Abstracts
This study had the objective of assessing the genetic divergence in giant missionary grass (Axonopus jesuiticus x A. scoparius) germplasm based on morphological and agronomic traits. Five accessions were evaluated in the field: V14337, V14403, V14404, V14405 and V14406. Three contrasting groups were formed using the UPGMA clustering method: V14337 and V14404 formed one group, V14403 and V14405 formed another, and V14406 was isolated from the other accessions. The most striking traits for the identification of the accessions were the height of the plant and the change color of the leaf. Only V14406 accession had purplish green leaves. The other four accessions differed with regards to plant height and dry matter production, with superiority of V14337 and V14404 accessions. The high similarity, as assessed by the mean Euclidean distance, suggests that V14337 and V14404 share the same genotype. The genotypic variability among accessions indicates their potential use in breeding programs.
biomass; canopy structure; giant missionary grass; morphological traits; nutritive value
Este estudo teve como objetivo acessar a divergência genética em germoplasma de grama-missioneira-gigante (Axonopus jesuiticus x A. scoparius) com base em caracteres morfológicos e agronômicos. Cinco acessos foram avaliados no campo: V14337, V14403, V14404, V14405 e V14406. Três grupos contrastantes foram formados pelo método de agrupamento UPGMA: V14337 e V14404 formaram um grupo, V14403 e V14405 formaram outro, e V14406 ficou isolado dos demais acessos. Os caracteres morfológicos mais evidentes para identificar os acessos foram a altura da planta e a alteração da cor da folha. Somente o acesso V14406 apresentou folhas verde-púrpuras. Os outros quatro acessos diferiram quanto à altura da planta e produção de matéria seca, com superioridade dos acessos V14337 e V14404. A elevada similaridade, acessada pela distância euclidiana média, sugere que V14337 e V14404 compartilham o mesmo genótipo. A variabilidade genotípica entre os acessos indica seu uso potencial em programas de melhoramento genético.
biomassa; estrutura do dossel; grama-missioneira-gigante; caracteres morfológicos; valor nutritivo
INTRODUCTION
Cultivated pastures are the basis for Brazilian beef production, and occupy an area of 101.4 million hectares. However, very few forage cultivars are commercially available. Thus the release of new improved cultivars is in high demand to diversify pastures and alleviate this problem (Jank et al. 2011Jank L, Valle CB and Resende RMS. 2011. Breeding tropical forages. Crop Breed and Appl Biotech vS1: 27-34.).
The development of cultivars is laborious and time consuming, requiring several stages that involve acquisition, characterization, preliminary evaluation, assessment of production with cutting and/or grazing, pre-launching and launching materials. During the characterization phase, the description and primary evaluation of the genetic material is performed for the classification, seed increase and selection of representative lines. In the preliminary evaluation, agronomic assessments are conducted in small plots, usually under cutting (Schultze-Kraft and 't Mannetje 2000). The evaluation may also describe the variation existent in a specific collection based on attributes of agronomic importance that are strongly influenced by the environment, such as the yield (Jaramillo and Baena 2000Jaramillo S and Baena M. 2000. Material de apoyo a la capacitación y conservación ex situ de recursos fitogenéticos, Cali: IPGRI, 209 p.).
In the south of Brazil, the main summer perennial grasses that are grown for livestock include the genera Axonopus andPaspalum (native) and Cynodon,Panicum and Pennisetum (exotic and improved). However, some works showed that another native grass, the giant missionary grass (Axonopus jesuiticus (Araújo) Valls x A. scoparius (Flüggé) Kuhlm.) is a good option of a warm season pasture (Deschamps and Tcacenco 2000Deschamps FC and Tcacenco FA. 2000. Parâmetros nutri-cionais de forrageiras nativas e exóticas no Vale do Itajaí, Santa Catarina. Pesqui Agropecu Bras 35: 457-465., Lajús et al. 2011Lajús CR, Scheffer-Basso SM, Miranda M, Denardin RBN and Valls JF. 2011. Morphophysiological characterization of giant missionary grass accessions. Rev Bras Zootecn 40: 2302-2307., Miranda et al. 2012Miranda M, Scheffer-Basso SM, Escosteguy P, Lajús CR, Scherer EE and Denardin RBN. 2012. Dry matter production and nitrogen use efficiency of giant missionary grass in response to pig slurry application. Rev Bras Zootecn 41: 537-543.), even for the crop-livestock (Balbinot Jr et al. 2009) or silvipastoral system (Soares et al. 2009Soares AB, Sartor LR, Adami PF, Arella AC, Fonseca L and Mezzalira JC. 2009. Influence of luminosity on the behavior of eleven perennial summer forage species. Rev Bras Zootecn 38: 443-451.).
This vegetative propagated natural triploid hybrid (2n= 32) potentially resulted from more than one natural crossing event, that occurred spontaneously in Alto Vale do Itajaí, State of Santa Catarina (SC), Brazil (A.P.S. Penãloza et al., unpublished data). It is also referred to asA. catharinensis Valls (Probst et al. 2009Probst R, Quadros SAF, Erpen JF and Vincenzi ML. 2009. Produção de mudas de espécies forrageiras no sistema hidropônico de leito flutuante (floating) com solução nutritiva à base de biofertilizante ou adubo solúvel. Acta Sci-Agr 31: 349-355.), but the publication of this name has not been formalized (Lajús et al. 2011Lajús CR, Scheffer-Basso SM, Miranda M, Denardin RBN and Valls JF. 2011. Morphophysiological characterization of giant missionary grass accessions. Rev Bras Zootecn 40: 2302-2307.). One of its parents, A. scoparius (2n= 20) (A.P.S. Penãloza et al., unpublished data), a cespitose grass, is found on roadsides, open fields, grasslands and forest edges, from México to Bolívia and Brazil (Dedecca 1956Dedecca DM. 1956. As espécies brasileiras do gênero Axonopus (Gramineae). Bragantia 15: 251-296., Giraldo-Cañas 2008Giraldo-Cañas D. 2008. Revisión del género Axonopus (Poaceae: Paniceae): primer registro del género en Europa y novedades taxonómicas. Caldasia 30: 301-314.). It is widely distributed in Brazil, reaching southward, along the coast to the State of Santa Catarina, where it is often grown as forage plant, especially in the Alto Vale do Itajaí region (Smith et al. 1982Smith LB, Wasshausen DC and Klein RM. 1982. Gramíneas. Gêneros: 85. Paspalum até 115. Zea. In: REITZ P (Ed), Flora Ilustrada Catarinense, Itajaí: Herbário Barbosa Rodrigues, p. 1127-1130.). A. jesuiticus is a native, stoloniferous and tetraploid grass (2n= 40) (Hickenbick et al. 1975Hickenbick MCM, Valls JFM, Salzano FM and Fernandes MIBM. 1975. Cytogenetic and evolutionary relationships in the genus Axonopus (Gramineae). Cytologia 40: 185-204.), which is used in the pasture formation in the south of Brazil.
From the samples collected in the State of Santa Catarina, five accessions were sent to Embrapa Genetic Resources and Biotechnology and evaluated by Lajús et al. (2011)Lajús CR, Scheffer-Basso SM, Miranda M, Denardin RBN and Valls JF. 2011. Morphophysiological characterization of giant missionary grass accessions. Rev Bras Zootecn 40: 2302-2307. with regard to the dynamics and allocation of dry matter in greenhouse condition. However, these authors did not evaluate the morphological floral traits, the chemical composition nor the canopy structure. The present work had the objective of characterizing the germplasm of giant missionary grass for morphological and agronomic traits to verify its genetic divergence.
MATERIALS AND METHODS
Five accessions of giant missionary grass from Active Germplasm Bank of Embrapa Genetic Resources and Biotechnology (BRA), identified as V14337 (BRA: 002020), V14403 (BRA-002429), V14404 (BRA-002437), V14405 (BRA-002445) and V14406 (BRA-002453), were evaluated. The initial denomination and other information about their origin were reported in Lajús et al. (2011)Lajús CR, Scheffer-Basso SM, Miranda M, Denardin RBN and Valls JF. 2011. Morphophysiological characterization of giant missionary grass accessions. Rev Bras Zootecn 40: 2302-2307.. The sites of collection of these accessions were as follows: V14337, Rio do Oeste, SC (27°11'33,76 S, 49°47'48 W and 365 m asl); V14403, Dona Emma, SC (26°59'05' S, 49°43'32' W and 390 m asl); V14404, Itajaí Experiment Station, SC (26°54'28' S, 48°39'43' W and 60 m asl); V14405, Presidente Getúlio, SC (27°03'02' S, 49°37'22' W and 242 m asl) and V14406, Ituporanga, SC (27° 24' 52' S, 49° 36' 9' W and 360 m asl). Specimens were deposited in the herbarium of the Universidade de Passo Fundo (UPF).
The plants were cultivated in UPF, Passo Fundo, State of Rio Grande do Sul (RS), Brazil (28° 15'S, 52° 24'W and 687 m asl). The climate is subtropical Cfa (Kuinchtner and Burial 2001Kuinchtner A and Burial GA. 2001. Clima do estado do Rio Grande do Sul segundo a classificação de Köppen e Thornthwaite. Disciplinarum Scientia 2: 171-182.), with a mean annual temperature of 17.5°C, relative humidity of 72% and mean annual rainfall of 1,787 mm. The soil at the site was classified as Oxisol (Embrapa 1999), and presented the following properties: pH of 5.5; organic matter= 3.4%; P= 16.8 mg/dm3; K= 230 mg/dm3; Ca= 4.7 cmolc/dm3; Mg= 2.4 cmolc/dm3; Cu= 1.68 mg/dm3; Zn= 1.09 mg/dm3; Al= 0.0 cmolc/dm3 and Mn= 14.7 mg/dm3. The rooted tillers were transplanted in September of 2009, at 50 cm distance, in rows of 10 m length, without replications. During 2009 and 2010, the weeds were mechanically controlled from the experimental area and the plants were cut at 10 cm height after the first flowering. Nitrogen fertilization, at 100 kg of N/ha, was performed in December of 2009 and October of 2010.
The evaluations began in March of 2011, when the plants were in full bloom. The height of the vegetative canopy (considered to be the space between the base of the plants and the inflection of the upper leaves at the top of the canopy), and the reproductive canopy (considered to be the space between the base of the plants and the highest summit of the inflorescence), were initially measured using a ruler, in ten plants per accession. Ten tillers and ten inflorescences were harvested and characterized for the following attributes: the color; diameter and length of the internodes of the reproductive tillers; presence/absence of hairs and pubescence in the stem (low, medium and high); number of leaves per tiller; the leaf angle (using a protractor), and type of internode (solid or hollow). The leaves were characterized according to the hairiness (presence/absence), color, apex shape, blade margin, and type of ligule (membranous/hairy-membranous). The last fully expanded leaf was measured for the length, width and rigidity index (ratio between the blade length and the natural length of the stretched blade; Rhodes and Collins 1981Rhodes I and Collins RP. 1981. Canopy structure. In: Hodgson J, Baker RD, Davies A, Laidlaw A and Leaver J (Eds), Measurement Handbook. Hurley: British Grassland Society, p. 139-156.). The rachis was evaluated for the type (wavy, flattened or excavated), length, number of nodes, length of internode and number of branches. The largest branch was measured for the length and number of spikelets; ten spikelets were evaluated for the length, width, number of florets, color of the glumes, lemma and palea, stamens and stigmas.
In the following year, the accessions were characterized for the canopy structure, dry matter production and nutritive value. In the middle of winter (August of 2011), the plants were uniformly cut to 10 cm, followed by nitrogen fertilization. On September 24th and November 30th of 2011, 61 days apart, the plants were cut to 10 cm. The cuttings were made for five samples of 0.25 m2 area in each row. The material was dried in a forced-air oven for 72 hours at 60°C, weighed, ground and analyzed using the NIRS method for crude protein (CP), acid detergent fiber (ADF) and neutral detergent fiber (NDF). After the last cutting, the plants were allowed to grow freely until they reached full bloom, which occurred in mid-autumn (April of 2012), when they were then evaluated for canopy structure. For this evaluation, a sample of 0.25 m2 for each row was cut close to the ground, and the material was placed on a bench, followed by cutting layers of 10 cm, as based on the description of Rhodes and Collins (1981)Rhodes I and Collins RP. 1981. Canopy structure. In: Hodgson J, Baker RD, Davies A, Laidlaw A and Leaver J (Eds), Measurement Handbook. Hurley: British Grassland Society, p. 139-156.. The leaves, stems and flowers of each layer were separated and dried in a forced-air oven for 72 hours at 60°C, followed by weighing for the calculation of the percentage change.
The data were analyzed using descriptive statistics, with the means and standard deviations. Genetic relationships among accessions were evaluated by generating a similarity matrix based on the mean Euclidean distance. This matrix was submitted to cluster analysis using the Unweighted Pair-group Method with Arithmetic Mean (UPGMA). The relative contribution of traits affecting genetic divergence was calculated by Singh's method (Singh 1981Singh D. 1981. The relative importance of characters affecting genetic divergence. Indian J Genetic Pl Br 41: 237-245.). The procedures were conducted through the Genes software (Cruz 2006Cruz CD. 2006. Programa Genes: análise multivariada e simulação, Editora UFV: Viçosa, 175 p.).
RESULTS AND DISCUSSION
All of the accessions presented the following: cespitose stoloniferous habit; elliptic, solid and glabrous internode; pubescent node; glabrous leaf; acute leaf apex; conspicuously serrate leaf margin; membranous-ciliate ligule; absence of auricles; wavy, flattened and excavated panicle rachis; one bisexual floret/spikelet; lemma without awn; purple stamens and stigmas; straw yellow lemma and palea. Other morphological traits exhibited variation among the accessions (Table I).
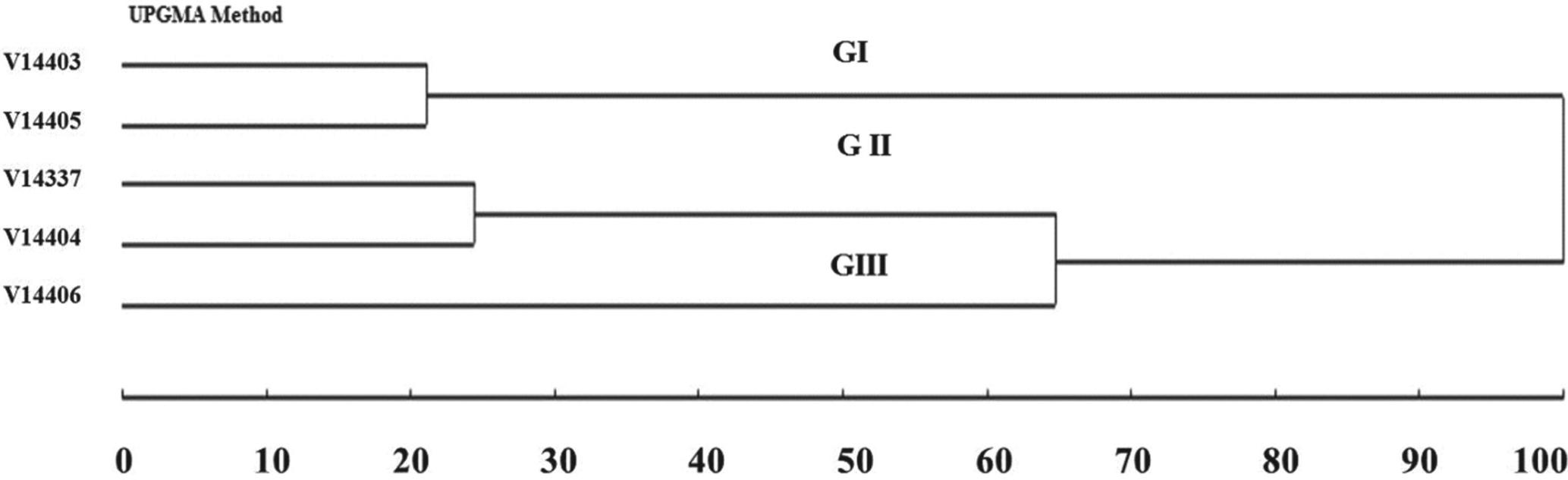
The UPGMA clustering revealed the formation of three groups: V14403 and V14405 (Group I), V14337 and V14404 (Group II) and V14406 (Group III) (Figure 1). The same arrangement was observed byLajús et al. (2011)Lajús CR, Scheffer-Basso SM, Miranda M, Denardin RBN and Valls JF. 2011. Morphophysiological characterization of giant missionary grass accessions. Rev Bras Zootecn 40: 2302-2307. who assessed the dynamics and partitioning of dry matter of the same accessions during continuous growth, which provided strength to the results. The accession V14406, isolated in Group III, was easily identified by the purplish color of the leaf blades. This attribute is characteristic of A. scoparius, one of the parent species of these hybrids. The greatest similarity was between V14403 and V14405 and between V14337 and V14404; the greatest dissimilarity occurred between V14405 and V14406 (Table II).
Dendrogram of dissimilarity among A. jesuiticus x A. scoparius accessions by the UPGMA clustering method based on mean Euclidean distances using 13 quantitative morphological traits (Table I).
The traits with the greatest relative contribution to the genetic divergence were the height of the reproductive and vegetative canopies, and the length of the largest panicle branch (Table III).
The accessions of group II (V14337 and V14404) showed the greatest height of the reproductive canopy (mean= 83.1 cm) (Table I). The accessions V14403 and V14405 presented the lowest values for the reproductive canopy (mean= 64.6 cm), the height of the vegetative canopy (mean= 46.1 cm) and the branch length (mean= 8.65 cm). Group III, consisting of accession V14406 and highlighted by the purplish color of the leaves and erect habit, exceeded the other accessions with regard to the length of the rachis of the panicle, the average number of nodes of the rachis, the number of branches/panicle and the number of spikelets/branch. This material presented leaves with a higher index of rigidity, a smaller leaf angle (27.5°) and the shortest spikelets. Lajús et al. (2011)Lajús CR, Scheffer-Basso SM, Miranda M, Denardin RBN and Valls JF. 2011. Morphophysiological characterization of giant missionary grass accessions. Rev Bras Zootecn 40: 2302-2307. indicated an angle of 80° for the leaves of such accessions, indicating incongruence with the results observed in the present work. However, the authors expressed the angle in relation to the abaxial side of the leaf, unlike our study in which the values were expressed on the adaxial surface. Therefore, the results were similar, as the values for accession V14406 ranged from 19.6 to 35° (mean 27.5°), indicating that the space between the stem and the abaxial side would be 80.5 to 65°, as reported by Lajús et al. (2011)Lajús CR, Scheffer-Basso SM, Miranda M, Denardin RBN and Valls JF. 2011. Morphophysiological characterization of giant missionary grass accessions. Rev Bras Zootecn 40: 2302-2307.. The leaf angle is an important element used to describe the plant canopy because it affects the structure of the pasture and animal bite size (Brinck et al. 2007Brinck GE, Casler MD and Hall MN. 2007. Canopy structure and neutral detergent fiber differences among temperate perennial grasses. Crop Sci 47: 2182-2189.).
From the results of the two cuttings made in the spring of 2011 (Table IV), three groups were formed (Figure 2), with the same components obtained from the morphological data (Figure 1). The accessions V14337 and V14404 were placed in the same group, with the shortest distance observed in the germplasm, and the greatest divergence observed between V14403 and V14404 (Table V). The variables with the greatest relative contribution to the genetic divergence were the dry matter yield obtained in the first (67.57%) and second (32.37%) cuttings (data not shown). Group I, composed of V14403 and V14405, was characterized by an average yield for the two cuttings of 1,684 kg of DM/ha, whereas the group with the highest value for this attribute was group II (V14337 and V14404), with an average of 2,286 kg of DM/ha. The lowest production was presented by V14406, with an average of 1,343 kg of DM/ha.
Dendrogram of dissimilarity among A. jesuiticus xA. scoparius accessions by UPGMA clustering method based on mean Euclidean distances using six agronomical traits (Table IV).
Traits Cutting 2 Height (cm) 45.0 ± 7.9 33.6 ± 6.3 46.6 ± 4.5 43.8 ± 7.9 36.0 ± 4.5 DMC (g/kg) 254 ± 7.5 266 ± 22.4 245 ± 22.6 236 ± 35.0 272 ± 21.5 DM (kg/ha) 2,808 ± 512 2,224 ± 538 3,016 ± 548 2,504 ± 1,065 2,587 ± 451 CP (g/kg) 131 ± 9.2 118 ± 6.3 116 ± 10.6 98 ± 15.8 129 ±10.1 NDF (g/kg) 737 ±7.0 726 ± 3.7 739 ± 24.6 724 ±14.5 721 ± 16.4 ADF (g/kg) 321 ± 13.9 324 ± 6.0 326 ± 13.0 324 ± 13.5 315 ± 12.7 DMC= dry matter content; DM= dry matter yield; CP= crude protein; ADF= acid detergent fiber; NDF= neutral detergent fiber.
The chemical composition indicated that all of the accessions were adequate for ruminant nutrition (Table IV). Among the accessions, V14406 had the highest crude protein content and the lowest content of acid detergent fiber. The ADF values were smaller than those reported byCrispim et al. (2003)Crispim SMA, Cardoso EL, Rodrigues CAG and Barioni W. 2003. Composição química da matéria seca de um campo de pastagem nativa submetido à queima, Pantanal, Mato Grosso do Sul, Brasil. Arch Latinoam Produc Anim 11: 157-162. forAxonopus purpusii (Mez) Chase, a native grass of the pastures of Pantanal, from 483 to 487 g/kg. Lima et al. (2001)Lima LMS, Alquini Y, Brito CJFA and Deschamps FC. 2001. Degradação ruminal dos tecidos vegetais e composição bromatológica de cultivares de Axonopus scoparius (Flüegge) Kuhlm. e Axonopus fissifolius (Raddi) Kuhlm. Cienc Rural 31: 509-515. characterized the chemical composition and degradability of a parent of giant missionary grass, A. scoparius, and found that the cultivar verde (with green leaf) proved more susceptible to ruminal digestion than the cultivar roxo (with purplish leaf). The chemical composition were close to the ones found in this study, of 60-130 (CP), 356-422 (ADF) and 637-675 (NDF) in the cultivar verde and 53-118 (CP), 406-410 (ADF) and 663-664 (NDF) in the cultivar roxo.
The percent contribution of the leaf, stem and inflorescence, factors used to describe the canopy structure (Rhodes and Collins 1981Rhodes I and Collins RP. 1981. Canopy structure. In: Hodgson J, Baker RD, Davies A, Laidlaw A and Leaver J (Eds), Measurement Handbook. Hurley: British Grassland Society, p. 139-156.), revealed high similarity between V14337 and V14404 (Figure 3). The highest layers of these accessions (70 to 90 cm) presented only inflorescences, and the leaves were concentrated between 10 and 40 cm from the bottom to the top. This distribution was similar to that observed for V14403 and V14406 (Figure 4), whereas a different distribution was verified for V14405, presenting approximately 80% of its leaves in the upper half layer, and the stem content showed the smallest variation among the accessions, contributing approximately 13 to 22% to the forage material. The density of leaves within the sward and the stem content are considered to be the main limiting factors affecting bite sizes (Stobbs 1973Stobbs TH. 1973. The effect of plant structure on the intake of tropical. II. Differences in sward structure, nutritive value, and bite size of animals grazing Setaria anceps and Chlorys gayana at various stages of growth. Aust J Agr Res 24: 821-829.), and a high allocation to the leaves is a desirable characteristic.
Vertical distribution of culm, leaf and inflorescence of the V14337 e V14404 accessions of A. jesuiticus x A. scoparius during the full bloom stage.
Vertical distribution of culm, leaf and inflorescence of the V14403, V14406 and V14405 accessions of A. jesuiticus xA. scoparius during the full bloom stage.
Based on the results obtained in this study, the hypothesis that the accessions V14337 and V14404 were the same material was strengthened. For the agronomic experiments conducted at the Experimental Station of Epagri Lages, State of Santa Catarina, the researchers used the accession V14404 under the name missionary giant, and it is likely to correspond to the same material received by Embrapa Genetics Resources and Biotechnology and recorded as V14337. The suspicion that these two accessions were the same material was reinforced by the results obtained in this study and by Lajús et al. (2011)Lajús CR, Scheffer-Basso SM, Miranda M, Denardin RBN and Valls JF. 2011. Morphophysiological characterization of giant missionary grass accessions. Rev Bras Zootecn 40: 2302-2307.. These authors compared the same five accessions of A. jesuiticus x A. scoparius and also found the greatest similarity between V14337 and V14404, which formed a group that was separate from the others. Furthermore, the similarity displayed by accessions V14403 and V14405, which were placed in the same group, could be related to the geographical proximity of their collection sites, Dona Emma and Presidente Getúlio, counties that border each other. However, the process of voluntary exchange of plant material between farmers should also be considered to explain the similarity between these accessions. The hybridization process that originated the giant missionary grass is linked to its perception by farmers, revealing distinct forms generated by spontaneous and parallels crosses of the same genitors (A.P.S. Penãloza et al., unpublished data).
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Universidade de Passo Fundo for fellowships and financial support.
REFERENCES
- Balbinot Junior AA, Moraes A, Veiga M, Pelissari A and Dieckow J. 2009. Integração lavoura-pecuária: intensificação de uso de áreas agrícolas. Cienc Rural 39: 1925-1933.
- Brinck GE, Casler MD and Hall MN. 2007. Canopy structure and neutral detergent fiber differences among temperate perennial grasses. Crop Sci 47: 2182-2189.
- Crispim SMA, Cardoso EL, Rodrigues CAG and Barioni W. 2003. Composição química da matéria seca de um campo de pastagem nativa submetido à queima, Pantanal, Mato Grosso do Sul, Brasil. Arch Latinoam Produc Anim 11: 157-162.
- Cruz CD. 2006. Programa Genes: análise multivariada e simulação, Editora UFV: Viçosa, 175 p.
- Dedecca DM. 1956. As espécies brasileiras do gênero Axonopus (Gramineae). Bragantia 15: 251-296.
- Deschamps FC and Tcacenco FA. 2000. Parâmetros nutri-cionais de forrageiras nativas e exóticas no Vale do Itajaí, Santa Catarina. Pesqui Agropecu Bras 35: 457-465.
- EMBRAPA-Empresa Brasileira de Pesquisa Agropecuária. 1999. Sistema de classificação de solos, Brasília: Centro Nacional de Pesquisa de Solos, 412 p.
- Giraldo-Cañas D. 2008. Revisión del género Axonopus (Poaceae: Paniceae): primer registro del género en Europa y novedades taxonómicas. Caldasia 30: 301-314.
- Hickenbick MCM, Valls JFM, Salzano FM and Fernandes MIBM. 1975. Cytogenetic and evolutionary relationships in the genus Axonopus (Gramineae). Cytologia 40: 185-204.
- Jank L, Valle CB and Resende RMS. 2011. Breeding tropical forages. Crop Breed and Appl Biotech vS1: 27-34.
- Jaramillo S and Baena M. 2000. Material de apoyo a la capacitación y conservación ex situ de recursos fitogenéticos, Cali: IPGRI, 209 p.
- Kuinchtner A and Burial GA. 2001. Clima do estado do Rio Grande do Sul segundo a classificação de Köppen e Thornthwaite. Disciplinarum Scientia 2: 171-182.
- Lajús CR, Scheffer-Basso SM, Miranda M, Denardin RBN and Valls JF. 2011. Morphophysiological characterization of giant missionary grass accessions. Rev Bras Zootecn 40: 2302-2307.
- Lima LMS, Alquini Y, Brito CJFA and Deschamps FC. 2001. Degradação ruminal dos tecidos vegetais e composição bromatológica de cultivares de Axonopus scoparius (Flüegge) Kuhlm. e Axonopus fissifolius (Raddi) Kuhlm. Cienc Rural 31: 509-515.
- Miranda M, Scheffer-Basso SM, Escosteguy P, Lajús CR, Scherer EE and Denardin RBN. 2012. Dry matter production and nitrogen use efficiency of giant missionary grass in response to pig slurry application. Rev Bras Zootecn 41: 537-543.
- Probst R, Quadros SAF, Erpen JF and Vincenzi ML. 2009. Produção de mudas de espécies forrageiras no sistema hidropônico de leito flutuante (floating) com solução nutritiva à base de biofertilizante ou adubo solúvel. Acta Sci-Agr 31: 349-355.
- Rhodes I and Collins RP. 1981. Canopy structure. In: Hodgson J, Baker RD, Davies A, Laidlaw A and Leaver J (Eds), Measurement Handbook. Hurley: British Grassland Society, p. 139-156.
- Schultze-Kraft R and 't Mannetje L. 2000. Evaluation of species and cultivars. In: 't Mannetje L and Jones RM (Eds), Field and laboratory methods for grassland and animal production research, Wallington: CABI International, p. 179-204.
- Singh D. 1981. The relative importance of characters affecting genetic divergence. Indian J Genetic Pl Br 41: 237-245.
- Smith LB, Wasshausen DC and Klein RM. 1982. Gramíneas. Gêneros: 85. Paspalum até 115. Zea. In: REITZ P (Ed), Flora Ilustrada Catarinense, Itajaí: Herbário Barbosa Rodrigues, p. 1127-1130.
- Stobbs TH. 1973. The effect of plant structure on the intake of tropical. II. Differences in sward structure, nutritive value, and bite size of animals grazing Setaria anceps and Chlorys gayana at various stages of growth. Aust J Agr Res 24: 821-829.
- Soares AB, Sartor LR, Adami PF, Arella AC, Fonseca L and Mezzalira JC. 2009. Influence of luminosity on the behavior of eleven perennial summer forage species. Rev Bras Zootecn 38: 443-451.
Publication Dates
-
Publication in this collection
10 Dec 2013 -
Date of issue
Mar 2014
History
-
Received
7 Feb 2013 -
Accepted
5 July 2013