLARKE FM AND MASTERS CJ. 1975. On the association of glycolytic enzymes with structural proteins of skeletal muscle. Biochim Biophys Acta 381: 37-46.
- EL-BACHA T, DE FREITAS MS AND SOLA-PENNA M. 2003. Cellular distribution of phosphofructokinase activity and implications to metabolic regulation in human breast cancer. Mol Gen Met 79: 294-299.
- FRUTON JS. 1972. Molecules and Life: Historical Essays on the Interplay of Chemistry and Biology. Wiley, New York.
- GIULIAN GG, MOSS RL AND GREASER M. 1983. Improved method for analysis and quantization of proteins on one-dimensional silver-stained slab gels. Anal Biochem 129: 277-287.
- HUE L, BEAULOYE C, MARSIN AS, BERTRAND L, HORMAN S AND RIDER MH. 2002. Insulin and ischemia stimulate glycolysis by acting on the same targets through different and opposing signaling pathways. J Mol Cell Cardiol 34: 1091-1097.
- KEMP RG. 1975. Rabbit muscle phosphofructokinase. Methods Enzymol 42: 71-77.
- KEMP RG AND FOE LG. 1983. Allosteric regulatory properties of muscle phosphofructokinase. Mol Cel Biochem 57: 147-154.
- KUO H-J, MALENCIK DA, LIOU R-S AND ANDERSON SR. 1986. Factors affecting the activation of rabbit muscle phosphofructokinase by actin. Biochemistry 25: 1278-1286.
- LAEMMLI UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685.
- LILLING G AND BEITNER R. 1990. Decrease in cytoskeleton bound phosphofructokinase in muscle induced by high intracellular calcium, serotonin and phospholipase A2 in vivo Int J Biochem 22: 857-863.
- LIOU R-S AND ANDERSON S. 1980. Activation of rabbit muscle phosphofructokinase by F-actin and reconstituted thin filaments. Biochemistry 19: 2684-2688.
- LIVNAT T, CHEN-ZION M AND BEITNER R. 1993. Uncoupling of mitochondrial oxidative phosphorylation abolishes the stimulatory action of insulin on the binding of glycolytic enzymes to muscle cytoskeleton. Int J Biochem 25: 993-997.
- LOWERY MS, ROBERTS SJ AND SOMERO GN. 1987. Effects of starvation on the activities and localization of glycolytic enzymes in the white muscle of the barred sand bass Paralabrax nebulifer Physiol Zool 60: 538-549.
- LOWRY OH, ROSEBROUGH NJ, FARR AL AND RANDALL RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
- LUTHER MA AND LEE JC. 1986. The role of phosphorylation in the interaction of rabbit muscle phosphofructokinase with F-actin. J Biol Chem 261: 1753-1759.
- MAIA JCC, GOMES SL AND JULIANI MH. 1983. Genes and antigens of parasites - a laboratory manual proceedings. Fiocruz/Brasil, pp. 144-157.
- NEWSHOLOME EA AND DIMITRIADIS G. 2001. Integration of biochemical and physiologic effects of insulin on glucose metabolism. Exp Clin Endocrinol Diabetes 109: S122-S134.
- PAETKAU V AND LARDY H. 1967. Phosphofructokinase - Correlation of physical and enzymatic properties. J Biol Chem 242: 2035-2042.
- PARDEE JD AND SPUDICH JA. 1982. Purification of muscle actin. Methods Enzymol 85: 164-181.
- PHILIPS D, BLAKE CCF AND WATSON HC. 1981. The enzymes of glycolysis: Structure, activity and evolution. Phil Trans R Soc Lon [Biol] 293: 1-214.
- ROBERTS SJ AND SOMERO GN. 1987. Binding of phosphofructokinase to filamentous actin. Biochemistry 26: 3437-3442.
- SALE EM, WHITE MF AND KAHN CR. 1987. Phosphorylation of glycolytic and gluconeogenic enzymes by the insulin receptor kinase. J Cell Biochem 33: 15-26.
- SCHIRMER T AND EVANS PR. 1990. Structural basis of the allosteric behavior of phosphofructokinase. Nature 343: 140-145.
- SOLA-PENNA M, SANTOS AC, SEREJO F, ALVES GG, EL-BACHA T, FABER-BARATA J, PEREIRA MF, DA POIAN AT AND SORENSON M. 2002. A radioassay for phosphofructokinase activity in cell extracts and purified enzyme. J Biochem Biophys Methods 50: 129-140.
- STANLEY WC AND CONNETT RJ. 1991. Regulation of muscle carbohydrate metabolism during exercise. Faseb J 5: 2155-2159.
- UYEDA K. 1979. Phosphofructokinase. Adv Enzymol 48: 193-244.
Correspondence to
Publication Dates
-
Publication in this collection
20 Aug 2004 -
Date of issue
Sept 2004
History
-
Received
08 Oct 2003 -
Accepted
26 May 2004