Abstracts
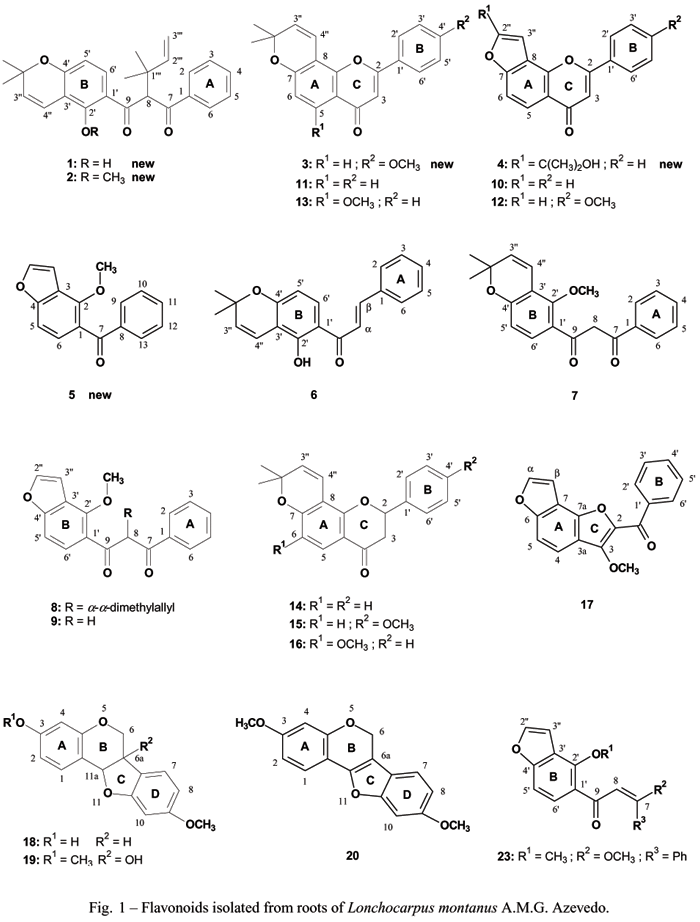
The analysis of root extracts from Lonchocarpus montanus A.M.G. Azevedo resulted in the isolation of twenty three compounds chiefly flavonoids of which five (four flavonoids and one benzophenone) are described for the first time. The molecular structures of the new compounds (1-5) were determined through spectral analysis (UV, IR, MS and NMR) as being: 2'-hydroxy-8-(<FONT FACE=Symbol>a,a</FONT>-dimethylallyl)-2", 2"-dimethylpyrano-(5",6":3',4')-dibenzoylmethane (1), 2'-methoxy-8-(<FONT FACE=Symbol>a, a</FONT>-dimethylallyl)-2", 2"-dimethylpyrano-(5",6":3',4')-dibenzoylmethane (2), 4'-methoxy-2",2"-dimethylpyrano-(5",6":8,7)-flavone (3), 2"-(1-hydroxy-1-methylethyl)-furano-(4",5":8,7)-flavone (4) and [2'-methoxy-furano-(4",5":3',4')-phenyl]-phenylmethanone (5). Additionally, fifteen fatty acids were detected through GC-MS analysis of the corresponding methyl esters [(CH3)2CH(CH2)8COOH and CH3(CH2)nCOOH (n = 6, 12-24)]. Quantitative RP-HPLC showed that the most abundant flavonoids in the petroleum ether and dichloromethane extracts were pongamol (19%) and lanceolatine B (8.0%), respectively. In the bioautography assay, the extracts, pongamol (9), lanceolatine B (10), isolonchocarpin (14), derriobtusone A (17) and medicarpine (18) were active against Staphilococus aureus whereas 9 also against Bacillus subtilis and Cladosporium cladosporioides. Compound 1, 2",2"-dimethylpyrano-(5",6":8,7)-flavone (11) and furano-(1200,1300:7,8)- 4'-methoxy flavone (12) were active against Fusarium oxysporium whereas 11 also against Rhizopus orizae. The extracts, compounds 9, 10, 17 and (E)-7-O-methoxypongamol (23) displayed high toxicity in the brine shrimp lethality assay.
Lonchocarpus montanus A.M.G. Azevedo; Leguminosae; dibenzoylmethanes; flavonoids; flavones; benzophenone; bioautography; Artemia salina lethality
A análise dos extratos das raízes de L. montanus A.M.G. Azevedo resultou no isolamento de vinte e três compostos principalmente flavonóides dos quais cinco são descritos pela primeira vez. As estruturas moleculares dos novos compostos (1-5) foram propostas através da análise dos espectros de UV, IV, EM e RMN como sendo: 2'-hidroxi-8-(<FONT FACE=Symbol>a, a</FONT>-dimetilalil)-2", 2"-dimetilpirano-(5", 6":3',4')-dibenzoilmetano (1), 2'-metoxi-8-(<FONT FACE=Symbol>a,a</FONT>-dimetilalil)-2", 2"-dimetilpirano-(5", 6":3',4')-dibenzoilmetano (2), 4'-metoxi-2", 2"-dimetilpirano-(5", 6":8,7)-flavona (3), 2"-(1-hidroxi-1-metiletil)-furano-(4", 5":8,7)-flavona (4) e [2'-metoxi-furano(4", 5":3',4')-fenil]-fenilmetanona (5). Adicionalmente quinze ácidos graxos foram detectados através da análise de CG-EM dos ésteres metílicos correspondentes [(CH3)2CH(CH2)8COOH e CH3(CH2)nCOOH (n = 6, 12-24)]. A análise quantitativa por CLAE mostrou que os flavonóides mais abundantes nos extratos éter de petróleo e diclorometânico foram pongamol (19%) e lanceolatina B (8.0%), respectivamente. Nos ensaios de bioautografia, os extratos, pongamol (9), laceolatina B (10), isolonchocarpina (14), derriobtusona A (17) e medicarpina (18) foram ativos contra Staphilococcus aureus enquanto 9, também contra Bacillus subtilis e Cladosporium cladosporióides. O composto 1, 2", 2"-dimetilpirano-(5", 6":8,7)-flavona (11) e furano-(2", 3":7,8)-4'-metoxiflavona (12) foram ativos contra Fusarium oxysporium, enquanto 11, também contra Rhizopus oryzae. Os extratos assim como os compostos 9, 10, 17 e (E)-7-O-metoxipongamol (23) apresentaram alta toxicidade no ensaio de letalidade com Artemia salina.
Lonchocarpus montanus A.M.G. Azevedo; Leguminosae; dibenzoilmetano; flavonoides; flavonas; benzofenona; bioautografia; letalidade com Artemia salina
CHEMICAL SCIENCES
Flavonoids of Lonchocarpus montanus A.M.G. Azevedo and biological activity
Aderbal F. MagalhãesI; Ana Maria G.A. TozziII; Eva G. MagalhãesI; Miriam SannomiyaI; Maria del Pilar C. SorianoI; Mary A.F. PerezI
IDepartamento de Química Orgânica, Instituto de Química/UNICAMP, Caixa Postal 6154, 13084-971 Campinas, SP, Brasil
IIDepartamento de Botânica, Instituto de Biologia/UNICAMP, Caixa Postal 6109, 13084-971 Campinas, SP, Brasil
Correspondence to Correspondence to: Aderbal Magalhães E-mail: aderbal@iqm.unicamp.br
ABSTRACT
The analysis of root extracts from Lonchocarpus montanus A.M.G. Azevedo resulted in the isolation of twenty three compounds chiefly flavonoids of which five (four flavonoids and one benzophenone) are described for the first time. The molecular structures of the new compounds (1-5) were determined through spectral analysis (UV, IR, MS and NMR) as being: 2'-hydroxy-8-(a,a-dimethylallyl)-2", 2"-dimethylpyrano-(5",6":3',4')-dibenzoylmethane (1), 2'-methoxy-8-(a, a-dimethylallyl)-2", 2"-dimethylpyrano-(5",6":3',4')-dibenzoylmethane (2), 4'-methoxy-2",2"-dimethylpyrano-(5",6":8,7)-flavone (3), 2"-(1-hydroxy-1-methylethyl)-furano-(4",5":8,7)-flavone (4) and [2'-methoxy-furano-(4",5":3',4')-phenyl]-phenylmethanone (5). Additionally, fifteen fatty acids were detected through GC-MS analysis of the corresponding methyl esters [(CH3)2CH(CH2)8COOH and CH3(CH2)nCOOH (n = 6, 12-24)]. Quantitative RP-HPLC showed that the most abundant flavonoids in the petroleum ether and dichloromethane extracts were pongamol (19%) and lanceolatine B (8.0%), respectively. In the bioautography assay, the extracts, pongamol (9), lanceolatine B (10), isolonchocarpin (14), derriobtusone A (17) and medicarpine (18) were active against Staphilococus aureus whereas 9 also against Bacillus subtilis and Cladosporium cladosporioides. Compound 1, 2",2"-dimethylpyrano-(5",6":8,7)-flavone (11) and furano-(1200,1300:7,8)- 4'-methoxy flavone (12) were active against Fusarium oxysporium whereas 11 also against Rhizopus orizae. The extracts, compounds 9, 10, 17 and (E)-7-O-methoxypongamol (23) displayed high toxicity in the brine shrimp lethality assay.
Key words:Lonchocarpus montanus A.M.G. Azevedo, Leguminosae, dibenzoylmethanes, flavonoids, flavones, benzophenone, bioautography, Artemia salina lethality.
RESUMO
A análise dos extratos das raízes de L. montanus A.M.G. Azevedo resultou no isolamento de vinte e três compostos principalmente flavonóides dos quais cinco são descritos pela primeira vez. As estruturas moleculares dos novos compostos (1-5) foram propostas através da análise dos espectros de UV, IV, EM e RMN como sendo: 2'-hidroxi-8-(a, a-dimetilalil)-2", 2"-dimetilpirano-(5", 6":3',4')-dibenzoilmetano (1), 2'-metoxi-8-(a,a-dimetilalil)-2", 2"-dimetilpirano-(5", 6":3',4')-dibenzoilmetano (2), 4'-metoxi-2", 2"-dimetilpirano-(5", 6":8,7)-flavona (3), 2"-(1-hidroxi-1-metiletil)-furano-(4", 5":8,7)-flavona (4) e [2'-metoxi-furano(4", 5":3',4')-fenil]-fenilmetanona (5). Adicionalmente quinze ácidos graxos foram detectados através da análise de CG-EM dos ésteres metílicos correspondentes [(CH3)2CH(CH2)8COOH e CH3(CH2)nCOOH (n = 6, 12-24)]. A análise quantitativa por CLAE mostrou que os flavonóides mais abundantes nos extratos éter de petróleo e diclorometânico foram pongamol (19%) e lanceolatina B (8.0%), respectivamente. Nos ensaios de bioautografia, os extratos, pongamol (9), laceolatina B (10), isolonchocarpina (14), derriobtusona A (17) e medicarpina (18) foram ativos contra Staphilococcus aureus enquanto 9, também contra Bacillus subtilis e Cladosporium cladosporióides. O composto 1, 2", 2"-dimetilpirano-(5", 6":8,7)-flavona (11) e furano-(2", 3":7,8)-4'-metoxiflavona (12) foram ativos contra Fusarium oxysporium, enquanto 11, também contra Rhizopus oryzae. Os extratos assim como os compostos 9, 10, 17 e (E)-7-O-metoxipongamol (23) apresentaram alta toxicidade no ensaio de letalidade com Artemia salina.
Palavras-chave:Lonchocarpus montanus A.M.G. Azevedo, Leguminosae, dibenzoilmetano, flavonoides, flavonas, benzofenona, bioautografia, letalidade com Artemia salina.
INTRODUCTION
The genus Lonchocarpus encompasses 150 species, including 24 native to Brazil. L. montanus is a new native species (A.M.G.A. Tozzi, unpublished data). The botanical classification was based on morphology of the flowers and fruits and the inflorescence structure, which has been considered an outstanding character. Accordingly L. montanus and L. obtusus Benth revealed a strong similarity and were allocated to the Unguiflora section in Lonchocarpus subgenus Lonchocarpus (A.M.G.A. Tozzi, unpublished data).L. obtusus has furnished flavonoids, including the new auronol derriobtusone A, (Do Nascimento et al.1976).The natural aurones and auronols comprise a very small group of flavonoids. L. montanus is an ornamental tree popularly known as "cabelouro" or "carrancudo". It is widely distributed through the states of Bahia, Goias, Minas Gerais and Tocantins.
Many flavonoids possess a remarkable spectrum of biological activities (Middleton et al. 2000). They have been reported to exhibit anti-cancer (Block 1992, Elangovan et al.1994, Middleton et al.2000), anti-viral (Selway 1986) and anti-inflammatory (Middleton et al. 2000, Middleton 1998, Gabor 1986) effects as well as to reducethe risk of cardiovascular diseases (Middleton et al.2000, Facino et al.1999, Hertog et al.1993, Mazur et al.1999). These activities are broadly attributed to their antioxidant properties.
This paper describes the isolation and characterization of four new flavonoids (1-4) and one new benzophenone derivative (5) together with eighteen known compounds (6-23) and a mixture of fatty acids (24-38), from root extracts of L. montanus. The results obtained after submitting extracts (petroleum ether and dichloromethane) and compounds 1, 6, 9-12, 14, 17, 18 to bioautography (against bacteria and fungi) as well as extracts (petroleum ether, dichloromethane and methanol) and compounds 9, 10, 17, to brine shrimp lethality are also included. The analysis of the extracts was performed through chromatography (CC, TLC, GC-MS and RP-HPLC).
MATERIALS AND METHODS
GENERAL PROCEDURES
Melting points were determined using a Mettler FP5 apparatus. The optical rotations were measured on a Carl Zeiss Jena Polamat A polarimeter or a Jasco J-720 spectropolarimeter. IR spectra (film or KBr) were recorded using a Perkin-Elmer Model 1600 FT-IR 1600 instrument. UV spectra were recorded on a HP Diode Array Spectrophotometer 8452 A spectrometer using MeOH as solvent. NMR spectra were recorded on Varian INOVA-500 (500 MHz for 1H and 125 MHz for 13C), Gemini 300 (300 MHz for 1H and 75 MHz for 13C) or Brucker AC 300 P (300 MHz for 1H and 75 MHz for 13C) spectrometers using CDCl3 as solvent and TMS as internal standard. The DEPT experiments were performed using polarization transfer pulses of 90º and 135º. EIMS, direct probe, HREIMS and MS/MS experiments were performed on a VG Auto Spec-Fisions Instrument by using electron ionization at 70 eV. GC-MS was performed on a Hewlett Packard (Model HP 5890 B SERIES II) instrument. Column chromatography (CC) separations were on silica gel 60 (70-230 mesh, Merck). TLC was performed on commercial plates (silica gel G and GF254, Merck) while preparative TLC used precoated 1000 m thick Merck silica gel 60 F254 glass plates. Compounds were detected by UV (l = 254 and 366 nm) irradiation and/or with an ethanolic solution of anisaldehyde, sulfuric acid and acetic acid (90mL:5mL:1mL), followed by heating. The solvent mixtures were prepared in volume ratios. Reversed-phase HPLC analyses were performed using a C-18 Nova Pak column, with diode-array detection. Identification of the peaks in the chromatogram of each extract (petroleum ether and dichloromethane) was carried out by co-injection with five flavonoids, 9, 10, 14, 17, 18 (one each time) isolated from the extracts of L. montanus roots and further purified by preparative HPLC.
GC-MS ANALYSIS OF METHYL ESTERS
The methyl fatty acids esters were analyzed by GC-MS using a HP 5970 mass selective detector coupled to a HP-gas chromatograph having a HP5 fused silica capillary column (30 m × 0.25 mm i.d., 0.25µm film). The instrument was operated with injector and detector temperatures of 260ºC and 280ºC, respectively, in the splitless (2µL injection) and constant flow mode. The temperature of the GC column was increased from 50 to 300ºC at a rate of 10ºC min-1 and held at 300ºC for 5min. The carrier gas was helium at a flow rate of 1.0mL min-1. Mass spectra were taken over the m/z 40-600 range with an ionizing voltage of 70eV. Linear retention time indices (RI) for the methyl fatty acids esters 25-39 were determined by comparing their retention times with those of n-paraffin standards and mass spectral data (Adams 1995). All mass spectra were identified by using an on-line library (Wiley 275) or authentic compounds (Table I).
QUANTITATIVE HPLC ANALYSIS
Light petroleum and dichloromethane root extracts from L. montanus were dissolved in acetonitrile.An aliquot (2µL) of each extract was analyzed on a Hewlett Packard model 1090, series II/M HPLC with a Waters Nova-Pak C-18,3µm, column, with mobile phase gradient of CH3CN-H2O (48-52) for 25 min and then to CH3CN-H2O (80-20) during 20 min at a flow rate of 0.8 mL min-1, keeping the column at room temperature. Detection was with a HP photodiode array detector. The identification of the peaks in the chromatograms of each extract (petroleum ether and dichloromethane) was carried out by individual co-injections with each one of the most abundant flavonoids (9, 10, 14, 17 and 18) isolated from the extracts of L. montanus, which were first purified by semi-preparative HPLC. Each compound was dissolved in acetonitrile and purified with a semi-preparative HPLC (Waters) system, consisting of an universal liquid chromatograph injector, a model 484 variable-wavelength detector (adjusted to 230 nm), a model 740 data module and a model 600E system controller and pump, with a Regis ODS, 5µm, 250 × 10 mm i.d. column, and a CH3CN-H2O gradient of 20-100% CH3CN in 45 min at a flow rate of 2.0 mLmin-1. The corresponding retention times were: 9 (7.8 min), 10 (8.5 min), 14 (29 min), 17 (13 min) and 18 (4.2 min).
CALIBRATION CURVES
Standard solutions of each quantified flavonoid (9, 10, 17) were prepared by serial dilutions in acetonitrile. Calibration curves were obtained by plotting the integrated peak areas at the maximum UV absorptionfor each compound versus concentration by performing linear regression analysis with correlation coefficients of 0.9999 for each analyzed compound. Quantification was made by external calibration in the petroleum ether extract the concentrations were found to be 9 (19%), 10 (10.6%) and 17 (13.6%) while in the dichloromethane extract they were: 9 (6.7%), 10 (8.0%) and 17 (6.4%).
BIOLOGICAL ACTIVITY
The bioautography was performed according to Saxena (Saxena et al. 1995), with some modifications (Magalhães et al. 1998), against eight fungi [Alternaria alternate (CCT 1250), Aspergillus fumigatus (CCT 01277), Aspergillus niger (CCT 1435), Candida albicans (CCT 0776), Cladosporium cladosporioides (CCT 5039), Fusarium oxysporium (CCT 3244), Penicilluim funiculosum (CCT 0490), Rhizopus orizae (CCT 4964)] and seven bacteria [Bacillus subtilis (CCT 0089), Escherichia colii (CCT 5050), Micrococcus luteus (CCT 2720), Rodococcus equi (CCT 0541), Salmonella typhimurium (CCT 0528), Staphylococcus aureus (CCT 4295), Streptococcus mutans (CCT 3440)]. The standards used for comparison were, cyclopyrox olamine for fungi and chloramphenicol for bacteria. The extracts (petroleum ether, dichloromethane and methanol) and compounds 9, 10, 14, 17 and 18 showed activity against Staphylococcus aureus, while 9 also against Bacillus subtilis and Cladosporium cladosporioides. Compounds 1, 11 and 12 showed activity against Fusarium oxysporium while 11 also against Rhizopus orizae.
The Brine shrimp lethality assay was performed according to McLaughlin (McLaughlin 1995). The extracts (petroleum ether, dichloromethane and methanol) and compounds 9, 10, 17 displayed high toxicity as revealed by very low LC50 values (Table II).
PLANT MATERIAL
Roots of L. montanus were collected in Taquatinga do Tocantins, Tocantins State, Brazil in March, 1998. The plant was identified by Dr. A.M.G. Azevedo from the Biology Institute of Campinas State University (UNICAMP), Campinas, SP, Brazil.A voucher specimen (B.A.S. Pereira and D. Alvarenga 1717 - UEC) is deposited at the herbarium of Campinas State University (UNICAMP).
EXTRACTION AND ISOLATION
Dried and pulverized roots (259 g) of L. montanus were successively extracted with petroleum ether (30-60ºC), CH2Cl2 and MeOH in a Soxhlet apparatus. After solvent evaporation, the petrol ether extract gave a viscous yellow oil (5.1 g), while the dichloromethane (3.5 g) and the methanol (6.0 g) extracts gave brown oils. Part of the petroleum ether extract (4.32 g) was fractionated by silica gel CC eluted first with petroleum ether/CHCl3 (1:1).The eluent polarity was gradually increased by addition of CHCl3 and then MeOH to furnish 295 fractions (30 mL each) which were reduced to 28 groupsafter TLC analysis. Most of the compounds were found in ten groups ranging from fractions 34 to 240. Asample of each was further fractionated by successive preparative TLC (silica gel) as described below and recovered from TLC plates by extraction with mixtures of CH2Cl2 and MeOH.
Fractions 51-75 (63 mg): preparative TLC [n-hexane/CH2Cl2/EtOAc (30:10:5, 2×) and n-hexane/CHCl3/ EtOAc (35:5:2)] gave 1 (5.7 mg) and 9 + demethylpongamol (30 mg).
Fractions 82-99 (100.3 mg): preparative TLC [CHCl3 (100%, 2×), n-hexane/CHCl3 (90:10, 4×), ether/n-hexane (90:10, 4×), petroleum ether/CH2Cl2/EtOAc (40:5:1) and n-hexane/CHCl3/EtOAc (50:10:1, 3×)] gave 5 (0.9 mg).
Fractions 115-119 (93.1 mg): preparative TLC [CHCl3 (100%) and petroleum ether/n-hexane (85:15, 7×)] gave 15 (1.9 mg) and 2 (1.7 mg).
Fractions 120-130 (96.4 mg): preparative TLC [(n-hexane/CH2Cl2/EtOAc (30:15:3, 6×)] gave 3 +demethylpongamol (1.6 mg).
Fractions 189-215 (48 mg): preparative TLC [n-hexane/EtOAc (70:30)] gave 4 (2.4 mg).
Fractions 34-41 (16 mg): preparative TLC [n-hexane/CHCl3/EtOAc (35:5:2)] gave 6 (1.1 mg).
Fractions 137-141 (98.7 mg): preparative TLC [n-hexane/CH2Cl2/MeOH (30:10:0.5, 2×)] gave 7 (2.1 mg).
Fractions 120-130 (98.6 mg): preparative TLC [petroleum ether/CH2Cl2/EtOAc (40:10:2) and n-hexane/ CHCl3/MeOH (35:5:1)] gave 8 (0.7 mg).
Fractions 120-130 (69.8 mg): preparative TLC [n-hexane/CHCl3/EtOAc (35:5:5, 2×), n-hexane/CHCl3 (94:6, 6×) and n-hexane/CHCl3(95:5, 5×)] gave 10 (8.1 mg).
Fractions 120-130 (100 mg): preparative TLC [n-hexane/CHCl3/EtOAc (35:5:5, 2×) and CH2Cl2/petroleum ether (95:5, 5×)] gave 11 (2.7 mg).
Fractions 145-153 (71.6 mg): preparative TLC [CHCl3/MeOH (99:1) and n-hexane/CHCl3/MeOH(15:15:1)] gave 18 (5.2 mg) and 13 (1.6 mg).
Fractions 103-112 (100 mg): preparative TLC [n-hexane/CHCl3/EtOAc (35:5:5) and n-hexane/EtOAc (97:3)] gave 14 (3.8 mg).
Fractions 115-119 (93.1 mg): preparative TLC [CHCl3 (100%) and petroleum/n-hexane (85:15, 7×)] gave 15 (1.9 mg).
Fractions 103-112 (100 mg): preparative TLC [n-hexane/CHCl3/EtOAc (35:5:5), Ag-TLC (5%) n-hexane/ CHCl3/EtOAc (35:5:0.3) and n-hexano/CHCl3/EtOAc (35:5:5)] gave 17 (15.0 mg).
Fractions 137-141 (98.7 mg): preparative TLC [n-hexane/CH2Cl2/MeOH (30:10:0.5, 2×) and n-hexane/ CHCl3/EtOAc (35:5:5)] gave 19 + 20 + 12 (2.5 mg).
Fractions 137-141 (78.3 mg): preparative TLC [n-hexane/CHCl3/MeOH (30:10:0.5, 2×) and n-hexane/ CHCl3/EtOAc (35:5:5)] gave 21 + 22 (3.0 mg).
Fractions 115-119 (116 mg) were flash chromatographed on silica gel, eluting with 2000 mL of petroleum ether/CH2Cl2 (70:30) and then washing with MeOH. After solvent evaporation, the methanolic fraction (28.1 mg) was submitted to preparative TLC [petroleum ether/CHCl3/EtOAc (40:10:2)] to give 16(2.7 mg).
The dichloromethane extract (2.4 g) was flash chromatographed on silica gel to give 501 fractions (30mL each) which were reduced to 49 groups after TLC analysis. A sample of each was further fractionatedby successive preparative TLC (silica gel) as described below and recovered from TLC plates by extraction with mixtures of CH2Cl2 and MeOH.
Fractions 154-161 (45.3 mg): preparative TLC (CHCl3 100%, 4×) gave 10 (7.8 mg) and 23 (4.6 mg).
Fractions 194-295 (116.4 mg): preparative TLC [n-hexane/EtOAc (35:5:5)] gave 18 (20.1 mg).
Fractions 126-135 (268.4 mg) were flash chromatographed on silica gel, eluting with n-hexane/CHCl3 (6:4). The eluent polarity was gradually increased by addition of CHCl3 and then MeOH to furnish 200 fractions (30 mL each) which were monitored by TLC. A sample of each was further fractionated by successive preparative TLC (silica gel) and recovered from TLC plates by extraction with mixtures of CH2Cl2 and MeOH.
Fractions 14-19 (32.8 mg): preparative TLC [CHCl3/MeOH (98:2, 3×)] gave 17 (15.7 mg).
Fractions 60-74 (51 mg): preparative TLC [CHCl3/n-hexane/MeOH (17:3:0.1, 3×)] gave 10 (20.7 mg) and 23 (13.0 mg).
NEW COMPOUNDS
2'-hydroxy-8-(a,a-dimethylallyl)-2", 2"-dimethylpyrano-(5",6":3',4')-dibenzoylmethane (1): yellow needles, [a]: D25 -8.68 (MeOH, c 0.008 gmL-1). HREIMS, m/z (%): 390.1831 (13) [M]+, 375.1609 (11) [M-Me]+, 307.0944 (100) [M-Me-68]+, 203.0641 (39) [M-187]+, 187.0363 (65) [M-Me-188]+, 105.0267 (51) [M-285]+, 77.0311 (28) [M-313]+.UV (MeOH): lmax (log e) = 253 (4.40), 319 (4.03), nm. IR (KBr): nmax = 3438, 2923, 2850, 1690, 1644, 1598, 1384, 1118 cm-1. 1H NMR (300 MHz): d and 13C NMR (75.4MHz): d, see Table III.
2'-methoxy-8-(a, a-dimethylallyl)-2", 2"-dimethylpyrano-(5", 6":3',4')-dibenzoylmethane (2): orange needles. HREIMS, m/z (%): 404.1988 (4) [M]+, 389.1768 (20) [M-Me]+, 217.0852 (66) [M-187]+. GC-MS, m/z (%): 404 (4) [M]+, 389 (19) [M-Me]+, 321 (13) [M-Me-68]+, 217 (100) [M-187]+, 105 (28) [M-299]+, 77 (30) [M-327]+. UV (MeOH): lmax (log e) = 248 (4.0). 1H NMR (300 MHz): d and 13C NMR (75.4 MHz): d, see Table III.
2'-hydroxy-furano-(4", 5":3',4')-dibenzoylmethane (demethylpongamol): GC-MS, m/z(%): 280 (23) M]+, 161 (16) [M-119]+, 160 (23) [M-120]+, 105 (100) [M-175]+, 77 (45) [M-175-CO]+, 51 (19) [M-175-[CO-C2H2]+.
4'-methoxy-2", 2"-dimethylpyrano-(5", 6":8,7)-flavone (3): colorless amorphous solid. GC-MS, m/z (%): 334 (13) [M]+, 319 (100) [M-Me]+, 187 (72) [M-Me-132]+, 159 (8) [M-Me-132-CO]+, 132 (9) [M-Me-187]+.1H NMR (500 MHz): d, see Table III.
2"-(1-hydroxy-1-methylethyl)-furano- (4",5":8,7)-flavone (4): colorless needles, HREIMS, m/z(%): 320.1050 (38) [M]+, 305.0816 (100) [M-Me]+, 203.0368 (17) [M-Me-102]+, 68.9970 (52)[M-252]+. MS, m/z(%): 320 (26) [M]+, 305 (100) [M-Me]+, 203 (25) [M-Me-102]+, 102 (33) [M-Me-203]+, 77 (22) [M-Me-228]+, 43 (99) [M-Me C17H10O3]+.1H NMR (500 MHz): d, see Table III.
2'-methoxyfurano(4",5": 3',4')-phenyl-phenylmethanone (5): colorless amorphous solid. MS, m/z(%): 252 [M]+ (absent), 175 (100) [M-77]+, 160 (24) [M-77-Me]+, 132 (5) [M-77-Me-CO]+, 105 (8) [M-147]+, 77 (28) [M-147-CO]+. UV (MeOH): lmax (log e)= 248 (3.77), 276 (3.36), 322 (2.79), nm. IR (film): nmax = 2924, 2853, 1651, 1585, 1472, 1257, 733 cm1.1H NMR (500 MHz): d, see Table III.
RESULTS AND DISCUSSION
The petroleum ether extract of L.montanusroots was submitted to successive chromatographic analysis (CC, TLC and preparative TLC), allowing the isolation of nineteen flavonoids (1-4, 6-20), one benzophenone derivative (5) (Fig. 1) and two steroids (21 and 22). A mixture of fatty acids was first methylated with diazomethane followed by GC-MS analysis, allowing the identification of fifteen methyl esters: 24 [CH3 (CH2) 6COOCH3], 25[(CH3)2CH (CH2)7COOCH3] and 26-38 [CH3(CH2)nCOOCH3 (n = 12-24)]. The dichloromethane extract gave the flavonoids 10, 17, 18and 23.
Compound 1 gave a molecular ion [M]+ at m/z 390.18314 in HREIMS which corresponds to C25H26O4 (calc. 390.18311). The NMR data (Table III) were very similar to those of the dibenzoylmethane derivative described earlier (Magalhães et al. 1997). In the 1HNMR spectrum, signals for a monosubstituted aromatic ring [d 7.91 (2H, d, J = 9.0 Hz, H-2, H-6), d 7.42 (2H, m, H-3,H-5) and d 7.50 (1H, m, H-4)], one a,a-dimethylallyl group [d 6.17 (1H, dd, J = 17.4 and 10.8 Hz, H-2"'), d 5.0 (1H, d, J = 17.4 Hz, H-3a"'), d 4.97 (1H, d, J = 10.8 Hz, H-3b"'), d 1.31 (3H, s) and d 1.29 (3H, s)], one uncoupled methynic hydrogen [d 5.42 (1H, s, H-8)], two ortho coupled aromatic hydrogens [d 7.70 (1H, d, J= 9.0 Hz, H-6') and d 6.35 (1H, d, J= 9.0 Hz, H')] and one hydrogen bonded hydroxy group [d 13.03 (1H, s)] were also observed. However instead of a furano ring, compound 1 has a 2,2-dimethylpyrano ring shown in the 1H NMR spectrum by the signals at d 5.57 (1H, d, J= 9.9 Hz, H-3"), d 6.68 (1H, d, J= 9.9 Hz, H-4"), d 1.45 (3H, s, 2"-CH) and d 1.43 (3H, s, 2"-CH). This was confirmed in the 13C NMR spectrum (Table III) by the signals at d 109.7 (C-3'), d 160.3 (C-4'), d 128.5 (C-3"), d 115.8 (C-4"), d 28.5 (2"-CH) and d 28.5 (2"-CH). The signals of two carbonyl groups (d 198.6 and d 194.2) in the 13CNMR is in accordance with a non-enolizable dibenzoylmethane skeleton (Parmar et al. 1989, Zeng et al. 1994, Fukai et al. 1994). The13CNMR spectrum and DEPT (90º and 135º) indicated the presence of nine quaternary carbons, eleven CH, one CH2 and four CH3. Based on these data, four structural possibilities were suggested concerning the alternative oxygenation pattern of the Bring (2',4'; 2',5'; 2',3'; 2',6'). In the nOe difference experiment, irradiation of H-4" (d 6.68) caused an enhancement of the signals at d 5.56 (H-3") and d 13.03 (chelated 2'-OH). These findings are in agreement with structure 1. Irradiation of H-8 (d 5.42) caused a higher enhancement of the signal at d 7.70 (H-6', 9.66%) than of the signal at d 7.91 (H-2 and H-6, 5.04%), suggesting that the preferential conformation is the one where the B ring benzoyl moiety and C-8/H-8 lie in the same plane (Parmar et al. 1989). Thus, C-8 is a stereogenic center, which was further confirmed by optical rotation measurement. Based on MS/MS experiments selecting the ions m/z 375 [307 (12%), 187 (100%)] and m/z307 [187(100%)], it was possible to suggest a fragmentation pathway (Fig. 2).
Compound 2 exhibited a molecular ion [M]+ at m/z404.198792 in HREIMS, which is fourteen mass units higher than 1, suggesting that the hydroxyl group was replaced by a methoxy group, for a molecular formula of C26H28O4 (calc. 404.198760). In the low resolution mass spectrum the base peak at m/z 217 [C13H13O3]+ originates from C-8/C-9 bond cleavage proving that the methoxy group is on B ring. The fragmentaion pathway (Fig. 2) is analogous to that of 1. NMR data (Table III) were also very similar those of 1 except for the lack of a signal corresponding to a hydrogen bonded hydroxy group at C-2' in the 1H NMR spectrum and the presence of a methoxy group (d 3.51, 3H, s, 2'-OCH) that was confirmed in the 13C NMR spectrum (d 66.0, 2'-OCH). The unusual chemical shift value observed for the methoxy group in the1H NMR spectrum evidenced that the methyl group stays out of the aromatic ring plane in order to relieve sterical hindrance with the a,a-dimethylallyl group at C-8, as can be demonstrated through the corresponding molecular model.
A minor compound together with 9 was detected through GC-MS. The corresponding mass spectrum (tR = 23.3 min) displayed a molecular ion [M]+ of m/z280 (23%) which is fourteen mass units lower than that of 9, according to the molecular formula C17H12O4. The peaks at m/z161 (16%) and m/z105 (100%) correspond to the fragments from the cleavage of C8-C9 and C7-C8 bonds, respectively, leading to the suggestion that this compound can be demethylpongamol, a new compound that is probably the biogenetic precursor of 1. Alternative modes of furano fusion with ring A however cannot be discarded.
Compound 3 also was isolated as a minor compound together with 12. The 1H NMR spectrum of the mixture showed a ratio of 17:83 (4:13). Among the signals corresponding to 3 there are those of a p-disubstituted aromatic ring [d 7.86 (2H, d, J= 8.5 Hz, H-2' and H-6') d 7.04 (2H, d, J= 8.5 Hz, H-3' and H-5')] and one methoxy group [d 3.91 (3H, s, 4'-OCH3)]. A typical flavone H-3 signal at d 6.69 (1H, s) was observed. GC-MS analysis furnished a chromatogram with two peaks [tR = 28.7 min (12); tR = 32.3 min (3)]. In the mass spectrum of 3, the molecular ion [M]+ at m/z 334 (10%) and the fragments at m/z 399 (100%), m/z 187 (80%), can be rationalized by the loss of one methyl radical from a 2,2-dimethylpyrano group followed by C ring RDA rearrangement. Based on the biosynthetic pathway of flavonoids (Cooper-Driver and Bhattacharya 1998), it could be suggested a close biosynthetic relation between 15 and 3.
Compound 4 gave a molecular ion [M]+ at m/z 320.1052 in HREIMS according to C20H1604 (calc. 320.1049). The 1H NMR spectrum (Table III) showed signals of one monosubstituted aromatic ring d 7.96-8.0 (2H, m, H-2', H-6') d 7.54-7.58 (3H, m, H-3', H-4', H-5')], two ortho coupled hydrogens [d 8.15 (1H, d, J= 9.0 Hz ) and d 7.53 (1H, dd, J= 9.0 and 1.0 Hz )], a flavone H-3 (d 6.90, s, 1H) and a long range coupled hydrogen [d 7.04 (1H, d, J= 1.0 Hz)]. In addition, the signals at d 1.76 [6H, s, 2"2"(CH3)2C-] and d 4.78 (1H, bs, 2"-C-OH) are similar to those corresponding to the isopropyl group in oroselol (Lee 1995). In the low resolution mass spectrum the most intense peaks at m/z 305 [100% (M+.-15)] and m/z 43 [99%, CH3CO+(M+.-15-C17H10O3)] can be rationalized by the loss of a methyl group followed by the loss of (4,5:8,7)-furanoflavone while the peak at m/z 203 (25%) corresponds to C ring RDA cleavage after the molecule loses a methyl group [(M+.-CH3)-C8H6].
Compound 5 did not give the molecular ion [M]+ in HREIMS even after lowering the energy of the ionizing electron bean. The 1HNMR spectrum (Table III) showed signals corresponding to one monosubstituted aromatic ring [d 7.97 (2H, d, J=8 Hz, H-9 and H-13), d 7.65 (1H, m, H-11) and d 7.52 (2H, t, J=8 Hz, H-10 and H-12)], one furan [d 6.92 (1H, d, J= 2.5 Hz, H-3") and d 7.65 (1H, m, H-2"], two ortho-coupled hydrogens [d 8.04 (1H, d, J= 8.5 Hz, H-6) and d 7.37 (1H, dd, J= 8.5 and 1 Hz, H-5)] and one aromatic methoxy group [d 3.81 (3H, s, 2-OCH3)]. In the low resolution mass spectrum the base peak at m/z175 (100%) corresponds to the fragment ion containing the B-ring, which results from the a carbonyl bond cleavage.
The known flavonoids (6-20, 23;see Table IV), b-sitosterol (21)and stigmasterol (22)were characterized by comparison of the respective spectral data with those found in the literature.
HPLC analyses of the petroleum ether and dichloromethane extracts allowed the identification of 9, 10, 14, 17 and 18. The most abundant flavonoids in the petroleum ether were found to be 9 (19%), 10 (10%) and 17 (13%) while in dichloromethane they were 9 (6.7%), 10 (8.0%) and 17 (6.4%).
The root extracts and the flavonoids 1, 6, 9-12, 14, 17 and 18 were submitted to bioautography assay (Saxena et al. 1995, Magalhães et al. 1998) against seven bacteria and eight fungi. Root extracts (petroleum ether and dichloromethane) and the flavonoids 9, 10, 14, 17 and 18 showed antibacterial activity against Staphilococcus aureus, whereas 9 was also active against Bacillus subtilis and Cladosporium cladosporioides. The flavonoids 1, 11 and 12 showed antifungal activity against Fusarium oxysporium whereas 11was also active against Rhizopus oryzae.
The petroleum ether, dichloromethane and methanol extracts and the flavonoids 9, 10, 17 and 23 were also submitted to the brine shrimp lethality bioassay (McLaughlin 1995) and displayed high toxicity as revealed by very low LC values (Table II).
Derriobtusone A (17) is a very rare auronol which was isolated only from L. obtusus. The occurrence of Derriobtusone A ( 17), among the most abundant compounds in L. montanus reinforces its allocation together with L. obtusus at Unguiflora section in Lonchocarpus subgenus Lonchocarpus. The structures of the most abundant flavonoids (9, 10, 17) furnished by L. montanus are closely related. These findings suggest a biosynthetic pathway consisted by alternative oxidative steps starting from the same chalcone (Fig. 3). However no aurone was found in L. montanus.
ACKNOWLEDGMENTS
The authors are gratefull to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for scholarships awarded to M. Sannomiya and M.D.C.P. Soriano, to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support and a scholarship awarded to M.A.F. Perez and to Dr. Carol Collins for critical reading and English revision. The authors are also very grateful to Dr. Luzia Koike for supervising the analysis of the methyl esters mixture.
Manuscript received on April 4, 2006; accepted for publication on February 7, 2007; presented by FERNANDO GALEMBECK
- ADAMS RP. 1995. Identification of Essential Oil Compounds by Gas Chromatography/Mass Spectrometry. Allured Publishing Corporation: Illinois, 469 p.
- BENTLEY MD, HASSANALI A, LWANDE W, NJOROGE PEW, OLE SITAYO EN AND YATAGAI M. 1987. Insect antifeedants from Tephrosia elata deflers Insect Sci Applic 8: 8588.
- BILTON JN, DEBNAM JR AND SMITH IM. 1976. 6a-hydroxypterocarpans from Red Clover. Phytochemistry 15: 14111417.
- BLOCK G. 1992. The data support a role for antioxidants in reducing cancer risk. Nutr Rev 50: 207213.
- COOPER-DRIVER GA AND BHATTACHARYA M. 1998. Role of Phenolic in Plant Evolution. Phytochemistry 49: 11651174.
- DELLE MONACHE F, SUAREZ LEC AND MARINI-BETTOLO GB. 1978. Flavonoids from the Seeds of Six Lonchocarpus Species. Phytochemistry 17: 18121813.
- DELLE MONACHE G, DE MELLO JF, MONACHE FD, MARINI-BETTOLO GB, DE LIMA GO AND COELHO JSB. 1974. XI/ New Prenylated Flavonoids. Gazz Chim Ital 104: 861865.
- DO NASCIMENTO MC AND MORS WB. 1972. Chalcones of the Root Bark of Derris Sericea Phytochemistry 11: 30233028.
- DO NASCIMENTO MC, DIAS RLV AND MORS WB. 1976. Flavonoids of Derris obtuse: Aurones and Auronols. Phytochemistry 15: 15531558.
- ELANGOVAN V, SEKAR N AND GOVINDASAMY S. 1994. Chemopreventive potential of dietary bioflavonoids against 20-methylcholanthrene-induced tumorigenesis. Cancer Lett 87: 107113.
- FACINO RM, CARINI M, ALDINI G, BERTI F, ROSSONI G, BOMBARDELLI E AND MORAZZONI P. 1999. Diet enriched with procyanidins enhances antioxidant activity and reduces myocardial post-ischemic damage in rats. Life Sci 64: 943949.
- FUKAI T, NISHIZAKA J AND NOMURA T. 1994. Five isoprenoid-substituted flavonoids from Glycyrrhiza eurycarpa Phytochemistry 35: 515519.
- GABOR M. 1986. Anti-inflammatory and anti-allergic properties of flavonoids. Prog Clin Biol Res 213: 471480.
- GARCEZ FR, SCRAMIN S, DO NASCIMENTO CM AND MORS WB. 1988. Prenylated Flavonoids as Evolutionary Indicators in the Genus Dahlstedtia. Phytochemistry 27: 10791083.
- GARG GP. 1979. New Component from Leaves of Pongamiaglabra Planta Med 37: 7374.
- HERTOG MGL, FESKENS EJM, KROMHOUT D, HOLLMAN PCH AND KATAN MB. 1993. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 342: 10071011.
- INGHAM JL. 1990. Systematic Aspects of Phytoalexin Formation within Tribe Phaseoleaeof the Leguminosae (Subfamily Papilionoideae). Biochem Systemat Ecol 18: 329343.
- KHALID SA AND WATERMAN PG. 1981. 8-C-prenylflavonoids from Seed of Tephrosia bracteolate Phytochemistry 20: 17191720.
- KUROSAWA K, OLLIS DW, SUTHERLAND IO AND GOTTLIEB OR. 1978. Variabilin, a 6a-hydroxypterocarpan from Dalbergiavariabilis Phytochemistry 17: 14171418.
- LEE YR. 1995. A Concise New Synthesis of Angular Furanocoumarins: Angelicin, Oroselone and Oroselol. Tetrahedron 51: 30873094.
- LYRA DA, DE MELLO JF, DELLE MONACHE G, DELLE MONACHE F AND MARINI-BETTOLLO GB. 1979. Flavonoids from Derrismollis Gazz Chim Ital 109: 9394.
- MAGALHÃES AF, TOZZI AMGA, SALES BHLN AND MAGALHÃES EG. 1996.Twenty-three Flavonoids from Lonchocarpus subglaucescens Phytochemistry 42: 14591471.
- MAGALHÃES AF, TOZZI AMGA, MAGALHÃES EG, BLANCO IS AND NOGUEIRA MA. 1997. Three Dibenzoylmethane Derivatives from Lonchocarpusspecies. Phytochemistry 46: 10291033.
- MAGALHÃES AF, TOZZI AMGA, MAGALHÃES EG, NOGUEIRA MA AND RONCANCIO VJF. 1998. Ensaios Biológicos com Extractos Obtenidos de Raíces de Lonchocarpuslatifolius(Willd) D.C. y de un Nuevo Dibenzoilmetano Aislado. Revista Ceres 45: 351358.
- MAGALHÃES AF, TOZZI AMGA, MAGALHÃES EG, NOGUEIRA MA AND QUEIROZ SCN. 2000. Flavonoids from Lonchocarpus latifolius Roots. Phytochemistry 55: 787792.
- MAHLI SS, BASU SP, SINHA KP AND BANERJE NC. 1989. Pharmacological Effects of Karanjin and Pongamol. Indian J Animal Sci 59: 657660.
- MAZUR A, BAYLE D, LAB C, ROCK E AND RAYSSIGUIER Y. 1999. Inhibitory effect of procyanidin-rich extracts on LDL-oxidation in vitro. Atherosclerosis 149: 421422.
- MBAFOR JT, ATCHADE AT, NKENGFACK AE, FOMUM ZT AND STERNER O. 1995. Furanoflavones from Root bark of Millettia sanagana Phytochemistry 40: 949952.
- MC LAUGHLIN JL. 1995. Tres Bioensayos Simples para Químicos de Productos Naturales. Rev Soc Ven Quim 18: 1318.
- MIDDLETON EJR. 1998. Effect of plant flavonoids on immune and inflammatory cell function. Adv Exp Med Biol 439: 175182.
- MIDDLETON EJR, KANDASWAMI C AND THEOHARIDES TC. 2000. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52: 651673.
- MIYASE T, UENO A, NORO T AND FUKUSHIMA S. 1980. Studies on the Constituents of Lespedeza cyrtobotrya Miq. I. The Structures of a New Chalcone and two New isoflav-3-enes. Chem Pharm Bull 28: 11721177.
- PARMAR VS, RATHORE JS, JAIN R, HEMDERSPM DA AND MALONE JF. 1989. Ocurrence of Pongamol as the Enol Structure in Tephrosia purpurea Phytochemistry 28: 591593.
- RAO EV AND RAJU NR. 1979. Ocurrence of (-)-Isolonchocarpin in the Roots of Tephrosia purpurea Phytochemistry 18: 15811582.
- RAO EV AND RAJU NR. 1984. 2 flavonoids from Tephrosia purpurea Phytochemistry 23: 23392342.
- SAXENA G, FARNER S, TOWERS GHN AND HANCOCK REW. 1995. Use of specific dyes in the detection of antimicrobial compounds from crude plant extracts using a thin layer chromatography agar overlay technique. Phytochem Anal 6: 125129.
- SELWAY JW. 1986. Antiviral activity of flavones and flavans. Prog Clin Biol Res 213: 521536.
- SINHA B, NATU AA AND NANAVATI DD. 1982. Prenylated Flavonoids from Tephrosia purpureaseeds. Phytochemistry 21: 14681470.
- SUBRAHMANYAM K, MADHUSUDHANA J AND JAGANNADHA RAO KV. 1977. Chemical Examination of the Heartwood of PongamiaglabraVent.: Isolation of Chromenochalkones and Synthesis of Pongachalkones I and II. Indian J Chem 15: 1215.
- TALAPATRA SK, MALLIK AS AND TALAPATRA B. 1980. Pongaglabol, a new Hydroxyfuranoflavone and Aurantiamide acetate, a Dipeptide from the Flowers of Pongamiglabra Phytochemistry 19: 11991202.
- VANETTEN HD, MATTHEWS PS AND MERCER EH. 1983. (+)-Maachiain and (+)-Medicarpin as Phytoalexins in Sophora japonicaand Identification of the (-)-isomers by Biotransformation. Phytochemistry 22: 22912295.
- WATERMAN PG AND MAHMOUD EN. 1985. Flavonoids from the Seed of Lonchocarpuscostaricensis Phytochemistry 24: 571574.
- ZENG L, FUKAI T, KANEKI T, NOMURA T, ZHANG RY AND LOU ZC. 1994. Four new isoprenoid-substituted dibenzoylmethane derivatives, Glyinflanins A, B, C and D from the roots of Glycyrrhiza inflate Heterocycles 34: 8597.
Publication Dates
-
Publication in this collection
27 Aug 2007 -
Date of issue
Sept 2007
History
-
Received
04 Apr 2006 -
Accepted
07 Feb 2007