Abstracts
This study focused on maximizing the extraction of total phenolics and flavonoids as well as the antioxidant activity measured by the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay from roasted yerba mate (Ilex paraguariensis) as a function of time (5, 7.5 and 10 min) and temperature of extraction (60, 75 and 90°C). The data were subjected to Response Surface Methodology and the results showed that polynomial equations were significant, did not present lack of fit, and presented adjusted determination coefficients above 98%, proving their suitability for prediction purposes. Using the desirability function, the optimum operating conditions to obtain a higher extraction of antioxidants was found to be 10 min of extraction at 90°C, and the tea prepared under these experimental conditions presented 427.74 mg of gallic acid equivalents per liter and 80.02% of inhibition of the DPPH radical. The flavonoid content was highly correlated (r = 0.9046, p < 0.001) to the antioxidant capacity.
Phenolic composition; correlation analysis; desirability function; phytochemicals; functional foods
Este trabalho objetivou maximizar a extração de fenólicos e flavonoides totais bem como a atividade antioxidante de erva-mate tostada (Ilex paraguariensis) frente ao radical livre 1,1-difenil-2-picrilhidrazila (DPPH) em função do tempo (5, 7,5 e 10 min) e a temperatura de extração (60, 75 e 90°C). Os dados foram submetidos à metodologia de superfície de resposta e os resultados mostraram que as equações polinomiais foram significativas, não apresentaram falta de ajuste e obtiveram coeficientes de determinação ajustados acima de 98%, comprovando a sua adequação para fins preditivos. Usando a função de desejabilidade, as condições ótimas para maximizar a extração de compostos antioxidantes foram de 10 min de extração a 90°C, e o chá preparado sob estas condições experimentais apresentou 427,74 mg de equivalentes de ácido gálico por litro e 80,02% de inibição da o radical DPPH. O teor de flavonoides foi altamente correlacionado (r = 0,9046, p < 0,001) com a atividade antioxidante.
Composição fenólica; análise de correlação; função de desejabilidade; fitoquímicos; alimentos funcionais
INTRODUCTION
Ilex paraguarienis A. St.-Hil. Var. paraguariensis (Aquifoliaceae) tea is widely consumed in South America, especially in Brazil, Argentina and Paraguay. The tea made from the leaves has several phytochemicals such as caffeic acid, chlorogenic acids, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, and 4,5-dicaffeoylquinic acid, catechins, amino acids, caffeine, quercetin, kaempferol, ascorbic acid, B1/B2vitamins, and rutin (Carini et al. 1998Carini M, Facino RM, Aldini G, Calloni M and Colombo L. 1998. Characterization of phenolic antioxidants from Maté (Ilex Paraguayensis) by liquid chromatography/mass spectrometry and liquid chromatography/tandem mass spectrometry. Rapid Commum Mass Spectrom 12: 1813-1819.,Dutra et al. 2010Dutra FLG, Hoffmann-Ribani R and Ribani M. 2010. Determination of phenolic compounds by isocratic high performance liquid chromatografic method during storage of yerba-mate. Quím Nova 33: 119-123., Berté et al. 2011Berté KA, Beux MR, Spada PK, Salvador M and Hoffmann-Ribani R. 2011. Chemical composition and antioxidant activity of yerba-mate (Ilex paraguariensis A.St.-Hil., Aquifoliaceae) extract as obtained by spray drying. J Agric Food Chem 59: 5523-5527.). All these compounds (solely or their interactions) are responsible for the alleged physiological effects of yerba-mate aqueous extract (tea) (Bracesco et al. 2011Bracesco N, Sanches AG, Contreras V, Menini T and Gugliucci A. 2011. Recent advances on Ilex paraguariensis research: minireview. J Ethnopharmacol 136: 378-384.).
Several in vitro, ex vivo, in vivo (using animals) and epidemiological protocols have indicated high antioxidant (Schinella et al. 2009Schinella G, Fantinelli JC, Tournier H, Prieto JM, Spegazzini E and Mosca SM. 2009. Antioxidant and cardioprotective effects of Ilex brasiliensis: A comparative study with Ilex paraguariensis (yerba mate). Food Res Int 42: 1403-1409., Reta et al. 2012Reta JV, Lanari MC and Valerga J. 2012. Polyphenol input to the antioxidant activity of yerba mate (Ilex paraguariensis) extracts. LWT - Food Sci Technol 45: 28-35.), antitumor (Mejía et al. 2010Mejía EG, Song YS, Heck CI and Ramírez-Mares MV. 2010. Yerba mate tea (Ilex paraguariensis): Phenolics, antioxidant capacity and in vitro inhibition of colon cancer cell proliferation. J Funct Foods 2: 23-34., Puangpraphant et al. 2011Puangpraphant S, Berhow MA and Mejia EG. 2011. Yerba Mate (Ilex Paraguariensis St. Hilaire) saponins inhibit human colon cancer cell proliferation. Food Chem 125: 1171-1178.), and anti-inflammatory (Arçari et al. 2011aArçari DP, Bartchewsky Jr W, Santos TW, Oliveira KA, Oliveira CC, Gotardo EM, Pedrazzoli JRJ, Gambero A, Ferraz LFC, Carvalho PO and Ribeiro M. 2011a. Anti-inflammatory effects of yerba maté extract (Ilex paraguariensis) ameliorate insulin resistance in mice with high fat diet-induced obesity. Mol Cell Endocrinol 335: 110-115.) effects of aqueous extracts of yerba mate, such as the commercial ready-to-drink beverages available in the marketplace in different countries, including the inhibition of high-density lipoprotein (HDL) oxidation induced by peroxyl radicals (Menini et al. 2007Menini T, Heck C, Schulze J, Mejia E and Gugliucci A. 2007. Protective action of Ilex paraguariensis extract against free radical inactivation of paraoxonase-1 in high-density lipoprotein. Planta Med 73: 1141-1147.), inhibitory ability against low-density lipoprotein (LDL) oxidation (Bracesco et al. 2003Bracesco N, Dell M, Rocha A, Behtash S, Menini T, Gugliucci A and Nunes E. 2003. Antioxidant Activity of a botanical extract preparation of Ilex paraguariensis: prevention of DNA double-strand breaks in Saccharomyces cerevisiae and human low-density lipoprotein oxidation. J Altern Complement Med 9: 379-387.), increase of gene expression of some antioxidant enzymes found in many organs (Matsumoto et al. 2009Matsumoto RLT, Bastos DHM, Mendonça S, Nunes VS, Bartchewsky W, Ribeiro ML and Carvalho PO. 2009. Effects of maté tea (Ilex paraguariensis) ingestion on mRNA expression of antioxidant enzymes, lipid peroxidation, and total antioxidant status in healthy young women. J Agric Food Chem 57: 1775-1780.), decrease in the serum concentration of LDL-cholesterol with a respective increase of the serum antioxidant capacity and glutathione content (Arçari et al. 2011bArçari DP, Porto VB, Rodrigues ERV, Varalda ER, Martins F, Lima RJ, Sawaya ACHF, Ribeiro ML and Carvalho PO. 2011b. Effect of mate tea (Ilex paraguariensis) supplementation on oxidative stress biomarkers and LDL oxidisability in normo- and hyperlipidaemic humans. J Funct Foods 3:190-197.).
Brazilians consumed more than 3 tons of dried tea leaves between 2009 and 2011, and roasted yerba-mate accounted for 70% of this total. The main reasons for the high consumption of such a tea are its sensory properties and tradition (Euromonitor International 2012). Beverage companies aim at delivering a required and demanded by consumers, and in this regard, consumers have become aware of the direct relationship between the regular consumption of foods rich in phenolic compounds, such as green tea or red wine, and the decreased risk of a range of cardiovascular and neurodegenerative diseases. In order to obtain a yerba-mate tea with optimized chemical composition as extracted by water, the most suitable statistical tool employed to maximize the compounds with the highest biological activity is the Response Surface Methodology (RSM). In accordance withBas and Boyaci (2007)Bas D and Boyaci IH. 2007. Modeling and Optimization I: Usability of response surface methodology. J Food Eng 78: 836-845., Granato et al. (2010a)Granato D, Castro IA, Ellendersen LSN and Masson ML. 2010a. Physical stability assessment and sensory optimization of a dairy-free emulsion using response surface methodology. J Food Sci 73: 149-155., Cruz et al. (2010)Cruz AG, Faria JAF, Walter EH, Andrade RR, Cavalcanti RN, Oliveira CA and Granato D. 2010. Processing optimization of probiotic yogurt containing glucose oxidase using response surface methodology. J Dairy Sci 93: 5059-5068. and Peña et al. (2014)Peña WEL, Andrade NJ, Soares NFF, Alvarenga VO, Rodrigues Junior S, Zuniga ADG, Granato D and Sant'Ana AS. 2014. Modelling Bacillus cereus adhesion on stainless steel surface as affected by temperature, pH and time. Int Dairy J 34: 153-158., RSM is a powerful mathematical technique based on regression analysis used to develop and improve (optimize) products and processes that have two or more factors that influence the response. RSM has been widely used by food companies to develop food products as well as to analyze, model and optimize processes (Granato et al. 2010bGranato D, Ribeiro JCB, Castro IA and Masson ML. 2010b. Sensory evaluation and physicochemical optimisation of soy-based desserts using response surface methodology. Food Chem 121: 899-906., Liu et al. 2012Liu C, Yu J, Wang J, Liu Z and Wang Q. 2012. Application of response surface methodology to optimise supercritical carbon dioxide extraction of oil from rapeseed (Brassica napus L.). Int J Food Sci Technol 47: 1115-1121., Delgado et al. 2012Delgado DA, Sant'Ana AS, Granato D and Massaguer PR. 2012. Inactivation of Neosartorya fischeri and Paecilomyces variotii on paperboard packaging material by hydrogen peroxide and heat. Food Control 23: 165-170., Ellendersen et al. 2012Ellendersen LSN, Granato D, Guergoletto KB and Wosiacki G. 2012. Development and sensory profile of a probiotic beverage from apple fermented with Lactobacillus casei. Eng Life Sci 12: 475-485.).
Food companies employ many technological operations and formulations to prepare the yerba-mate leaves, which create intrinsic differences in the chemical composition and pharmacological activities of commercial teas and, therefore, there is not a global process optimized by means of statistical techniques employed to extract phenolic compounds that display antioxidant activity from roasted yerba-mate. Consumers do not have access to neither chemical composition nor antioxidant capacity assays but they do have access to kitchen thermometers and chronometers, thus it would be interesting to investigate the effects of temperature and extraction time on the chemical composition and on the in vitroantioxidant activity of yerba-mate teas. In fact, no study was found regarding the response surface optimization of phenolic compounds and antioxidant activity of roasted yerba-mate teas. Based on these considerations, this study aimed at optimizing the experimental extraction conditions to obtain a tea made from roasted yerba-mate leaves (Ilex paraguariensis) that presents a high content of total phenolic compounds, flavonoids and in vitrofree-radical scavenging activity.
MATERIALS AND METHODS
Chemicals
Folin–Ciocalteu reagent and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich (St. Louis, MO, USA), and catechin, gallic acid, methanol, sodium nitrite, aluminum chloride, sodium hydroxide, and sodium carbonate were of analytical grade.
Experimental Design and Tea Preparation
In order to assess the effect of temperature (60, 75 or 90°C) and extraction time (5, 7.5 or 10 min) on the extraction of total phenolic compounds, flavonoids and on the antioxidant activity towards DPPH radical, a full factorial design (32) was applied and two replicates in the center point were added to the experiment to fit the surface plot for the responses and to estimate the pure error of the multiple regression models (Myers et al. 2009Myers RH, Montgomery DC and Anderson-Cook CM. 2009. 3rd ed., Response surface methodology: process and product optimization using designed experiments, Wiley: New York.), totaling 11 tea preparations. The ranges of time and temperature of extraction adopted in this work were based on a preliminary study.
Grounded roasted yerba mate leaves (brand Leão Junior®, place of production: Paraná, Brazil; species:Ilex paraguariensis A.St.-Hil., Aquifoliaceae) were acquired in the commerce of São Paulo, Brazil. A detailed quality control report about the botanical authenticity of the plant material was performed by the company. In order to prepare each tea sample (Table I), a total of 2.0 grams of the plant material was extracted with 100 mL of distilled water (ratio 1:50 of plant material:solvent) in a 500 mL glass beaker. Firstly, the water was heated up to each temperature value and added to a flask containing the plant material. The temperature inside the flask was regulated with a thermostatic bath with temperature control and the extraction procedure was carried out under magnetic stirring. The yerba mate and the water were thermostabilized at the specific temperatures, and the total extraction time was in accordance with the experimental design (Table I). The initial time was set when yerba mate was added to the water. The mixture was then filtered and immediately put into Falcon tubes and frozen at -18°C until further analysis.
DPPH Free-Radical Scavenging Activity Assay
The free-radical scavenging activity of tea samples towards the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical was determined in triplicate using the method proposed by Brand-Williams et al. (1995)Brand-Williams W, Cuvelier ME and Berset C. 1995. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci Technol 28: 25-30., with minor modifications. Briefly, a 100-µL aliquot of yerba mate tea (diluted seven times in distilled water) was mixed with 3.9 mL of a methanolic DPPH solution (125 µmol/L). The mixture was left to react in the dark for 30 min at 25°C, and then the absorbance was read at a wavelength of 517 nm using a spectrophotometer (Model 432, Femto Ltda, São Paulo, Brazil). Methanol was used as the negative control (blank). The DPPH free radical scavenging activity of each tea sample was calculated using Equation 1:
Determination of Total Phenolic Content
The total phenolic compound content of each tea sample (diluted three times in distilled water) was determined in triplicate using the Folin-Ciocalteu method (Singleton and Rossi 1965Singleton VL and Rossi Jr JA. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult 16: 144-158.). The absorbance was measured using a spectrophotometer (Model 432, Femto Ltda, São Paulo, Brazil) at the wavelength of 725 nm. The total phenolic content was determined by a standard curve (total phenolic concentration = 126.85 x absorbance; r = 0.9869; p < 0.001) of gallic acid (0 to 165 mg/L), and the results were expressed as mg of gallic acid equivalents per liter of tea (mg GAE/L).
Determination of Total Flavonoid Content
The total flavonoid content of the teas (diluted three times in distilled water) was determined using a modified colorimetric method employing aluminum chloride (Jia et al. 1999Jia Z, Tang M and Wu J. 1999. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64: 555-559.). The absorbance was measured at a 510 nm wavelength against a prepared blank (water) using a spectrophotometer (Model 432, Femto Ltda, São Paulo, Brazil). The flavonoid content was determined using a standard curve of catechin (0–250 mg/L) and the results, determined from a regression equation (flavonoid concentration = 476.55 × absorbance; r = 0.9996; p < 0.001), were expressed as milligrams of catechin equivalents per liter of tea (mg CTE/L). Data presented are the averages of three independent measurements.
STATISTICAL ANALYSES
Data were presented as mean ± pooled standard deviation. RSM is applied when at least a pair of samples differ (p < 0.05) from each other, that is, when the p-value from ANOVA is below 0.05. In this sense, differences among samples were checked by using one-way analysis of variance (ANOVA) followed by Fisher Least Significant Difference (LSD)post-hoc test (Granato et al. 2014Granato D, Calado VMA and Jarvis B. 2014. Observations on the use of statistical methods in food science and technology. Food Res Int 55: 137-149.). Prior to this analysis, the homogeneity of variances and normality of each response variable were confirmed by using the Hartley and the Shapiro-Wilk tests, respectively (Granato et al. 2011aGranato D, Castro IA, Piekarski FVBW, Benincá C and Masson ML. 2011a. Influence of passion fruit juice on colour stability and sensory acceptability of non-sugar Yacon-based pastes. Braz Arch Biol Technol 54: 149-159.). P-values below 0.05 were regarded as significant. The quality of the mathematical models fitted by RSM was evaluated by ANOVA, based on the F-test and on the percentage of total explained variance (R2) and also on the adjusted determination coefficient (R2 adj), which provide a measurement of how much of the variability in the observed response values could be explained by the experimental factors and their linear and quadratic interactions (Granato et al. 2010bGranato D, Ribeiro JCB, Castro IA and Masson ML. 2010b. Sensory evaluation and physicochemical optimisation of soy-based desserts using response surface methodology. Food Chem 121: 899-906.) A second-order polynomial quadratic equation (Equation 2) was used to fit the results:
where: Y is the predicted response, β0 is a constant, βj, βij and βjj are the regression coefficients and xj and xi are the levels of the independent variables (temperature and extraction time). Experimental data were then fitted to the selected regression model to achieve a proper understanding of the correlation between each factor and different responses. This was obtained by estimating the numerical values of the model terms (regression coefficients), whose significance was statistically judged in accordance with the t-statistic at a confidence interval of 95%. Non-significant (p > 0.05) terms were deleted from the initial equation and data were refitted to the selected model. The goodness-of-fit of the regression model was evaluated by the correlation coefficient (r), the coefficient of determination (R2), and also by the probability value (p-value) of the regression and lack-of-fit. To visualize the effects of temperature and extraction time and the relationships between the factors and the response variables, 2D contour plots of the fitted regression equations were generated using the Statistica v. 7 software (Statsoft, USA). A simultaneous optimization using the desirability function was performed in order to maximize the total phenolic compounds, the flavonoid content and also to maximize the antioxidant capacity of teas towards the DPPH radical (Derringer and Suich 1980Derringer G and Suich R. 1980. Simultaneous Optimization of Several Response Variables. J Qual Technol 12: 214-219.).In order to assess the correlation between response variables, the mean values of each tea preparation were initially checked for normal distribution by using the Shapiro-Wilk test. Secondly, the Pearson's (parametric data) and Spearman's rank (non-parametric data) coefficients were calculated for each pairwise of variables and the p-value for each coefficient was provided.
RESULTS AND DISCUSSION
The total phenolic compounds of yerba mate tea preparations varied from 349.92 to 428.31 mg GAE/L and the total flavonoid content ranged from 268.06 to 421.75 mg CTE/L of tea, corroborating the results obtained in previous studies for yerba mate and other types of teas (Seeram et al. 2008Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M and Heber D. 2008. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J Agric Food Chem 56: 1415-1422., Kodama et al. 2010Kodama DH, Gonçalves AESS, Lajolo FM and Genovese MI. 2010. Flavonoids, total phenolics and antioxidant capacity: comparison between commercial green tea preparations. Ciênc Tecnol Aliment 30: 1077-1082.,Reta et al. 2012Reta JV, Lanari MC and Valerga J. 2012. Polyphenol input to the antioxidant activity of yerba mate (Ilex paraguariensis) extracts. LWT - Food Sci Technol 45: 28-35., Zielinski et al. in press). The DPPH free radical scavenging activity was relatively high and values ranged from 52.38 to 80.65% of inhibition of the radical (Table I).
The experimental data, for all response variables, were homoscedastic (p ≥ 0.19) and there were significant (p < 0.001) differences among samples when the one-factor analysis of variances was employed, which is a basic requirement for RSM application. Response surface plots are presented in Figure 1.
Response surface plots to show the effect of extraction time (min) and temperature (°C) on the total content of phenolic compounds (A), flavonoids(B), and antioxidant activity towards DPPH(C) of roasted yerba mate leaves.
The linear correlation analysis showed that the total phenolic compounds correlated well with the content of total flavonoids (r = 0.7596, p < 0.01, n = 11), while the antioxidant activity of yerba mate tea infusions was significantly correlated to the content of total flavonoids, according to the linear regression analysis (Figure 2). Likewise, Grujic et al. (2012)Grujic N, Lepojevic Z, Srdjenovic B, Vladic J and Sudji J. 2012. Effects of different extraction methods and conditions on the phenolic composition of mate tea extracts. Molecules 17: 2518-2528. verified a correlation between the DPPH free radical scavenging activity and the total phenolic compounds (r = 0.85) and the flavonoid content (r = 0.74). The antioxidant activity flavonoids is due to the number and acidity of their phenolic hydroxyl groups and to the resonance between the free electron pair on the phenolic oxygen and the benzene ring, which increases electron delocalization, conferring a nucleophilic character upon the substitution position adjacent to the hydroxyl group (Cheynier 2006Cheynier V. 2006. In: Flavonoids: chemistry, biochemitry and applications; Anderson OM and Markham KR (Eds), New York: Boca Raton, p. 263-370.).
Linear Regression Analysis between the total flavonoid content and the antioxidant capacity towards the DPPH radical.
The total content of phenolic compounds was significantly correlated with the free-radical scavenging activity towards DPPH radical (ρ = 0.6895, p = 0.02; n = 11). These results are in accordance with those reported elsewhere (Cao et al. 2007Cao W, Chen W, Sun S, Guo P, Song J and Tian CJ. 2007. Investigating the antioxidant mechanism of violacein by density functional theory method. Mol Struct Theochem 817: 1-4.,Hartwig et al. 2012Hartwig VG, Brumovsky LA, Fretes RM and Boado LS. 2012. A novel procedure to measure the antioxidant capacity of yerba maté extracts. Ciênc Tecnol Aliment 32: 126-133.). As also outlined by Cheynier (2006)Cheynier V. 2006. In: Flavonoids: chemistry, biochemitry and applications; Anderson OM and Markham KR (Eds), New York: Boca Raton, p. 263-370., Cao et al. (2007)Cao W, Chen W, Sun S, Guo P, Song J and Tian CJ. 2007. Investigating the antioxidant mechanism of violacein by density functional theory method. Mol Struct Theochem 817: 1-4. and Granato et al. (2011b)Granato D, Castro IA and Katayama FCU. 2011b. Phenolic composition of South American red wines classified according to their antioxidant activity, retail price and sensory quality. Food Chem 129: 366-373. it is widely accepted that the antioxidant capacity measured by various in vitromethods depends on several intrinsic factors and experimental conditions: quantity and interactions among phenolic compounds present in the test material, the concentration and chemical type of the free radical, the time employed in the assay, the dilution of the sample, pH, solubility, stereochemical structure, among others.
According to the data shown in Table II, it is possible to observe that the regression models proposed for the responses were highly significant (p < 0.0001). Indeed, no lack-of-fit (p ≥ 0.105) was found and both R2 and R2 adj were higher than 98%, indicating that the empirical models fit the experimental data satisfactorily and may be used for prediction purposes. For the total flavonoid content (R2 = 99.89, R2 adj = 99.63, p < 0.0001) and phenolic compounds (R2 = 99.72, R2 adj = 99.29, p < 0.0001), the quadratic and linear effects of extraction time and temperature as well as the linear and quadratic effects of the interaction between extraction time and temperature were significant (p < 0.05). For the DPPH model (R2 = 98.91, R2 adj = 98.19, p < 0.0001), the linear and quadratic effects of temperature as well as the quadratic effect of extraction time and the interaction between the linear effect of temperature and the quadratic effect of extraction time were deemed significant to represent the experimental data.
In practice, high values (> 70%) of determination coefficients (R2) are reasonable indicators of suitability of regression models to describe the influence of the independent variables (extraction time and temperature) on the dependent variables (flavonoids, phenolic compounds and DPPH) (Bas and Boyaci 2007Bas D and Boyaci IH. 2007. Modeling and Optimization I: Usability of response surface methodology. J Food Eng 78: 836-845.). It is possible to observe that the modeling of experimental data allowed for the generation of useful mathematical equations for general use, within the experimental range tested in this study, to predict the behavior of the system under different factor combinations. Indeed, food companies that want to develop mate teas from roasted leaves with a functional appeal (high antioxidants content or high concentration of phenolic compounds, for example) may use the approach employed in the study to test new ranges of extraction time and temperature or even other technological conditions to enhance the extraction of antioxidants from yerba mate leaves.
It is noteworthy that the modeling of the in vitroantioxidant activity of roasted yerba-mate teas is an important result seeing that food companies could use this statistical approach as the basis for developing products with a functional claim based on the antioxidant activity measured byin vitro protocols. Herein, the development of functional beverages with high content of phenolic compounds and antioxidant activity has been widely studied (Owczarek et al. 2004Owczarek L, Jasiñska U, Osiñska M and Skapska S. 2004. Juices and beverages with a controlled phenolic content and antioxidant capacity. Pol J Food Nutr Sci 13: 261-268.,Mello et al. 2009Mello ACB, Freitas RJS, Waszczynskyj N, Koehler HC, Wille GMFC and Berté KAS. 2009. Bebida gaseificada de erva-mate verde/Gasified drink of green yerba-mate. Bol Centro Pesqui Process Aliment 27: 19-26., Soccol et al. 2012Soccol CR, Lima IFP, Lindner JD, Parada JL and Soccol VT. 2012. Development of an innovative nutraceutical fermented beverage from herbal mate (Ilex paraguariensis A.St.-Hil.) extract. Int J Mol Sci 13: 788-800.) and the industry could benefit from these studies, that is, new functional food products, especially bioactive beverages, could be developed.
Another aspect that should also be mentioned here is the fact that many studies are conducted to improve (not optimize) the extraction conditions of flavonoids and phenolic compounds from medicinal herbs that exert antioxidant activity by using the one-at-a-time approach, that is, only one variable (for example: time, temperature, agitation, etc) is tested to render a higher content of phenolic compounds/antioxidant activity (Nishiyama et al. 2010Nishiyama MF, Costa MAF, Costa AM, Souza CGM, Boer CG, Bracht CK and Peralta RM. 2010. Chá verde brasileiro (Camellia sinensis var. asamica): efeitos do tempo de infusão, acondicionamento da erva e forma de preparo sobre a eficiência de extração dos bioativos e sobre a estabilidade da bebida. Ciênc Tecnol Aliment 30: 191-196.). This method seems to be attractive but does not depict the interaction between/among factors that influence the responses, leading to non-realistic optimization conditions. In this regard, RSM is considered to be the best statistical approach to assess the influence of different experimental factors and their linear and quadratic interactions on analytical responses and also to model and optimize the extraction conditions of phenolics that display important biological activities (Li et al. 2010Li B, Xu Y, Jin YX, Wu YY and Tu YY. 2010. Response surface optimization of supercritical fluid extraction of kaempferol glycosides from tea seed cake. Ind Crop Prod 32: 123-128., Chen et al. 2012Chen B, Zhou W, Ning M, Wang Z, Zou W, Zhang H and Wang Q. 2012. Evaluation of antitumour activity of tea carbohydrate polymers in hepatocellular carcinoma animals. Int J Biol Macromol 50: 1103-1108.).
Nishiyama et al. (2010)Nishiyama MF, Costa MAF, Costa AM, Souza CGM, Boer CG, Bracht CK and Peralta RM. 2010. Chá verde brasileiro (Camellia sinensis var. asamica): efeitos do tempo de infusão, acondicionamento da erva e forma de preparo sobre a eficiência de extração dos bioativos e sobre a estabilidade da bebida. Ciênc Tecnol Aliment 30: 191-196. used the ‘one-at-a-time’ approach to assess the effects of the infusion time, type of packaging of the herb (in bulk or in sachets) and brewing condition on the extraction of bioactive phenolic compounds from Brazilian green tea and authors verified that the use of the herb in bulk and 5 min of infusion was the most favorable condition for the extraction of bioactive compounds. On the other hand, Li et al. (2010)Li B, Xu Y, Jin YX, Wu YY and Tu YY. 2010. Response surface optimization of supercritical fluid extraction of kaempferol glycosides from tea seed cake. Ind Crop Prod 32: 123-128. used RSM to model the extraction of kaempferol from tea seed cake as a function of extraction time, temperature, pressure and ethanol content. The authors verified that the best combination of those factors to maximize the extraction of kaempferol was an extraction time of 150 min, pressure of 20 MPa, temperature of 80°C, and 60% aqueous ethanol solution. Likewise, Chen et al. (2012)Chen B, Zhou W, Ning M, Wang Z, Zou W, Zhang H and Wang Q. 2012. Evaluation of antitumour activity of tea carbohydrate polymers in hepatocellular carcinoma animals. Int J Biol Macromol 50: 1103-1108. used a Box–Behnken design followed by RSM analysis to maximize the extraction of a tea carbohydrate that presents a considerable antitumor activity. For this purpose, authors employed temperature, extraction time and solvent–solid ratio as independent variables and verified that the best experimental conditions to obtain an optimized extraction of carbohydrates were an extraction temperature of 90°C, extraction time 30 min, and solvent–solid ratio 5:1.
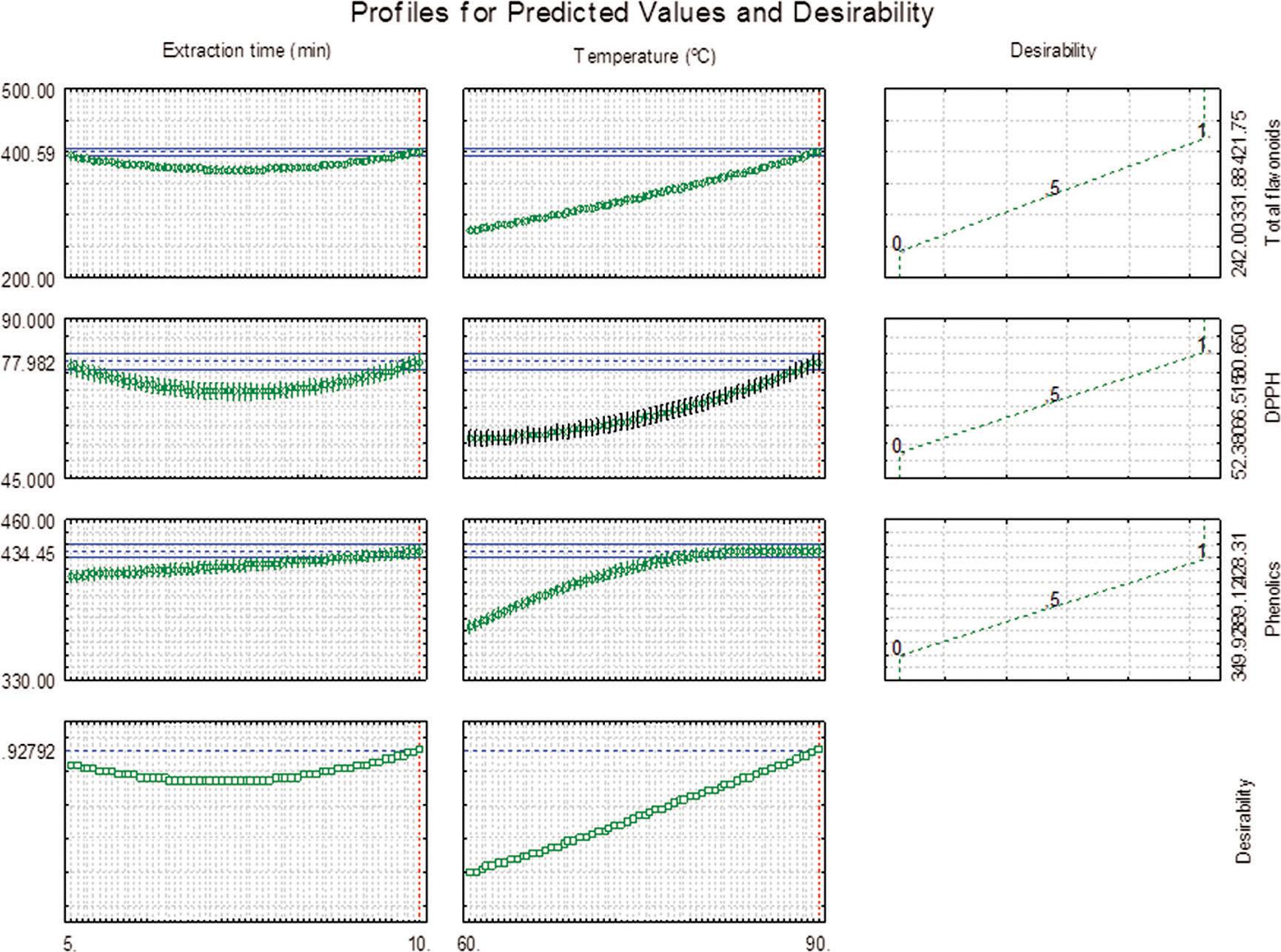
The final result for the simultaneous optimization using the desirability function (desirability index of 0.9279) suggested that the tea made from yerba-mate leaves extracted at 90°C for 10 min was the most adequate experimental conditions to achieve the best solution for this combination of variables (Figure 3). Indeed, this result corroborates the findings from the initial experimental data, where tea sample #8 (90°C, 10 min of extraction) presented the highest content of phenolics, flavonoids and also antioxidant capacity.
Optimization of bioactive compounds from roasted yerba mate leaves using the desirability function (Derringer and Suich approach).
CONCLUSIONS
Response Surface Methodology was used to model and optimize the experimental conditions to extract phenolic compounds (including flavonoids) from roasted yerba-mate leaves that exert antioxidant activity towards DPPH radical. The best combination of extraction time and temperature were found to be 10 minutes at 90°C, which rendered a mean phenolic content of 427.74 mg GAE/L and 80.02% of inhibition of the DPPH radical. The total flavonoid content correlated closely to the antioxidant capacity, corroborating the fact that this phenolic class is responsible for the beneficial health effects of yerba-mate tea consumption. RSM proved to be effective in optimizing the extraction conditions of bioactive phenolic compounds from roasted I. paraguariensis leaves and results may be used to have an idea about the antioxidant activity and content of total phenolic compounds and flavonoids when different combination of extraction time and temperature are used to prepare teas.
ACKNOWLEDGMENTS
This project was conducted at and supported by UniFMU, and authors are grateful for the administrative support provided by Ricardo Cecílio (UniFMU) to make this project viable.
REFERENCES
- Arçari DP, Bartchewsky Jr W, Santos TW, Oliveira KA, Oliveira CC, Gotardo EM, Pedrazzoli JRJ, Gambero A, Ferraz LFC, Carvalho PO and Ribeiro M. 2011a. Anti-inflammatory effects of yerba maté extract (Ilex paraguariensis) ameliorate insulin resistance in mice with high fat diet-induced obesity. Mol Cell Endocrinol 335: 110-115.
- Arçari DP, Porto VB, Rodrigues ERV, Varalda ER, Martins F, Lima RJ, Sawaya ACHF, Ribeiro ML and Carvalho PO. 2011b. Effect of mate tea (Ilex paraguariensis) supplementation on oxidative stress biomarkers and LDL oxidisability in normo- and hyperlipidaemic humans. J Funct Foods 3:190-197.
- Bas D and Boyaci IH. 2007. Modeling and Optimization I: Usability of response surface methodology. J Food Eng 78: 836-845.
- Berté KA, Beux MR, Spada PK, Salvador M and Hoffmann-Ribani R. 2011. Chemical composition and antioxidant activity of yerba-mate (Ilex paraguariensis A.St.-Hil., Aquifoliaceae) extract as obtained by spray drying. J Agric Food Chem 59: 5523-5527.
- Bracesco N, Dell M, Rocha A, Behtash S, Menini T, Gugliucci A and Nunes E. 2003. Antioxidant Activity of a botanical extract preparation of Ilex paraguariensis: prevention of DNA double-strand breaks in Saccharomyces cerevisiae and human low-density lipoprotein oxidation. J Altern Complement Med 9: 379-387.
- Bracesco N, Sanches AG, Contreras V, Menini T and Gugliucci A. 2011. Recent advances on Ilex paraguariensis research: minireview. J Ethnopharmacol 136: 378-384.
- Brand-Williams W, Cuvelier ME and Berset C. 1995. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci Technol 28: 25-30.
- Cao W, Chen W, Sun S, Guo P, Song J and Tian CJ. 2007. Investigating the antioxidant mechanism of violacein by density functional theory method. Mol Struct Theochem 817: 1-4.
- Carini M, Facino RM, Aldini G, Calloni M and Colombo L. 1998. Characterization of phenolic antioxidants from Maté (Ilex Paraguayensis) by liquid chromatography/mass spectrometry and liquid chromatography/tandem mass spectrometry. Rapid Commum Mass Spectrom 12: 1813-1819.
- Chen B, Zhou W, Ning M, Wang Z, Zou W, Zhang H and Wang Q. 2012. Evaluation of antitumour activity of tea carbohydrate polymers in hepatocellular carcinoma animals. Int J Biol Macromol 50: 1103-1108.
- Cheynier V. 2006. In: Flavonoids: chemistry, biochemitry and applications; Anderson OM and Markham KR (Eds), New York: Boca Raton, p. 263-370.
- Cruz AG, Faria JAF, Walter EH, Andrade RR, Cavalcanti RN, Oliveira CA and Granato D. 2010. Processing optimization of probiotic yogurt containing glucose oxidase using response surface methodology. J Dairy Sci 93: 5059-5068.
- Delgado DA, Sant'Ana AS, Granato D and Massaguer PR. 2012. Inactivation of Neosartorya fischeri and Paecilomyces variotii on paperboard packaging material by hydrogen peroxide and heat. Food Control 23: 165-170.
- Derringer G and Suich R. 1980. Simultaneous Optimization of Several Response Variables. J Qual Technol 12: 214-219.
- Dutra FLG, Hoffmann-Ribani R and Ribani M. 2010. Determination of phenolic compounds by isocratic high performance liquid chromatografic method during storage of yerba-mate. Quím Nova 33: 119-123.
- Ellendersen LSN, Granato D, Guergoletto KB and Wosiacki G. 2012. Development and sensory profile of a probiotic beverage from apple fermented with Lactobacillus casei. Eng Life Sci 12: 475-485.
- Euromonitor International. 2012. Tea in Brazil. Available at, http://www.euromonitor.com/tea-in-brazil/report. Access, 10 December 2012.
» http://www.euromonitor.com/tea-in-brazil/report - Granato D, Calado VMA and Jarvis B. 2014. Observations on the use of statistical methods in food science and technology. Food Res Int 55: 137-149.
- Granato D, Castro IA, Ellendersen LSN and Masson ML. 2010a. Physical stability assessment and sensory optimization of a dairy-free emulsion using response surface methodology. J Food Sci 73: 149-155.
- Granato D, Castro IA and Katayama FCU. 2011b. Phenolic composition of South American red wines classified according to their antioxidant activity, retail price and sensory quality. Food Chem 129: 366-373.
- Granato D, Castro IA, Piekarski FVBW, Benincá C and Masson ML. 2011a. Influence of passion fruit juice on colour stability and sensory acceptability of non-sugar Yacon-based pastes. Braz Arch Biol Technol 54: 149-159.
- Granato D, Ribeiro JCB, Castro IA and Masson ML. 2010b. Sensory evaluation and physicochemical optimisation of soy-based desserts using response surface methodology. Food Chem 121: 899-906.
- Grujic N, Lepojevic Z, Srdjenovic B, Vladic J and Sudji J. 2012. Effects of different extraction methods and conditions on the phenolic composition of mate tea extracts. Molecules 17: 2518-2528.
- Hartwig VG, Brumovsky LA, Fretes RM and Boado LS. 2012. A novel procedure to measure the antioxidant capacity of yerba maté extracts. Ciênc Tecnol Aliment 32: 126-133.
- Jia Z, Tang M and Wu J. 1999. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64: 555-559.
- Kodama DH, Gonçalves AESS, Lajolo FM and Genovese MI. 2010. Flavonoids, total phenolics and antioxidant capacity: comparison between commercial green tea preparations. Ciênc Tecnol Aliment 30: 1077-1082.
- Li B, Xu Y, Jin YX, Wu YY and Tu YY. 2010. Response surface optimization of supercritical fluid extraction of kaempferol glycosides from tea seed cake. Ind Crop Prod 32: 123-128.
- Liu C, Yu J, Wang J, Liu Z and Wang Q. 2012. Application of response surface methodology to optimise supercritical carbon dioxide extraction of oil from rapeseed (Brassica napus L.). Int J Food Sci Technol 47: 1115-1121.
- Matsumoto RLT, Bastos DHM, Mendonça S, Nunes VS, Bartchewsky W, Ribeiro ML and Carvalho PO. 2009. Effects of maté tea (Ilex paraguariensis) ingestion on mRNA expression of antioxidant enzymes, lipid peroxidation, and total antioxidant status in healthy young women. J Agric Food Chem 57: 1775-1780.
- Mejía EG, Song YS, Heck CI and Ramírez-Mares MV. 2010. Yerba mate tea (Ilex paraguariensis): Phenolics, antioxidant capacity and in vitro inhibition of colon cancer cell proliferation. J Funct Foods 2: 23-34.
- Mello ACB, Freitas RJS, Waszczynskyj N, Koehler HC, Wille GMFC and Berté KAS. 2009. Bebida gaseificada de erva-mate verde/Gasified drink of green yerba-mate. Bol Centro Pesqui Process Aliment 27: 19-26.
- Menini T, Heck C, Schulze J, Mejia E and Gugliucci A. 2007. Protective action of Ilex paraguariensis extract against free radical inactivation of paraoxonase-1 in high-density lipoprotein. Planta Med 73: 1141-1147.
- Myers RH, Montgomery DC and Anderson-Cook CM. 2009. 3rd ed., Response surface methodology: process and product optimization using designed experiments, Wiley: New York.
- Nishiyama MF, Costa MAF, Costa AM, Souza CGM, Boer CG, Bracht CK and Peralta RM. 2010. Chá verde brasileiro (Camellia sinensis var. asamica): efeitos do tempo de infusão, acondicionamento da erva e forma de preparo sobre a eficiência de extração dos bioativos e sobre a estabilidade da bebida. Ciênc Tecnol Aliment 30: 191-196.
- Owczarek L, Jasiñska U, Osiñska M and Skapska S. 2004. Juices and beverages with a controlled phenolic content and antioxidant capacity. Pol J Food Nutr Sci 13: 261-268.
- Peña WEL, Andrade NJ, Soares NFF, Alvarenga VO, Rodrigues Junior S, Zuniga ADG, Granato D and Sant'Ana AS. 2014. Modelling Bacillus cereus adhesion on stainless steel surface as affected by temperature, pH and time. Int Dairy J 34: 153-158.
- Puangpraphant S, Berhow MA and Mejia EG. 2011. Yerba Mate (Ilex Paraguariensis St. Hilaire) saponins inhibit human colon cancer cell proliferation. Food Chem 125: 1171-1178.
- Reta JV, Lanari MC and Valerga J. 2012. Polyphenol input to the antioxidant activity of yerba mate (Ilex paraguariensis) extracts. LWT - Food Sci Technol 45: 28-35.
- Schinella G, Fantinelli JC, Tournier H, Prieto JM, Spegazzini E and Mosca SM. 2009. Antioxidant and cardioprotective effects of Ilex brasiliensis: A comparative study with Ilex paraguariensis (yerba mate). Food Res Int 42: 1403-1409.
- Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M and Heber D. 2008. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J Agric Food Chem 56: 1415-1422.
- Singleton VL and Rossi Jr JA. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult 16: 144-158.
- Soccol CR, Lima IFP, Lindner JD, Parada JL and Soccol VT. 2012. Development of an innovative nutraceutical fermented beverage from herbal mate (Ilex paraguariensis A.St.-Hil.) extract. Int J Mol Sci 13: 788-800.
- Zielinski AAF, Haminiuk CWI, Alberti A, Nogueira A, Demiate IM and Granato D. In press. A comparative study of the phenolic compounds and the in vitro antioxidant activity of different Brazilian teas using multivariate statistical techniques. Food Res Int. DOI: http://dx.doi.org/10.1016/j.foodres.2013.09.010
» http://dx.doi.org/10.1016/j.foodres.2013.09.010
Publication Dates
-
Publication in this collection
Apr 2014 -
Date of issue
June 2014
History
-
Received
15 Jan 2013 -
Accepted
15 July 2013