Abstracts
The substantial therapeutic potential of tempol (4-hydroxy-2,2,6,6-tetramethyl-1-piperidinyloxy) and related cyclic nitroxides as antioxidants has stimulated innumerous studies of their reactions with reactive oxygen species. In comparison, reactions of nitroxides with nitric oxide-derived oxidants have been less frequently investigated. Nevertheless, this is relevant because tempol has also been shown to protect animals from injuries associated with inflammatory conditions, which are characterized by the increased production of nitric oxide and its derived oxidants. Here, we review recent studies addressing the mechanisms by which cyclic nitroxides attenuate the toxicity of nitric oxidederived oxidants. As an example, we present data showing that tempol protects mice from acetaminophen-induced hepatotoxicity and discuss the possible protection mechanism. In view of the summarized studies, it is proposed that nitroxides attenuate tissue injury under inflammatory conditions mainly because of their ability to react rapidly with nitrogen dioxide and carbonate radical. In the process the nitroxides are oxidized to the corresponding oxammonium cation, which, in turn, can be recycled back to the nitroxides by reacting with upstream species, such as peroxynitrite and hydrogen peroxide, or with cellular reductants. An auxiliary protection mechanism may be down-regulation of inducible nitric oxide synthase expression. The possible therapeutic implications of these mechanisms are addressed.
cyclic nitroxides; tempol; antioxidants; nitric oxide-derived oxidants; inflammation; acetaminopheninduced hepatotoxicity
O considerável potencial terapêutico de tempol (4-hidroxi-2,2, 6,6-tetrametil-1piperiniloxila) e nitróxidos cíclicos relacionados como antioxidantes tem estimulado inúmeros estudos de suas reações com espécies reativas derivadas de oxigênio. Em comparação, as reações de nitróxidos com oxidantes derivados do óxido nítrico têm sido investigadas menos frequentemente. Todavia, essas reações são relevantes porque o tempol é também capaz de proteger animais de injúrias associadas a condições inflamatórias, as quais são caracterizadas por uma aumentada produção de óxido nítrico e derivados oxidantes. Aqui, discutimos estudos recentes abordando os mecanismos pelos quais nitróxidos cíclicos atenuam a toxicidade de oxidantes derivados do óxido nítrico. Como um exemplo, apresentamos dados que demonstram que o tempol protege camundongos do dano hepatotóxico promovido por altas doses de acetaminofeno e discutimos o possível mecanismo de proteção. Com base nos estudos sumarizados, é proposto que nitróxidos atenuam a injúria tecidual em condições inflamatórias devido principalmente a sua capacidade de reagir rapidamente com ambos, dióxido de nitrogênio e radical carbonato. Em conseqüência, os nitróxidos são oxidados ao cátion oxamônio correspondente, o qual, por sua vez, pode ser reciclado ao nitróxido através de reações com espécies precursoras, como peroxinitrito e peróxido de hidrogênio, ou com redutores celulares. Um possível mecanismo auxiliar de proteção é a regulação negativa da expressão da sintase do óxido nítrico induzível. As possíveis implicações terapêuticas desses mecanismos são abordadas.
nitróxidos cíclicos; tempol; antioxidantes; oxidantes derivados do óxido nítrico; inflamação; hepatotoxicidade induzida por acetaminofeno
BIOMEDICAL AND MEDICAL SCIENCES
Cyclic nitroxides inhibit the toxicity of nitric oxide-derived oxidants: mechanisms and implications
Ohara Augusto * Member Academia Brasileira de Ciências ; Daniel F. Trindade; Edlaine Linares; Sandra M. Vaz
Departamento de Bioquímica, Instituto de Química, Universidade de São Paulo Caixa Postal 26077, 05513-970 São Paulo, SP, Brasil
Correspondence to Correspondence to: Ohara Augusto E-mail: oaugusto@iq.usp.br
ABSTRACT
The substantial therapeutic potential of tempol (4-hydroxy-2,2,6,6-tetramethyl-1-piperidinyloxy) and related cyclic nitroxides as antioxidants has stimulated innumerous studies of their reactions with reactive oxygen species. In comparison, reactions of nitroxides with nitric oxide-derived oxidants have been less frequently investigated. Nevertheless, this is relevant because tempol has also been shown to protect animals from injuries associated with inflammatory conditions, which are characterized by the increased production of nitric oxide and its derived oxidants. Here, we review recent studies addressing the mechanisms by which cyclic nitroxides attenuate the toxicity of nitric oxidederived oxidants. As an example, we present data showing that tempol protects mice from acetaminophen-induced hepatotoxicity and discuss the possible protection mechanism. In view of the summarized studies, it is proposed that nitroxides attenuate tissue injury under inflammatory conditions mainly because of their ability to react rapidly with nitrogen dioxide and carbonate radical. In the process the nitroxides are oxidized to the corresponding oxammonium cation, which, in turn, can be recycled back to the nitroxides by reacting with upstream species, such as peroxynitrite and hydrogen peroxide, or with cellular reductants. An auxiliary protection mechanism may be down-regulation of inducible nitric oxide synthase expression. The possible therapeutic implications of these mechanisms are addressed.
Key words: cyclic nitroxides, tempol, antioxidants, nitric oxide-derived oxidants, inflammation, acetaminopheninduced hepatotoxicity.
RESUMO
O considerável potencial terapêutico de tempol (4-hidroxi-2,2, 6,6-tetrametil-1piperiniloxila) e nitróxidos cíclicos relacionados como antioxidantes tem estimulado inúmeros estudos de suas reações com espécies reativas derivadas de oxigênio. Em comparação, as reações de nitróxidos com oxidantes derivados do óxido nítrico têm sido investigadas menos frequentemente. Todavia, essas reações são relevantes porque o tempol é também capaz de proteger animais de injúrias associadas a condições inflamatórias, as quais são caracterizadas por uma aumentada produção de óxido nítrico e derivados oxidantes. Aqui, discutimos estudos recentes abordando os mecanismos pelos quais nitróxidos cíclicos atenuam a toxicidade de oxidantes derivados do óxido nítrico. Como um exemplo, apresentamos dados que demonstram que o tempol protege camundongos do dano hepatotóxico promovido por altas doses de acetaminofeno e discutimos o possível mecanismo de proteção. Com base nos estudos sumarizados, é proposto que nitróxidos atenuam a injúria tecidual em condições inflamatórias devido principalmente a sua capacidade de reagir rapidamente com ambos, dióxido de nitrogênio e radical carbonato. Em conseqüência, os nitróxidos são oxidados ao cátion oxamônio correspondente, o qual, por sua vez, pode ser reciclado ao nitróxido através de reações com espécies precursoras, como peroxinitrito e peróxido de hidrogênio, ou com redutores celulares. Um possível mecanismo auxiliar de proteção é a regulação negativa da expressão da sintase do óxido nítrico induzível. As possíveis implicações terapêuticas desses mecanismos são abordadas.
Palavras-chave: nitróxidos cíclicos, tempol, antioxidantes, oxidantes derivados do óxido nítrico, inflamação, hepatotoxicidade induzida por acetaminofeno.
INTRODUCTION
In spite of the substantial evidence indicating that oxidative mechanisms contribute to the pathogenesis of many human diseases, multiple large prospective intervention trials with classical antioxidants, such as vitamin C, vitamin E and b-carotene, failed to have a significant impact upon disease risk and progression (Brennan and Hazen 2003, Kris-Etherton et al. 2004). Among the many reasons responsible for such inconclusive results, an early lack of appreciation for the roles of nitric oxide-derived oxidants in pathologic processes and the limited actions of classical antioxidants should be included (Brennan and Hazen 2003, Szabo et al. 2007). Thus, it is conceivable that a better understanding of the mechanisms by which non-classical antioxidants, such as uric acid and tempol, protect animals during conditions of oxidative stress may contribute to the design of new antioxidant strategies to treat human diseases (Augusto et al. 2002).
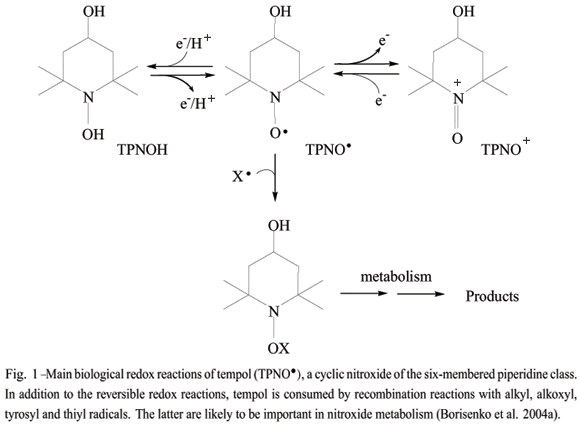
Tempol (4-hydroxy-2,2,6,6-tetramethyl-1-piperidinyloxy) and other cyclic nitroxides are particularly effective at reducing oxidative injury in cell and animal models (for recent reviews, see Soule et al. 2007, Kagan et al. 2007). Also known as aminoxyls and nitroxyls, these compounds are stable free radicals due to the three electron bond between N and O and the presence of a-substituents (usually methyl groups) that preclude radical-radical dismutation (Fig. 1). Due to their stability and paramagnetic nature, cyclic nitroxides have been extensively employed as probes in biophysical studies.In addition, nitroxides are multifunctional antioxidants because of their ability to react with diverse biological oxidants and reductants while being recycled through oxammonium cation- (TPNO+) and hydroxylamine-derivatives (TPNOH), respectively (Fig. 1). Eventually, nitroxides can be consumed by recombination reactions with certain radicals, such as thiyl radicals, and thesereactions are likely to be important in nitroxide metabolism (Fig. 1) (Borisenko et al. 2004a).
The most cited antioxidant mechanism of nitroxides is their superoxide dismutase activity, probably because it was the first property elucidated at the molecular level (Krishna et al. 1992). Other investigated nitroxide antioxidant mechanisms include inhibition of Fenton chemistry by the ability to oxidize transition metal ions, termination of radical chain reactions by radical recombination and acceptance of electrons from the mitochondrial electron transport chain (Soule et al. 2007, Kagan et al. 2007). In comparison, reactions of cyclic nitroxides with nitric oxide-derived oxidants have been less frequently investigated and discussed. This is relevant, however, because tempol has also been shown to protect animals from injuries associated with inflammatory conditions (reviewed in Thiemermann 2003), which are characterized by increased production of nitric oxideand its derived oxidants (reviewed in Radi 2004).
Thus, it is timely to critically review recent studies that address the mechanisms by which cyclic nitroxides in general, and tempol in particular, attenuate the toxicity of nitric oxide-derived oxidants. This protection is accompanied by decreased levels of protein tyrosine nitration in tissues, exemplified in mice treated with an overdose of acetaminophen, which will be examined and discussed in consideration of recent mechanistic studies (Bonini et al. 2002, Goldstein et al. 2003a, b, 2004,2006, Fernandes et al. 2005, Linares et al., in press, Vaz and Augusto 2006). The possible therapeutic implications of these mechanisms will also be addressed.
PROTECTIVE EFFECTS OF TEMPOL ARE PARALLELED BY DECREASED LEVELS OF PROTEIN TYROSINE NITRATION
It has long been recognized that nitric oxide, in addition to its signaling properties, can act as a cytotoxic effector when produced at high rates by either inflammatory stimuli-induced nitric oxide synthase (iNOS) or overstimulation of the constitutive forms. The cytotoxic effects of nitric oxide are largely dependent on the formation of oxidants that are more reactive towards biomolecules than itself, such as peroxynitrite (ONOOH/ ONOO-), nitrogen dioxide  and carbonate radical
and carbonate radical  . Peroxynitrite is produced from the diffusion-controlled reaction of nitric oxide
. Peroxynitrite is produced from the diffusion-controlled reaction of nitric oxide  with the superoxide radical
with the superoxide radical  , whereas nitrogen dioxide and carbonate radicals are produced in yields of 35% each from the reaction of peroxynitrite with carbon dioxide (Fig. 2). In addition, nitrogen dioxide can be produced from nitric oxide reaction with oxygen in hydrophobic environments where these gases concentrate, peroxynitrite reaction with hemeproteins and nitrite oxidation by hemeperoxidase enzymes (reviewed in Augusto et al. 2002, Radi 2004). On the other hand, nitrite is a biological product and precursor of nitric oxide (Gladwin et al. 2005).
, whereas nitrogen dioxide and carbonate radicals are produced in yields of 35% each from the reaction of peroxynitrite with carbon dioxide (Fig. 2). In addition, nitrogen dioxide can be produced from nitric oxide reaction with oxygen in hydrophobic environments where these gases concentrate, peroxynitrite reaction with hemeproteins and nitrite oxidation by hemeperoxidase enzymes (reviewed in Augusto et al. 2002, Radi 2004). On the other hand, nitrite is a biological product and precursor of nitric oxide (Gladwin et al. 2005).
The first recognized molecular footprint of the formation of nitric oxide-derived oxidants in vivo was the occurrence of 3-nitrotyrosine residues in tissue proteins (Beckman et al. 1994). Presently, it is known that biological nitration can occur at tyrosine and tryptophan residues, lipids and nucleic acid bases, but tyrosine nitration continues to attract the most attention because of its high occurrence in proteins and the sensitive detection of 3-nitrotyrosine in biological samples by immunological techniques. As a post-translational modification, protein tyrosine nitration has been evaluated as a potential indicator of acute and chronic disease states and a predictor of disease risk. In the case of infectious diseases, the occurrence of protein tyrosine nitration in infected tissues and cells has been considered evidence for theinvolvement of nitric oxide-derived oxidants in phagocyte microbicidal mechanisms (reviewed in Radi 2004).
It is important to mention that biological protein nitration occurs with low yields because the dominant reactions of nitric oxide and derived oxidants are those with abundant biological reagents, such as oxygen, carbon dioxide and biothiols (Lancaster 2006). Thus, protein thiol oxidation, glutathiolation and nitrosation are also important post-translational protein modifications caused by nitric oxide-derived oxidants. However, in addition to protein nitration, only protein thiol nitrosation can directly reveal production of nitric oxide-derived oxidants.
Protein tyrosine nitration under physiological conditions occurs by a free radical mechanism that involves recombination between nitrogen dioxide and protein tyrosyl radicals (Augusto et al. 2002, Radi 2004). Thus, efficient protein tyrosine nitration requires systems that direct or indirectly produce tyrosyl radicals in additionto nitrogen dioxide. Examples of such systems are peroxynitrite/carbon dioxide and hemeperoxidases, suchas myeloperoxidase (MPO)/ and eosinophil peroxidase(EPO)/hydrogen peroxide/nitrite. More recently, other hemeproteins, such as COX-2 (Palazzolo-Ballance et al. 2007) and iNOS (Marechal et al. 2007), have been proposed as catalysts of biological protein tyrosine nitration. All of these reactants and enzymes are considered to be important players in inflammatory processes. Thus, it is not surprising that in protecting experimental animals from injury associated with inflammatory conditions, tempol also decreased tissue protein nitration levels (reviewed in Thiemermann 2003).
In line with the general literature trend, however, the protective effects of tempol were mainly attributed to its superoxide dismutase activity that precludes hydroxyl radical and peroxynitrite formation. The possibility of tempol scavenging both hydroxyl radicals and peroxynitrite was also discussed (Thiemermann 2003). However, nitroxides are not particularly efficient superoxide dismutase (SOD) mimics (Goldstein et al. 2003b) and it was unlikely that local concentrations of administered tempol could surpass superoxide dismutase isoenzymes, which are abundant in most physiological environments. Likewise, administered tempol was unlikely to compete with endogenous targets for hydroxyl radicals, which react with most biomolecules at diffusion-controlled rates. A reaction between tempol and peroxynitrite also appeared unlikely because we had previously shown that peroxynitrite did not react directly with desferal and its derived nitroxide (Denicola et al. 1995). Thus, it was relevant to examine the interaction of tempol with peroxynitrite, peroxynitrite/carbon dioxide (Bonini et al. 2002) and MPO/hydrogen peroxide/nitrite in vitro (Vaz and Augusto 2006), cell culture (Fernandes et al. 2005) and mice (this work; also, Linares et al. in press). These studies, taken together with parallel studies by Goldstein and co-workers (Goldstein et al. 2003a, b, 2004, 2006), provided important information about the mechanisms by which tempol and related nitroxides can attenuate the toxicity of nitric oxide-derived oxidants.
TEMPOL DIVERTS PEROXYNITRITE REACTIVITY TOWARDS PROTEINS FROM NITRATION TO NITROSATION
In our initial studies in vitro, we demonstrated that tempol does not react directly with peroxynitrite but, instead, reacts with peroxynitrite-derived radicals to deactivate them while being oxidized to the oxammonium cation, which is recycled back to tempol by oxidizing the remaining peroxynitrite to nitric oxide and oxygen. Tempol recycling is not continuous because part of it is consumed by reactions with protein-derived radicals (Fig. 2). Thus, tempol diverts peroxynitrite reactivity from nitrating to nitrosating mechanisms in vitro (Bonini et al. 2002) and in cells (Fernandes et al. 2005). Ourconclusion was extended by parallel studies of Goldstein and co-workers (Goldstein et al. 2003a, b, 2004, 2006), who determined the second order rate constants of tempol and tempo and their corresponding oxammonium cation- and hydroxylamine-derivatives with a series of oxidants, including nitric oxide-derived oxidants (Table I). Tempo, the tempol analogue that lacks the4-OH group (Fig. 1), was prioritized because its oxammonium cation is more stable and easier to study kinetically. All of the data collected in Table I will be discussed later.
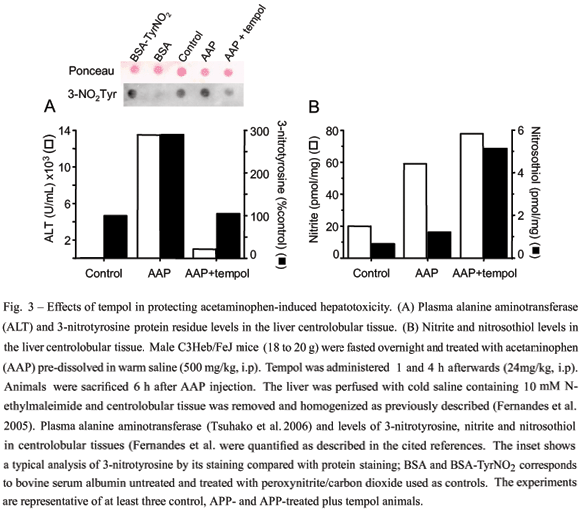
To examine whether tempol mechanisms in vivo relate with those operating in vitro (Fig. 2), we selected mice treated with an overdose of acetaminophen as our first animal model. Although a safe and effective analgesic at therapeutic levels, acetaminophen overdosescause hepatotoxicity, which, in severe cases, leads to liver failure in experimental animals and humans. Despite substantial efforts, the mechanism of acetaminophen overdose hepatotoxicity is still not completely understood. At first, emphasis was placed on its metabolism to N-acetyl-p-benzoquinone mediated by cytochromeP450. More recently, nitric oxide-derived oxidants produced by an inflammatory response emerged as a potential secondary mediator of hepatocyte cell death (Denicola and Radi 2005). Indeed, accumulating evidence indicates that leukocyte infiltration, inflammatory cytokines, iNOS expression and increased nitric oxide production contribute to acetaminophen-promoted liver injury (Knight et al. 2002, Ishida et al. 2002). Our results confirmed that administration of an overdose of acetaminophen (500 mg/kg; i.p) to mice caused liver damage because the plasma of the treated animals presented activity of a characteristic liver enzyme, alanine aminotransferase (ALT) (Fig. 3). Acetaminophen-induced liver damage was markedly inhibited by tempol, which also reduced protein 3-nitrotyrosine while increasing nitrite and nitrosothiol levels in the centrolobular liver tissue (Fig. 3). Such behavior in an animal model is analogous to that observed for bovine serum albumin and cells treated with a bolus of peroxynitrite in the presenceof tempol (Fig. 2) (Fernandes et al. 2005). Thus, our results support a role for peroxynitrite in acetaminophen-induced hepatotoxicity (Fig. 3).
INHIBITION OF IN VIVO LEISHMANICIDAL MECHANISM BY TEMPOL
The second animal model we investigated was a chronic infection model, the relatively resistant C57/Bl6 miceinfected with Leishmania amazonensis (Linares et al. in press). Previously, based on several lines of evidence, we proposed that C57Bl/6 mice are able to control cutaneous L. amazonensis infection mainly because their footpad macrophages are activated at an early infection stage to produce peroxynitrite-derived radicals that are crucial to parasite elimination (Linares et al. 2001). Thus, tempol could worsen infection because of its ability to neutralize peroxynitrite-derived radicals.
Since L. amazonensis is a chronic model, tempol was administered in the drinking water at 2 mM throughout the experiments (1-15 weeks) and it was shown to reach infected footpads by EPR analysis of tissue homogenates. This analysis revealed the presence of tempol in the homogenates as two of the forms shown in Fig. 1, the nitroxide ( ~ 30%) and the hydroxylamine-derivative ( ~ 70%). At the time of maximum infection (6 weeks), tempol increased footpad lesion size (120%) and parasite burden (150%). In lesion extracts, tempol decreased overall nitric oxide products and expression of inducible nitric oxide synthase to about 80% of control animals. Nitric oxide-derived products that are formed by radical mechanisms, such as 3-nitrotyrosine and nitrosothiol, decreased to about 40% of control mice. The effect of tempol in decreasing iNOS expression is probably due to its capacity to reduce NF-kB activation, as shown before in an acute model of inflammation in mice, carrageenan-induced pleuritis (Cuzzocrea et al. 2004). Taken together, our results indicated that tempol worsened L. amazonensis infection by a dual mechanism involving down-regulation of iNOS expression and scavenging of nitric oxide-derived oxidants (Linares et al. in press).
It is instructive to compare the results obtainedwith the above mice models. In the acute model, inhibition of iNOS expression is not apparent because nitrite levels are increased rather than decreased by tempol (Fig. 3). Although in both models tempol decreased protein tyrosine nitration levels in the affected tissues, nitrosothiol levels increased in the acute model but decreased in the chronic model. Considering our in vitro studies (Fig. 2), we could infer that peroxynitrite is likely to be involved in acetaminophen-induced liver damage but not in parasite killing. At this point, however, this conclusion appears premature. On the one hand, the data are limited and levels of nitric oxide-derived products, such as nitrate, nitrite and nitrosothiols, in animal tissues are influenced by many factors that remain to be fully characterized (Bryan et al. 2004). On the other hand, it is presently accepted that formation of both nitrosothiols and 3-nitrotyrosine occurs by radical mechanisms (Bryan et al. 2004, Lancaster 2006), which, in principle, can be inhibited by tempol (Figs. 1 and 2). A shift of peroxynitrite reactivity from nitration to nitrosation by tempol may become detectable only when local tempol concentrations are high enough to produce a sufficient amount of oxammonium cations to oxidize peroxynitrite to nitric oxide, as occurs upon a bolus addition of peroxynitrite to proteins and cells (Fig. 2) and, apparently, in the acute acetaminophen-induced model of inflammation (Fig. 3). In fact, tempol plus its hydroxylamine-derivative level measured in the footpads of mice infected with L. amazonensis was found to be around 1 µM, whereas, in the blood of acetaminophen-treated mice, it was about 10 times higher (data not shown) because the animals received two doses of tempol (24 mg/kg, i.p.) 1 and 4 h after acetaminophen administration (Fig. 3). Certainly, further studies are required to establish whether changes in the profile of nitric oxide-derived products by tempol may become a strategy to discriminate between different nitric oxide-derived oxidants in vivo. A step in this direction was to study the effects of tempol on hemeperoxidase-mediated protein nitration.
INHIBITION OF HEMEPEROXIDASE-MEDIATED PROTEIN TYROSINE NITRATION BY TEMPOL
Recently, we examined the effects of tempol on protein nitration mediated by myeloperoxidase (MPO), a mammalian enzyme that plays a central role in innate immune defense and various inflammatory processes. For comparative purposes, some experiments were also performed with HRP, an abundant plant peroxidase that is frequently used as a model of mammalian peroxidases. As the nitration target, we employed RNase, a model protein that contains 6 tyrosines, no tryptophan and all of 8 cysteines involved in intramolecular disulfide bonds (Vaz and Augusto 2006). Our results showed that tempol efficiently inhibits peroxidase-mediated RNasenitration. For instance, 10 µM tempol was able to inhibit by 90% the yield of 290 µM 3-nitrotyrosine produced from 370 µM RNase. Also, we showed that tempol reacts with MPO-I and HRP-I with second order rate constants that are orders of magnitude higher (106 and 103 M-1 s-1, respectively) than that reported for ferryl-myoglobin (101 M-1 s-1) (Krishna et al. 1996). Nevertheless, the determined rate constants were not high enough for tempol (10 µM) competing with nitrite(1 mM) for MPO-I and HRP-I. This was confirmed by substrate (nitrite and hydrogen peroxide) consumption and product (3-nitrotyrosine and oxygen) formationquantification and modeling by kinetic simulations. It was concluded that the inhibitory effect of tempol was mainly due to its reaction with nitrogen dioxide to produce the oxammonium cation, which, in turn, recycled back to tempol by reacting with hydrogen peroxide and superoxide radicals to produce oxygen and regenerate nitrite (Fig. 4). Another relevant tempol reaction was with RNase tyrosyl radicals, which consumed the nitroxide and precluded its continuous recycling (Vaz andAugusto 2006). Overall, this mechanism presents similarities to the one proposed for tempol diversion ofperoxynitrite/carbon dioxide reactivity in vitro and cells (Fig. 2).
These studies were important because a significant fraction of nitric oxide-derived oxidants produced invivo are likely to depend on reactions catalyzed bymammalian hemeperoxidases, such as MPO and eosinophil peroxidase (EPO) (Radi 2004, Szabo et al. 2007).In addition, the peroxidase activity of prostaglandin endoperoxide H synthase (Palazzolo-Ballance et al. 2007) and the oxygenase domain of inducible nitric oxide synthase (Marechal et al. 2007) have been recently proposed as possible sources of nitrogen dioxide in mammals. Since MPO is a very reactive peroxidase, our demonstration that tempol efficiently inhibits myeloperoxidase-mediated protein nitration should also hold forother mammalian peroxidases.
CONCLUSIONS AND THERAPEUTIC IMPLICATIONS
Most of the discussed reactions of tempol and its redox active forms, the oxammonium cation and the hydroxylamine (Fig. 1), with biological intermediates and their corresponding second order rate constants are collected in Table I. As we observed in the case of mice infected with L. amazonensis (Linares et al. in press), the predominant form of tempol appears to be the hydroxylamine because of the reducing physiological environment (Linares et al. in press). Since tempol is usually more reactive than the hydroxylamine towards biological intermediates (Table I), it is likely to be more important to scavenge them. The hydroxylamine derivatives certainly react with oxidants in vivo but they may bebetter suited to function as reservoirs of nitroxides. Thus, we propose that nitroxides attenuate the toxic effects of nitric oxide-derived oxidants mainly because of their ability to react rapidly with nitrogen dioxide and carbonate radicals (k > 108M-1 s-1) that would otherwise attack a variety of biological targets, causing oxidative and nitro-oxidative damage (Fig. 4). In addition, cyclic nitroxides may down-regulate iNOS expression, as demonstrated in the case of tempol administration to mice infected with L. amazonensis (Linares et al. in press). Inreacting with nitric oxide-derived radicals, nitroxidesare oxidized to the corresponding oxammonium cation,which, in turn, may be recycled back to the nitroxide by reacting with upstream species, such as peroxynitrite and hydrogen peroxide, to regenerate nitric oxide,superoxide radicals and oxygen (Fig. 4, Table I). Invivo, it is likely that oxammonium cation can be recycled by physiological reductants but these reactions have yet to be explored (Goldstein et al. 2003a). The most abundant physiological reductants, such as GSH and NAD(P)H, once oxidized may be repaired by enzymatic systems but, in the case of a pronounced redox imbalance, reductant-derived radicals may participate in oxidative reactions and contribute to tissue damage (Fig. 4). Such reactions may be responsible for thetoxicity associated with high doses of nitroxides (Soule et al. 2007, Kagan et al. 2007).
Because tempol is quite effective at reducing inflammatory injury in genuine physiological conditions, specifically in animal models (Thiemermann 2003), it is conceivable that targeting nitroxides to cells and sitesof increased nitric oxide-derived oxidant productionmay become a potential therapeutic strategy. A proof of the concept is found in the promising results obtained by targeting nitroxides to mitochondria and mitochondrial membranes to reduce oxygen-derived radical levels (Dhanasekaran et al. 2005, Kagan et al. 2007). On the other hand, the long-known tempol radioprotective effects are being explored for use in both clinical radiation oncology and radiological emergency situations, such as nuclear power plant disasters or terrorist attacks (Soule et al. 2007). Relevantly, a pilot study showed that topical tempol application resulted in significant prevention of radiation-induced alopecia in patients receiving cranial radiation.
In conclusion, this overview supports the development of therapeutic strategies based on nitroxides as antioxidants. Because of the dual role of nitric oxide-derived oxidants in inflammatory tissue injury and microorganism control, however, a potential increased risk of infection should be contemplated.
ACKNOWLEDGMENTS
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Projeto Institutos do Milênio: Redoxoma).
Manuscript received on October 25, 2007; accepted for publication on January 21, 2008; contributed by OHARA AUGUSTO* * Member Academia Brasileira de Ciências
- AUGUSTO O, BONINI MG, AMANSO AM, LINARES E, SANTOS CXC AND DE MENEZES SL. 2002. Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radic Biol Med 32: 841859.
- ARONOVITCH Y, GODINGER D, ISRAELI A, KRISHNA MC, SAMUNI A AND GOLDSTEIN S. 2007. Dual activity of nitroxides as pro- and antioxidants: catalysis of coppermediated DNA breakage and H2O2 dismutation. Free Radic Biol Med 42: 13171325.
- BAR-ON P, MOHSEN M, ZHANG R, FEIGIN E, CHEVION M AND SAMUNI A. 1999. Kinetics of nitroxide reaction with iron(II). J Am Chem Soc 121: 80708073.
- BECKMANN JS, YE YZ, ANDERSON PG, CHEN J, ACCAVITTI MA, TARPEY MM AND WHITE CR. 1994. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe-Seyler 375: 8188.
- BONINI M, MASON RP AND AUGUSTO O.2002. The mechanism by which 4-hydroxy-2,2,6,6-tetramethylpiperidene-1-oxyl (tempol) diverts peroxynitrite decomposition from nitrating to nitrosating species. Chem Res Toxicol 15: 506511.
- BORISENKO GG, MARTIN I, ZHAO Q, AMOSCATO AA, TYURINA YY AND KAGAN VE. 2004a. Glutathione propagates oxidative stress triggered by myeloperoxidase inHL-60cells. Evidence for glutathionyl radical-induced peroxidation of phospholipids and cytotoxicity. J Biol Chem 279: 2345323462.
- BORISENKO GG, MARTIN I, ZHAO Q, AMOSCATO AA AND KAGAN VE. 2004b. Nitroxides scavenge myeloperoxidase-catalyzed thiyl radicals in model systems and in cells. J Am Chem Soc 126: 92219232.
- BRENNAN ML AND HAZEN SL. 2003. Amino acid and proteinBoxidation in cardiovascular disease. Amino Acids 25: 365374.
- BRYAN NS, RASSAF T, MALONEY RE, RODRIGUEZ CM, SAIJO F, RODRIGUEZ JR AND FEELISCH M. 2004. Cellular targets and mechanisms of nitros(yl)ation: aninsight into their nature and kinetics in vivo. Proc Natl Acad Sci USA 101: 43084313.
- CUZZOCREA S, PISANO B, DUGO L, IANARO A, PATEL NS, CAPUTI AP AND THIEMERMANN C. 2004. Tempol reduces the activation of nuclear factor-kappaB in acute inflammation. Free Radic Res 38: 813819.
- DENICOLA A, SOUZA JM, GATTI RM, AUGUSTO O AND RADI R. 1995. Desferrioxamine inhibition of the hydroxyl radical-like reactivity of peroxynitrite: role of the ydroxamic groups. Free Radic Biol Med. 19: 1119.
- DENICOLA A AND RADI R. 2005. Peroxynitrite and drugdependent toxicity. Toxicol 208: 273288.
- DHANASEKARAN A, KOTAMRAJU S, KARUNAKARAN C, KALIVENDI SV, THOMAS S, JOSEPH J AND KALYANARAMAN B. 2005. Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide. Free Radic Biol Med 39: 567583.
- FERNANDES DC, MEDINAS DB, ALVES MJ AND AUGUSTO O. 2005. Tempol diverts peroxynitrite/carbon dioxide reactivity toward albumin and cells from protein-tyrosine nitration to protein-cysteine nitrosation. Free Radic Biol Med 38: 189200.
- GLADWIN MT ET AL. 2005. The emerging biology of the nitrite anion. Nat Chem Biol 1: 308314.
- GOLDSTEIN S, SAMUNI A AND RUSSO A. 2003a. Reaction of cyclic nitroxides with nitrogen dioxide: the intermediacy of the oxoammonium cations. J Am Chem Soc 125: 83648370.
- GOLDSTEIN S, MERENYI G, RUSSO A AND SAMUNI A. 2003b. The role of oxoammonium cation in the SODmimic activity of cyclic nitroxides. J Am Chem Soc 125: 789795.
- GOLDSTEIN S, SAMUNI A AND MERENYI G. 2004. Reactions of nitric oxide, peroxynitrite, and carbonate radicals with nitroxides and their corresponding oxoammonium cations. Chem Res Toxicol 17: 250257.
- GOLDSTEIN S, SAMUNI A, HIDEG K AND MERENYI G. 2006. Structure-activity relationship of cyclic nitroxides as SOD mimics and scavengers of nitrogen dioxide and carbonate radicals. J Phys Chem A 110: 36793685.
- ISHIDA Y, KONDO T, OHSHIMA T, FUJIWARA H, IWAKURA Y AND MUKAIDA N. 2002. A pivotal involvement of IFN-gamma in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J 16: 12271236.
- KAGAN VE, JIANG J, BAYIR H AND STOYANOVSKY DA. 2007. Targeting nitroxides to mitochondria: location, location, location, and... concentration: highlight commentary on "Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide". Free Radic Biol Med 43: 348350.
- KNIGHT TR, HO YS, FARHOOD A AND JAESCHKE H.2002. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine leivers: Protection by glutathione. J Pharmacol Exp Ther 302:468475.
- KRIS-ETHERTON PM, LICHTENSTEIN AH, HOWARD BV, STEINBERG D AND WITZTUM JL. 2004. Antioxidant vitamin supplements and cardiovascular disease. Circulation 110: 637641.
- KRISHNA MC, GRAHAME DA, SAMUNI A, MITCHELL JB AND RUSSO A. 1992. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide.Proc Natl Acad Sci USA 89: 55375541.
- KRISHNA MC, SAMUNI A, TAIRA J, GOLDSTEIN S, MITCHELL JB AND RUSSO A. 1996. Stimulation by nitroxides of catalase-like activity of hemeproteins. Kinetics and mechanism. J Biol Chem 271: 2601826025.
- LANCASTER JR JR. 2006. Nitroxidative, nitrosative, and nitrative stress: kinetic predictions of reactive nitrogen species chemistry under biological conditions. Chem Res Toxicol 19: 11601174.
- LINARES E, GIORGIO S, MORTARA RA, SANTOS CXC, YAMADA AT AND AUGUSTO O. 2001. Role of peroxynitrite in macrophage microbicidal mechanisms in vivo revealed by protein nitration and hydroxylation. FreeRadic Biol Med 30: 12341242.
- LINARES E, GIORGIO S AND AUGUSTO O. In press. Inhibition of in vivo leishmanicidal mechanisms by tempol. Nitric oxide downregulation and oxidant scavenging. Free Radic Biol Med.
- MARECHAL A, MATTIOLI TA, STUEHR DJ AND SANTOLINI J. 2007. Activation of peroxynitrite by inducible nitric-oxide synthase: a direct source of nitrative stress. J Biol Chem 282: 1410114112.
- PALAZZOLO-BALLANCE AM, SUQUET C AND HURST JK. 2007. Pathways for intracellular generation of oxidants and tyrosine nitration by a macrophage cell line. Biochemistry 46: 75367548.
- RADI R. 2004. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci USA 101: 40034008.
- SAMUNI A, GOLDSTEIN S, RUSSO A, MITCHELL JB, KRISHNA MC AND NETA P. 2002. Kinetics and mechanism of hydroxyl radical and OH-adduct radical reactions with nitroxides and with their hydroxylamines. J Am Chem Soc 124: 87198724.
- SOULE BP, HYODO F, MATSUMOTO K, SIMONE NL, COOK JA, KRISHNA MC AND MITCHELL JB. 2007. The chemistry and biology of nitroxide compounds. Free Radic Biol Med 42: 16321650.
- SZABO C, ISCHIROPOULOS H AND RADI R. 2007. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6: 662680.
- THIEMERMANN C.2003. Membrane-permeable radical scavengers (tempol) for shock, ischemia-reperfusion injury, and inflammation. Crit Care Med 31: S7684.
- TSUHAKO MH, AUGUSTO O, LINARES E, DAGLI ML AND PEREIRA CA. 2006. Association between nitric oxide synthesis and vaccination-acquired resistance to murine hepatitis virus by spf mice. Free Radic Biol Med 41: 15341541.
- VAZ SM AND AUGUSTO O. 2006. The mechanism by which tempol inhibits peroxidase-mediated protein nitration. Free Radic Biol Med 41S, S142.
Correspondence to:
Publication Dates
-
Publication in this collection
10 Mar 2008 -
Date of issue
Mar 2008
History
-
Accepted
21 Jan 2008 -
Received
25 Oct 2007