Abstracts
High fidelity calcium carbonate and hydroxyapatite (bio) inorganic replicas of the fibrous network of the dried fruit of Luffa cylindrica are described, utilizing a facile synthetic route. The loofa sponge is a highly complex macroscopic architectural template, an inexpensive and sustainable resource. In the context of the morphosynthesis, the capability of replication of the loofa sponge opens the possibility of the use of biodiversity in obtaining new materials. We would like to emphasize that the template proposed in this paper, makes possible the preparation of inorganic replicas with a very desirable size, on the centimeter scale. This fact is innovative with respect to inorganic replicas described in the literature, which predominate at the micrometric scale, limited to the original size of the template.
morphosynthesis; biomimetic synthesis; morphology; calcite; hydroxyapatite
Réplicas (bio) inorgânicas de carbonato de cálcio e de hidroxiapatita, com elevada fidelidade à morfologia fibrosa do fruto seco da espécie Luffa cylindrica são descritas, utilizando uma rota de síntese simples e de baixo custo. A esponja vegetal é um molde macroscópico com arquitetura altamente complexa, de baixo custo e de fonte renovável. Dentro do contexto da morfossíntese, a capacidade de replicação da Luffa cylindrica acena com a possibilidade de uso da biodiversidade na obtenção de novos materiais. Enfatizamos que o molde proposto neste trabalho possibilita a preparação de réplicas inorgânicas com o tamanho desejado, em uma escala de centímetros. Este fato é inovador em relação as réplicas inorgânicas descritas na literatura, nas quais predominam a escala micrométrica, limitadas ao tamanho original do molde.
morfossíntese; síntese biomimética; morfologia; calcita; hidroxiapatita
CHEMICAL SCIENCES
Morphosynthesis: high fidelity inorganic replica of the fibrous network of loofa sponge (Luffa cylindrica)
Italo O. Mazali; Oswaldo L. Alves* * Member Academia Brasileira de Ciências
Laboratório de Química do Estado Sólido, Instituto de Química - UNICAMP Cx. Postal 6154, 13083-970 Campinas, SP, Brasil
Correspondence Correspondence to Prof. Oswaldo Luiz Alves E-mail: oalves@iqm.unicamp.br
ABSTRACT
High fidelity calcium carbonate and hydroxyapatite (bio) inorganic replicas of the fibrous network of the dried fruit of Luffa cylindrica are described, utilizing a facile synthetic route. The loofa sponge is a highly complex macroscopic architectural template, an inexpensive and sustainable resource. In the context of the morphosynthesis, the capability of replication of the loofa sponge opens the possibility of the use of biodiversity in obtaining new materials. We would like to emphasize that the template proposed in this paper, makes possible the preparation of inorganic replicas with a very desirable size, on the centimeter scale. This fact is innovative with respect to inorganic replicas described in the literature, which predominate at the micrometric scale, limited to the original size of the template.
Key words: morphosynthesis, biomimetic synthesis, morphology, calcite, hydroxyapatite.
RESUMO
Réplicas (bio) inorgânicas de carbonato de cálcio e de hidroxiapatita, com elevada fidelidade à morfologia fibrosa do fruto seco da espécie Luffa cylindrica são descritas, utilizando uma rota de síntese simples e de baixo custo. A esponja vegetal é um molde macroscópico com arquitetura altamente complexa, de baixo custo e de fonte renovável. Dentro do contexto da morfossíntese, a capacidade de replicação da Luffa cylindrica acena com a possibilidade de uso da biodiversidade na obtenção de novos materiais. Enfatizamos que o molde proposto neste trabalho possibilita a preparação de réplicas inorgânicas com o tamanho desejado, em uma escala de centímetros. Este fato é inovador em relação as réplicas inorgânicas descritas na literatura, nas quais predominam a escala micrométrica, limitadas ao tamanho original do molde.
Palavras-chave: morfossíntese, síntese biomimética, morfologia, calcita, hidroxiapatita.
INTRODUCTION
Morphosynthesis consist of the chemical construction and patterning of inorganic materials with unusual and complex architecture (Ozin 1997, Walsh et al. 1999, Mann 2000). The complex inorganic architectural replication is constructed hierarchically on a length scale from the nanometer to the millimeter level. The controlled synthesis of inorganic materials with specific morphology is an important aspect in the development of new materials in many fields such as catalysis, electronics, nanocomposites, etc. From a materials perspective, biomimetic syntheses have permitted the replication of a range of organized inorganic forms using, as templates, bacterial colonies (Li et al. 2003), insect wings (Cook et al. 2003), spider silk (Huang et al. 2003) and pollen grains (Hall et al. 2003), for example. Most of the morphosynthesis routes or biomimetic syntheses involve precipitation reactions in self-assembly organic media, such as micelles, surfactants and microemulsions (Mann 2000). Sponge-like microspheres of calcium carbonate (vaterite) were prepared by passive evaporation of the volatile components of a supersaturated water-in-oil microemulsion (Walsh et al. 1999). The biomimetic synthesis of calcium carbonate, one of the most abundant biominerals, has received much attention owing to its wide application in such industrial fields as paper, paint, cosmetics, and ceramics (Yu et al. 2004). Calcite has been reported to be effective in fluoride removal from industrial wastewater and drinking water (Yang et al. 1999, Fan et al. 2003). Recently, the coating of calcium carbonate powder with fluoride for wettability enhancement was proposed as a new foaming agent for foamed metal production (Nakamura et al. 2002). Thus, the calcium carbonate waste from water treatment can be recycled as a foaming agent. Calcium carbonate is also a known biomaterial with potential bioactivity (Yu et al. 2004).
The development of advanced materials for biomedical applications is among the most important problems facing modern materials engineering (Kawachi et al. 2000). The greatest potential for bone substitution is shown by materials based on hydroxyapatite, which can develop tight bonding with bone tissue, exhibits osteoconductive behavior, is stable toward bioresorption, and has no adverse effects on the human organism (Finisie et al. 2001, Orlovskii et al. 2002). Synthetic hydroxyapatite precursors are produced by a variety of ceramic processing routes including precipitation, sol-gel and hydrothermal processing. The most important parameters for application of calcium carbonate and hydroxyapatite are specific surface area, particle morphology, chemical and phase composition and microstructure.
However, biomimetic material will only be technologically relevant if inexpensive routes with high product yields can be developed (Walsh et al. 1999). In this context, Hall et al. (2003) described a facile and inexpensive method for replicating the complex surface morphology of flower and tree pollen grains. These replicas consist of silica, calcium carbonate and calcium phosphate minerals.
The aim of this work was to evaluate the capability of the biomimetic synthesis of calcium carbonate and hydroxyapatite inorganic replicas of the fibrous network of loofa sponge (Luffa cylindrica, cucurbitaceous family). The loofa sponge is a highly complex macroscopic architectural template, an inexpensive and sustainable resource. The loofa sponge is cultivated, unlike the sponge produced with cellulose that is extracted from trees. The plant is cultivated in many countries, including Brazil, where its cultivation has an increasing economic importance. The fruits of Luffa cylindrica are smooth and cylindrical shaped. The fibers are composed of 60% cellulose, 30% hemicellulose, and 10% lignin and can be used by industry for many purposes, such as packaging, insulating, or filling materials. The absorbent capacity of the fibers for deionized water is 13.6 g/g (Bal et al. 2004). Traditionally, it is used for bathing and dish washing and, recently, the fibers were used for environmental reclamation (Iqbal and Edyvean 2004).
MATERIALS AND METHODS
The dried fruit of Luffa cylindrica was cut into discs of ca. 10 cm diameter, 3 cm thick, washed withabundant distilled water and then dried at 60ºC. Calcium carbonate and hydroxyapatite inorganic replicas were prepared according to a previously described procedure (Hall et al. 2003). Aqueous solutions of CaCl2 (3.0 mol L-1, pH 8), of Na2CO3 (3.0 mol L-1, pH 13) and of Na2HPO4 (2.0 mol L-1, pH 10) were first prepared as stock solutions. For both inorganic replicas, the sponge was immersed in CaCl2 solution for 12 h, at room temperature. After this period, the sponge was removed from the solution and washed with distilled water to remove excess CaCl2 solution. Calcium carbonate and hydroxyapatite were produced on the fibrous network by reimmmersion of the sponge in Na2CO3 (12 h) or Na2HPO4 (12 h) solutions, respectively. The sponge was again washed with distilled water and submitted to pyrolysis at 600ºC for 24 h under air atmosphere after heating rate of 5ºC min-1, to produce inorganic replicas.
In order to study the decomposition of the fibrous network of loofa sponge, thermogravimetric analyses were carried out (TGA, Shimadzu 50WS) using an air flow rate of 20 mL min-1 and a heating rate of 10ºC min-1. Powder X-ray diffraction (XRD) patterns were obtained using a Shimadzu XRD6000 diffractometer, with Ni filters and CuKa radiation, using 30 kV and 20 mA, calibrated with Si, at a 1º min-1 rate. Infrared spectra (IR) of KBr pellets were measured with a Perkin Elmer 1600 FTIR in the 1400-400 cm-1 range, with a resolution of 2 cm-1. The Raman spectra were recorded on a Renishaw System 3000 Raman Imaging Microscope (ca. 1mm spatial resolution) using a He-Ne laser (632.8 nm) and 8 mW of power before the entrance optics. Scanning electron microscopy (SEM) was performed by using a JEOL JSM T-300 Microscope. The BET surface areas were determined by N2 adsorption using a Micromeritics 2360 instrument.
RESULTS AND DISCUSSION
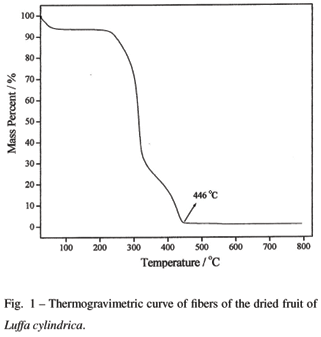
Fig. 1 shows three mass loss steps in the TGA curve of fibers of the dried fruit of Luffa cylindrica with total mass loss around 98.5%. The first one is related to the elimination of water, with a mass loss of approximately 7%. The mass losses for the subsequent events are 91.5%, and can be associated with the pyrolysis of the fibrous network of loofa sponge. The thermal decomposition of the fibrous network of loofa sponge is completed at 446ºC in the TGA curve, and yields a fine black powder, related to the presence of residual carbon. Thermal decomposition carried out at 600ºC for 24 yields a fine white powder constituted of potassium carbonate (major component) and calcium carbonate, as determined by energy dispersive X-ray fluorescence spectrometry. For this reason, after production of the calcium carbonate or hydroxyapatite on the fibrous network, the pyrolysis of the vegetable template was carried out at 600ºC for 24 h.
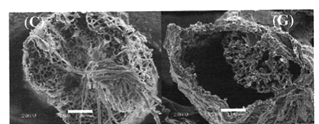
Fig. 2a and 2b show the very complex macroscopic morphology of Luffa cylindrica. Cross-sectional observation of the Luffa cylindrica, by SEM (Fig. 2c and 2d), reveals that the microspongy structure is formed by multicellular fibers bonded together with a large lumen and containing small punctuations along the fibers as interconnections (Bal et al. 2004). Fig. 2e and 2f show the high-fidelity calcium carbonate macroscopic replica of Luffa cylindrica. The hydroxyapatite replica is similar. The inorganic replicas preserve the microspongy structure, as illustrated in Fig. 2g and 2h. The capacity of replication of the morphology of the loofa sponge is due to microspongy fibrous system that offers good accessibility to fluid associated with the high retention capacity of the fibers for aqueous solutions. Both inorganic replicas present a reduction in the volume between 30% and 40% in relation to the original template and they are fragile, although, sufficiently resistant to permit handling.
Powdered samples of calcium carbonate and hydroxyapatite replicas exhibit specific surface areas with 1.10 m2 g-1 and 2.75 m2 g-1, respectively. Recently, experiments for fluoride removal from aqueous solutions were carried out with calcium carbonate and hydroxyapatite with specific surface areas of 0.057 m2 g-1 and 0.052 m2 g-1, respectively (Fan et al. 2003).
The three common polymorphs of calcium carbonate (CaCO3) are vaterite, aragonite and calcite, in order of increasing thermodynamic stability. Because of their different crystal structures the three phases can be discriminated using a vibrational technique, i.e. infrared and Raman spectroscopy (Kontoyannis and Vagenas 2000, Dickinson and McGrath 2001, Vagenas et al. 2003). The stronger and more highly resolved bands at ~ 1085 cm-1 are unable to be used in the analysis of the mixed systems due to extensive overlap between the three polymorphs in this region. The Raman active bands at 711 cm-1, 700 cm-1 and 750 cm-1 for calcite, aragonite and vaterite, respectively, were used for quantitative analysis. IR bands chosen for differentiation between polymorphs occur at 713 cm-1 for calcite, at 700 and 713 cm-1 for aragonite, and at 745 cm-1 for vaterite. Analysis by powder X-ray diffraction uses the reflection peak, in 2q, at 29.5º for calcite, 25.0º for vaterite, and 45.9º for aragonite (Kontoyannis and Vagenas 2000, Dickinson and McGrath 2001). The calcium carbonate replica is featured by the presence of a Raman band at 708 cm-1 (Fig. 3a) and an IR band at 713 cm-1 (Fig. 3b), assigned to in-plane bending vibration modes of CO32- in the crystalline lattice of calcite. In the XRD pattern (Fig. 4), the presence of a peak at 29.3º (2q) confirms the occurrence of the calcite polymorph of calcium carbonate.
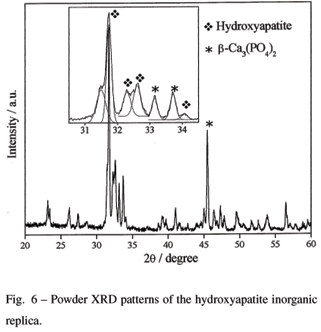
The Raman spectrum of the calcium phosphate replica (Fig. 5a) exhibits a very strong band at 962 cm-1, unequivocally attributed to the totally symmetric n1(PO4)A1 stretching mode of free tetrahedral phosphate ion in the hydroxyapatitephase, Ca5(PO4)3(OH). The Raman bands observed in the frequency regions 400-460 cm-1, 570-625 cm-1, and 1010-1095 cm-1 are assigned, respectively, to n2-, n4- and n3-type internal PO43- modes (Aza et al. 1997, Silva and Sombra 2004). The synthesis of hydroxyapatite as a major phase in the calcium phosphate replica is also confirmed by the XRD pattern (Saeri et al. 2003) (Fig. 6). The calcium phosphate replica presents two minor phases: b-Ca3(PO4)2 (identified by XRD in Fig. 6) and b-Ca2P2O7. The occurrence of this latter phase was confirmed by the presence of the IR band at 725 cm-1 (Fig. 5b), which is characteristic of the b-Ca2P2O7n(P-O-P)sym mode (Waal and Hutter 1994).
The SEM, IR, Raman and XRD results confirm the morphosynthesis of high fidelity inorganic replicas of the fibrous network of the dried fruit of Luffa cylindrica. As emphasized previously, the controlled synthesis of inorganic materials with specific morphology is an important aspect in the development of new materials. In this context, the capability of replication of the loofa sponge is consistent with the possible of use of biodiversity, with special prominence for Brazilian biodiversity, in the obtaining of new materials. We would like to emphasize that the template proposed in this paper, shows the possibility of preparation of inorganic replicas with desirable sizes, on the centimeter scale. This fact is innovative with regard to inorganic replicas described in the literature, in which the micrometric scale predominates, being limited by the original size of the template.
CONCLUSION
High fidelity calcium carbonate and hydroxyapatite replicas of the fibrous network of the dried fruit of Luffa cylindrica are described, utilizing a facile synthetic route and an inexpensive and sustainable resource template. The capacity of replication of the morphology of the loofa sponge is due to its microspongy fibrous system that offers good accessibility to fluid associated with the high retention capacity of the fibers for aqueous solutions. In the context of the morphosynthesis, the capability of replication of the loofa sponge presents the possibility of use of the biodiversity in the obtaining of new materials.
ACKNOWLEDGMENTS
The authors are grateful to Prof. D.L.A. Faria and Prof. M.L.A. Temperini (Instituto de Química, Universidade de São Paulo, Brasil) for the Raman measurements and to Prof. C.H. Collins (Instituto de Química, Universidade de Campinas, Brasil) forEnglish revision. This is a contribution of Millennium Institute for Complex Materials (ConselhoNacional de Desenvolvimento Científico e Tecnológico/CNPq).
Manuscript received on August 16, 2004; accepted for publication on October 23, 2004; contributed by OSWALDO L. ALVES
- AZA PN, GUITIÁN F, SANTOS C, AZA S, CUSCÓ R AND ARTÚS L. 1997. Vibrational properties of calcium phosphate compounds. 2. Comparison between hydroxyapatite and b-tricalcium phosphate. Chem Mater 9: 916-922.
- BAL KE, BAL Y AND LALLAM A. 2004. Gross morphology and absorption capacity of cell-fibers from the fibrous vascular system of Loofah (Luffa cylindrica). Textile Res J 74: 241-247.
- COOK G, TIMMS PL AND SPICKERMANN CG. 2003. Exact replication of biological structures by chemical vapor deposition of silica. Angew Chem Int Ed 42: 557-559.
- DICKINSON SR AND MCGRATH KM. 2001. Quantitative determination of binary and tertiary calcium carbonate mixtures using powder X-ray diffraction. Analyst 126: 1118-1121.
- FAN X, PARKER DJ AND SMITH MD. 2003. Adsorption kinetics of fluoride on low cost materials. Wat Res 37: 4929-4937.
- FINISIE MR, JOSUÉ A, FÁVERE VT AND LARANJEIRA MCM. 2001. Synthesis of calcium-phosphate and chitosan bioceramics for bone regeneration. An Acad Bras Cienc 73: 525-532.
- HALL SR, BOLGER H AND MANN S. 2003. Morphosynthesis of complex inorganic forms using pollen grain templates. Chem Commun 22: 2784-2785.
- HUANG LM, WANG HT, HAYASHI CY, TIAN BZ, ZHAO DY AND YAN YS. 2003. Single-strand spider silk templating for the formation of hierarchically ordered hollow mesoporous silica fibers. J Mater Chem 13: 666-668.
- IQBAL M AND EDYVEAN RGJ. 2004. Alginate coated loofa sponge discs for the removal of cadmium from aqueous solutions. Biotechnol Lett 26: 165-169.
- KAWACHI EY, BERTRAN CA, REIS RR AND ALVES OL. 2000. Bioceramics: tendencies and perspectives of an interdisciplinary area. Quím Nova 23: 518-522.
- KONTOYANNIS CG AND VAGENAS NV. 2000. Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy. Analyst 125: 251-255.
- LI Z, CHUNG SW, NAM JM, GINGER DS AND MIRKIN CA. 2003. Living templates for the merarchical assembly of gold nanoparticles. Angew Chem Int Ed 42: 2306-2309.
- MANN S. 2000. The chemistry of form. Angew Chem Int Ed 39: 3392-3406.
- NAKAMURA T, GNYLOSKURENKO SV, SAKAMOTO K, BYAKOVA AV AND ISHIKAWA R. 2002. Development of new foaming agent for metal foam. Mater Trans 43: 1191-1196.
- ORLOVISKII VP, KOMLEV VS AND BARINOV SM. 2002. Hydroxyapatite and hydroxyapatite-based ceramics. Inorg Mater 38: 973-984.
- OZIN GA. 1997. Morphogenesis of biomineral and morphosynthesis of biomimetic forms. Acc Chem Res 30: 17-27.
- SAERI MR, AFSHAR A, GHORBANI M, EHSANI N AND SORREEL CC. 2003. The wet precipitation process of hydroxyapatite. Mater Lett 57: 4064-4069.
- SILVA CC AND SOMBRA ASB. 2004. Raman spectroscopy measurements of hydroxyapatite obtained by mechanical alloying. J Phys Chem Solids 65: 1031-1033.
- VAGENAS NV, GATSOULI A AND KONTOYANNIS CG. 2003. Quantitative analysis of synthetic calcium carbonate polymorphs using FT-IR spectroscopy. Talanta 59: 831-836.
- WAAL D AND HUTTER C. 1994. Vibrational spectra of a solid solution of cadmium and calcium pyrophosphate. Mater Res Bull 29: 1129-1135.
- WALSH D, LEBEAU B AND MANN S. 1999. Morphosynthesis of calcium carbonate (vaterite) microsponges. Adv Mater 11: 324-328.
- YANG M, HASHIMOTO T, HOSHI N AND MYOGA H. 1999. Fluoride removal in a fixed bed packed with granular calcite. Wat Res 33: 3395-3402.
- YU J, LEI M, CHENG B AND ZHAO X. 2004. Effects of PAA additive and temperature on morphology of calcium carbonate particles. J Solid State Chem 177: 681-689.
Publication Dates
-
Publication in this collection
01 Feb 2005 -
Date of issue
Mar 2005
History
-
Accepted
23 Oct 2004 -
Received
16 Aug 2004