Abstracts
Chagasin may be considered a potential plant-incorporated protectant (PIP) protein due to its deleterious effects on insect pests. However, extensive safety studies with PIP's are necessary before introducing them into the target plant. Thus, a short-term feeding trial in rats with high doses of r-chagasin was conducted to provide evidences about its safety. Three test diets containing casein + r-chagasin (0.25, 0.5 and 1% of total protein) were offered to rats (10 days). The test diets did not show adverse effects upon the development, organ weight, hematological parameters and serum protein profiles of rats, providing preliminary information on the safety of r-chagasin.
chagasin; recombinant protein; hazard potential; short-term rat feeding trial
Chagasina pode ser considerada como uma proteína com potencial para protetor incorporado a planta (PPI), devido aos seus efeitos deletérios sobre insetos praga. No entanto, estudos extensivos de segurança com PPI são necessários antes de introduzi-las na planta alvo. Assim, um experimento de alimentação de curto prazo em ratos com doses elevadas de r-chagasina foi conduzido para fornecer evidências sobre a sua segurança. Três dietas teste contendo caseína + r-chagasina (0,25; 0,5 e 1% de proteína total) foram oferecidas aos ratos (10 dias). As dietas teste não apresentaram efeitos adversos sobre o desenvolvimento, o peso de órgãos, parâmetros hematológicos e perfis de proteínas séricas dos ratos, fornecendo informações preliminares sobre a segurança da r-chagasina.
chagasina; proteína recombinante; potencial de perigo; experimento de alimentação de curto prazo em rato
AGRARIAN SCIENCES
A brief report on some health aspects of rats fed with crescent levels of recombinant chagasin, a potential plant defense protein
Osmundo B. Oliveira NetoI, II; Davi F. FariasIII; Ilka M. VasconcelosIII; Norma S. PaesI; Ana C. S. MonteiroIV; Maria C. M. da SilvaI; Luciane M. GuimarãesI; Ana F.U. CarvalhoV; Maria F. Grossi-de-SáI, VI
IEmbrapa Recursos Genéticos e Biotecnologia, Parque Estação Biológica, Final W5 Norte, Asa Norte, 70770-900 Brasília, DF, Brasil
IICentro Universitário Euro-Americano (Unieuro), SCES, Tr 0 s/n lt 1, 70201-001 Brasília, DF, Brasil
IIIDepartamento de Bioquímica e Biologia Molecular, Universidade Federal do Ceará, Av. Mister Hull, s/n, 60455-970 Fortaleza, CE, Brasil
IVInstituto Nacional de Câncer, Divisão de Laboratórios de Oncologia Experimental, Rua Andre Cavalcanti, 37, Bairro de Fátima, 20231-050 Rio de Janeiro, RJ, Brasil
VDepartamento de Biologia, Universidade Federal do Ceará, Av. Mister Hull, s/n, 60455-970 Fortaleza, CE, Brasil
VIPós-Graduação em Ciências Genômicas e Biotecnologia, Universidade Católica de Brasília, SGAN Quadra 916, Módulo B, W5 Norte, Asa Norte, 70790-160 Brasília, DF, Brasil
Correspondence to Correspondence to: Maria Fatima Grossi-de-Sá E-mail: fatimasa@cenargen.embrapa.br
ABSTRACT
Chagasin may be considered a potential plant-incorporated protectant (PIP) protein due to its deleterious effects on insect pests. However, extensive safety studies with PIP's are necessary before introducing them into the target plant. Thus, a short-term feeding trial in rats with high doses of r-chagasin was conducted to provide evidences about its safety. Three test diets containing casein + r-chagasin (0.25, 0.5 and 1% of total protein) were offered to rats (10 days). The test diets did not show adverse effects upon the development, organ weight, hematological parameters and serum protein profiles of rats, providing preliminary information on the safety of r-chagasin.
Key words: chagasin, recombinant protein, hazard potential, short-term rat feeding trial.
RESUMO
Chagasina pode ser considerada como uma proteína com potencial para protetor incorporado a planta (PPI), devido aos seus efeitos deletérios sobre insetos praga. No entanto, estudos extensivos de segurança com PPI são necessários antes de introduzi-las na planta alvo. Assim, um experimento de alimentação de curto prazo em ratos com doses elevadas de r-chagasina foi conduzido para fornecer evidências sobre a sua segurança. Três dietas teste contendo caseína + r-chagasina (0,25; 0,5 e 1% de proteína total) foram oferecidas aos ratos (10 dias). As dietas teste não apresentaram efeitos adversos sobre o desenvolvimento, o peso de órgãos, parâmetros hematológicos e perfis de proteínas séricas dos ratos, fornecendo informações preliminares sobre a segurança da r-chagasina.
Palavras-chave: chagasina, proteína recombinante, potencial de perigo, experimento de alimentação de curto prazo em rato.
INTRODUCTION
Chagasin is a recently described cysteine proteinase (CP) inhibitor of 12 kDa molecular mass isolated from the pathogenic protozoan Trypanosoma cruzi (Monteiro et al. 2001). Recently, Monteiro et al. (2008) have reported that a recombinant form of chagasin (r-chagasin) can inhibit in vitro the major cysteine proteinase (EC 3.4.22) activity of the digestive tract of the common bean weevil Acanthoscelides obtectus (Say), resulting in deleterious effects upon this bruchid. In general, the control of these pest insects requires a heavy use of chemical pesticides, which cause considerable risks to human health, environment and non-target organisms, as well as the onset of insecticide resistance and high costs (Schmale et al. 2006). An alternative to chemical control methods is the development of genetically resistant plants, which have been shown to be more cost-effective and sustainable to pest management (Grossi-de-Sa et al. 2007).
An important approach for the safety assessment of agricultural products generated by biotechnology with the expression of transgenic proteins is the risk assessment of the candidate novel proteins (CNP's). Since some proteins introduced into crops using recombinant DNA technology are not natural constituents of foods or feeds previously consumed, the effects of these proteins for humans or animals is unknown. Therefore, it is very important to assess the potential hazard of CNP's before their introduction into the target plant (Delaney et al. 2008). Thus, the present study aimed to assess the short-term effects of diets containing increasing levels of r-chagasin (0.25, 0.5 and 1.0% of total protein) on some health aspects of growing rats. The analyzed parameters included growth rate, organ weight alterations, hematological data and serum protein profiles of rats.
MATERIALS AND METHODS
EXPRESSION AND PURIFICATION OF R-CHAGASIN
R-Chagasin was expressed in the periplasmic space of Eschericia coli MC1061 with the plasmid pHD313/Tc18 as an expression vector. The purification process followed the methodology described by Monteiro et al. (2008).
ANIMAL FEEDING TRIAL
Twenty outbred male Wistar rats (21 days old) were obtained from the animal facilities at BIOAGRI (Planaltina, Brazil). Four diets were prepared to contain 100 g protein/Kg diet: the control diet containing casein (CAS) as reference protein and three test diets containing casein and r-Chagasin at 0.25 g (CHS0.25), 0.50 g (CHS0.5) and 1.0 g (CHS1.0) protein/ Kg diet, corresponding to 0.25%, 0.5% and 1.0%, respectively, of total dietary protein. The diets were isoproteic and isocaloric and contained 40% maize starch, 10% potato starch, 15% glucose, 15% corn oil, 5% vitamin mix, 5% mineral mix and 10% protein. The rats (50.4 ± 4.2 g) were individually housed in metabolic cages (Nalgene®, Nalge Co., USA), kept at 22 °C in 12:12 h light-dark cycle and adapted to experimental conditions by receiving a fully balanced semi-synthetic casein diet for 10 days. After the adaptation period, rats' mean body weight was 81.5 ± 0.83 g. The animals were then divided into 4 groups (n = 6) and pair-fed for 10 days with control and test diets (15.0 g/rat/day) and water supplied ad libitum. Rats' weight, diet spillage, refused diet and clinical observations were recorded daily. At the end of the trial the rats were euthanized by halothane inhalation followed by cervical dislocation, the internal organs were dissected, weighted and subjected to a complete gross pathology examination. All procedures were approved by the Laboratory Animal Research Ethics Committee at BIOAGRI.
HEMATOLOGICAL AND SERUM BIOCHEMICAL DATA
At the end of the feeding trial (day 10), blood was collected by cardiac puncture and serum samples were separated by low-speed centrifugation (1,500 x g, for 15 min). The hematological parameters, measured with an automated analyzer (Sysmex M-2000, TOA Medical Electronics Co., Ltd., Hyogo, Japan) were: red blood cell count (RBC), hemoglobin (Hb), hematocrit (Ht), mean corpuscular volume (MCV), mean corpuscular hemoglobin and mean corpuscular hemoglobin concentration (MCHC). The serum biochemical parameters, determined according to instructions of BIOCLIN, Quibasa Ltda (Minas Gerais, Brazil), were: total protein (TP), albumin/globulin ratio (A/G) and albumin (Alb).
STATISTICAL ANALYSIS
The data were subjected to one-way analysis of variance (ANOVA) and the significant difference among means determined by Tuckey's test (p < 0.05).
RESULTS AND DISCUSSION
Recently, proteins introduced into crops via genetic modification by recombinant DNA techniques have been studied for their unintended effects upon non-target organisms (Delaney et al. 2008). In the present study, the recombinant chagasin protein was included in the diets of growing rats at 0.25, 0.5 or 1.0% of total protein, which is equivalent to 250, 500 or 1000 mg/ Kg diet, respectively. The chagasin concentrations in all test diets were much greater than that expressed in a genetically modified plant, which varies from 0.01 to 0.1% of total protein (Kilic and Akay 2007). Furthermore, the proportion of recombinant proteins in the diet used in the present work was close to that of PHA-E (1.5%) and GNA (1.25%) lectins (Poulsen et al. 2007a, b) used in animal feeding trial with GM rice (Oryza sativa L.), greater than that used for the toxin Cry1Ab in transgenic rice (10 mg/ Kg diet) (Schroder et al. 2007) and well above that of diets containing cotton seed (Gossypum hirsutum L.) bran (0.28 mg of Cry1F/ Kg diet and 0.096 mg of Cry1Ac/ Kg diet) (Dryzga et al. 2007).
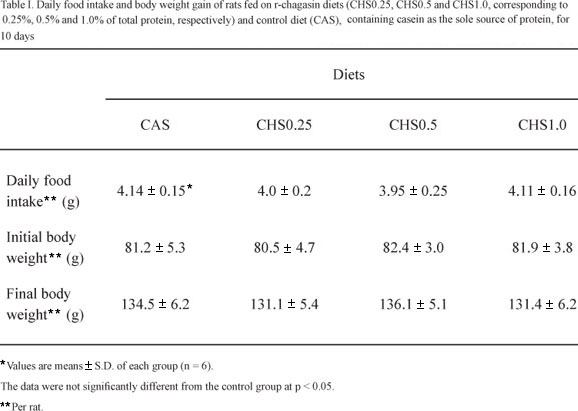
The results of the feeding trials with rats showed that the dietary intake and the growth of rats fed on test diets were similar to those fed on control diet as observed in Table I. The average dietary consumption per rat (g/day) of r-chagasin diets (4.0 ± 0.2 to CHS0.25, 3.95 ± 0.25 to CHS0.5 and 4.11 ± 0.16 to CHS1.0) was similar (p > 0.05) to that of casein group (4.14 ± 0.15 g), showing that the inclusion of r-chagasin in the diet did not reduce daily food intake. This finding suggests the lack of toxic and/or antinutritional effects in the recombinant protein since it is well known that rats reject diets when they are nutritionally deficient, when they show undesirable organoleptic properties and/or high levels of antinutrients (Liener 1980). At the end of the experimental period, the body weights of rats fed on the test diets (CHS0.25, 131.1 ± 5.4 g; CHS0.5, 136.1 ± 5.1 g and CHS1.0, 131.4 ± 6.2 g) were similar (p > 0.05) to those of rats fed on the CAS diet (134.5 ± 6.2 g). Thus, the body weight gain of rats fed on r-chagasin diets was similar to those of rats fed on casein as the sole source of protein. It is likely that the lack of deleterious effects of chagasin on rats' growth can be explained by its specificity towards cysteine proteinases (CP) (Monteiro et al. 2001, 2008). These enzymes are not part of the enzymatic machinery involved in the digestive process of mammals (Isenman et al. 1999). Therefore, CP inhibitors should be of little importance for these organisms. On the other hand, CP constitutes one class of digestive enzymes widely distributed among coleopteran species (Murdock et al. 1987) although few studies have reported the successful control of coleoptera species using CP inhibitors. One such example involves the constitutive expression of the rice cysteine proteinase inhibitor, oryzacystatin, in transgenic poplar trees, thereby conferring resistance to the coleopteran pest Chrysomela tremulae (Fabricius) (Leplé et al. 1995).
The presence of purified r-chagasin in casein-based diets did not promote significant organ weight alterations (data not show) or visual structural damage (stomach, small intestine, cecum and colon, kidneys, liver and pancreas) when compared to those of rats fed on CAS diet, suggesting that dietary r-chagasin did not cause any toxic effect upon these organs. The same has been described to occur with mice fed for 28 days on the entomotoxic proteins Cry34Ab1 and Cry35Ab1 (Juberg et al. 2009). Likewise, no significant difference was observed for any hematological and serum biochemical parameters in either treatment when compared to control group (Table II). Serum biochemical data of rats on diet containing 0.25%, 0.5% or 1.0% of r-chagasin revealed that total serum protein was similar to that of rats fed on the CAS diet. Similarly, the concentrations of serum albumin and globulin were not affected by chagasin diets at any of the different levels of inclusion tested (average values of 4.0 ± 0.2 g/dL and 2.0 ± 0.2 g/dL, respectively) when compared to control (4.2 ± 0.3 g/dL and 2.1 ± 0.3 g/dL, respectively). Thus, regarding hematological and serum biochemistry parameters, no treatment-related changes were detected. Serum albumin level has been shown to be a good predictor for the clinical state of animals (Harkness and Wagner 1989). Thus, the results of this study indicate that dietary r-chagasin does not show significant toxicity even when administered at the level of 1.0 g r-chagasin/kg diet.
CONCLUSIONS
The present study provided preliminary evidence on the safety of the recombinant protein r-chagasin and enhances its biotechnological potential as a safe tool for the control of important crop pests. Further studies on the structural homology of chagasin with other toxic, allergenic or antinutritional proteins, as well as long-term toxicity studies, must be performed to guarantee total safety.
ACKNOWLEDGMENTS
This work was supported by grants from the Brazilian government, Empresa Brasileira de Pesquisa de Agropecuária (EMBRAPA), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Thanks to Dr Magnus Abrahamson (University of Lund, Sweden) for providing the plasmid pHD313/Tc18 and to BIOAGRI for the conduction of animal trials.
Manuscript received on March 30, 2010
Accepted for publication on December 29, 2010
- DELANEY B ET AL. 2008. Evaluation of protein safety in the context of agricultural biotechnology. Food Chem Toxicol 46: S71-S97.
- DRYZGA MD, YANO BL, ANDRUS AK AND MATTSSON JL. 2007. Evaluation of the safety and nutritional equivalence of a genetically modified cottonseed meal in a 90-day dietary toxicity study in rats. Food Chem Toxicol 45: 1994-2004.
- GROSSI-DE-SA MF ET AL. 2007. Susceptibility of Anthonomus grandis (Cotton boll weevil) and Spodoptera frugiperda (Fall armyworm) to a Cry1Ia-type toxin from a Brazilian Bacillus thuringiensis strain. J Biochem Mol Biol 40: 773-782.
- HARKNESS JE AND WAGNER JE. 1989. The biology and medicine of rabbits and rodents, 2nd ed., Philadelphia: Lea & Febiger, 210 p.
- ISENMAN L, LIEBOW C AND ROTHMAN S. 1999. The endocrine secretion of mammalian digestive enzymes by exocrine glands. Am J Physiol Endocrinol Metab 276: 223-232.
- JUBERG DR, HERMAN RA, THOMAS J, BROOKS KJ AND DELANEY B. 2009. Acute and repeated dose (28 day) mouse oral toxicology studies with Cry34Ab1 and Cry35Ab1 Bt proteins used in coleopteran resistant DAS-59122-7 corn. Regul Toxicol Pharmacol 54: 154-163.
- KILIC A AND AKAY MT. 2008. A three generation study with geneticallymodified Bt corn in rats: Biochemical and histopathological investigation. Food Chem Toxicol 46: 1164-1170.
- LEPLÉ JC, BONADÉ-BOTTINO M, AUGUSTIN S AND PILATE G. 1995. Toxicity to Chrysomela tremulae (Coleoptera: Chrysomelidae) of transgenic poplars expressing a cysteine proteinase inhibitor. Mol Breed 1: 319-328.
- LIENER IE. 1980. Heat-labile antinutritional factors. In: SUMMERFIELD RJ AND BUNTING AH (Eds), Advances in legumes science, London: Royal Botanical Gardens, London, UK, p. 157-170.
- MONTEIRO ACS, ABRAHAMSON M, LIMA APCA, VANNIER- SANTOS MA AND SCHARFSTEIN J. 2001. Identification, characterization and localization of chagasin, a tight-binding cysteine protease inhibitor in Trypanosoma cruzi. J Cell Sci 114: 3933-3942.
- MONTEIRO ACS ET AL. 2008. A recombinant form of chagasin from Trypanosoma cruzi: inhibitory activity on insect cysteine proteinases. Pest Manag Sci 64: 755-760.
- MURDOCK LL, BROOKHART G, DUNN PE, FOARD DE, KELLEY S, KITCH L, SHADE RE, SHUKLE RH AND WOLFSON JL. 1987. Cysteine digestive proteinases in Coleoptera. Comp Biochem Physiol B Biochem Mol Biol 87: 783-787.
- POULSEN M ET AL. 2007a. Safety testing of GM-rice expressing PHA-E lectin using a new animal test design. Food Chem Toxicol 45: 364-377.
- POULSEN M ET AL. 2007b. A 90-day safety study in Wistar rats fed genetically modified rice expressing snowdrop lectin Galanthus nivalis (GNA). Food Chem Toxicol 45: 350-363.
- SCHMALE I, WACKERS FL, CARDONA C AND DORN S. 2006. Biological control of the bean weevil, Acanthoscelides obtectus (Say) (Col.: Bruchidae), by the native parasitoid Dinarmus basalis (Rondani) (Hym.: Pteromalidae) on small-scale farms in Colombia. J Stored Prod Res 42: 31-41.
- SCHRODER M ET AL. 2007. A 90-day safety study of genetically modified rice expressing Cry1Ab protein (Bacillus thuringiensis toxin) in Wistar rats. Food Chem Toxicol 45: 339-349.
Correspondence to:
Publication Dates
-
Publication in this collection
14 Feb 2012 -
Date of issue
Mar 2012
History
-
Received
30 Mar 2010 -
Accepted
29 Dec 2010