Abstracts
In this study, we analyzed diet, sexual dimorphism and bromeliad use in three populations of the hylid frog Phyllodytes luteolus from restinga habitats along the Brazilian coast. We found 13 arthropods categories in 161 stomachs. Ants and termites were the dominant prey items. The similar trophic niche across populations suggests this species has a conservative diet. We found sexual dimorphism regarding body size and jaw width. We recordedP. luteolus in five bromeliad species, but predominantly inAechmeablanchetiana (35.6% of individuals recorded). We recorded solitary individuals in 44% of occupied bromeliads, and never found two males sharing the same bromeliad. The data is suggestive that populations ofP. luteolus has a conservative diet independent of area, with ants and termites the being most relevant prey items. The sexual dimorphism in jaw and the solitary males may suggest that this species have territorial behavior.
Atlantic forest; bromeliad; diet; sexual dimorphism; territorial behavior
Neste estudo, analizamos a dieta, dimorfismo sexual e uso de bromélias em três populações do hilídeo Phyllodytes luteolus de habitats de restinga ao longo da costa brasileira. Encontramos 13 categorias de artrópodes em 161 estômagos. Formigas e cupins foram as presas dominantes. O nicho trófico semelhante registrado entre as populações sugere que essa espécie possui uma dieta conservativa. Encontramos dimorfismo sexual referentes ao tamanho do corpo e largura da mandíbula. Observamos P. luteolus em cinco espécies de bromélias, mas predominantemente em Aechmeablanchetiana (35.6% dos indivíduos encontrados). Encontramos indivíduos solitários em 44% das bromélias ocupadas, e nunca dois machos dividindo a mesma bromelia. Os dados sugerem que as populações de P. luteolus possuem uma dieta conservadora independente da localidade, com formigas e cupins como as presas mais relevantes. O dimorfismo sexual no tamanho das mandíbulas e os machos solitários podem sugerir que esta espécie possui comportamento territorial.
Mata Atlântica; bromélia; dieta; dimor fismo sexual; comportamento territorial
INTRODUCTION
Anurans commonly consume arthropods as a primary food resource (Duellman and Trueb 1986Duellman WE and Trueb L. 1986. Biology of amphibians. 2ª ed., Baltimore and London: McGraw-Hill, 670 p., Lima and Moreira 1993Lantyer-SilvaAsf, SoléM and ZinaJ. 2014. Repro ductive biology of a bromeligenous frog endemic to the Atlantic Forest: Asparasphenodon arapapa Pimenta, Napoli and Haddad, 2009 (Anura: Hylidae). An Acad Bras Cienc86: 865-880., Ferreira et al. 2012Ferreira RF, Schineider Jap and Teixeira RL. 2012. Diet, fecundity and use of bromeliads by Phyllodytes luteolus (Anura: Hylidae) in southeastern Brazil. J Herpetol 46(1): 19-24.), and kinds and sizes of prey consumed may differ (Sabagh et al. 2012Rocha Cfd, Van Sluys M, Ornellas AB, Siqueira AE,Conde Cfv, Bittencourt EB, Oliveira Mgn, Barros MC and Magalhães Sap. 1996. The effects of fire on natural populations of Vrisea neoglutinosa in a relict restinga of Espírito Santo state. Bromélia 3: 16-26. , Maia-Carneiro et al. 2013Lima APand Moreira G. 1993. Effects of prey size and foraging mode on the ontogenetic change in feeding niche of Colostethus stepheni (Anura: Dendrobatidae). Oecologia 95(1): 93-102., Coco et al. 2014Coco L,Borges-Junior Vnt,Fusinatto LA, Kiefer MC, Oliveira JC, Araujo PG, Costa BM, Van Sluys M and Rocha Cfd. 2014. Feeding habits of the leaf litter frog Haddadus binotatus (Anura, Craugastoridae) from two Atlantic Forest areas in southeastern Brazil. An Acad Bras Cienc 86: 239-249.) between populations. This may be due to prey availability, which can be different among areas, differences in periods of collection, altitudes, and/or phylogeny (Sabagh et al. 2012). Anurans can also use different types of microhabitat, such as leaf-litter on the forest floor, rocks, streams, ponds, lakes, trees, and bromeliads (Eterovick 1999Eterovick PC. 1999. Use and sharing of calling and retreat sites by Phyllodytes luteolus in a modified environment. J Herpetol1(1): 17-22., Almeida-Gomes et al. 2008Almeida-Gomes M et al. 2008. Herpetofauna of an Atlantic rainforest area (Morro São João) in Rio de Janeiro State, Brazil. An Acad Bras Cienc 80: 291-300. , Duré et al. 2009, Martins et al. 2010Marra RV, Van Sluys M and Rocha Cdf. 2004. Food habits of Eleutherodactylus parvus (Anura: Leptodac tylidae) at Atlantic rainforest area, Southeastern Brazil. Herpetol Rev 35(2): 135-137. , Ferreira et al. 2012). Bromeligenous frogs reproduce, forage and complete their entire life cycle inside bromeliads (sensuPeixoto 1995Papp MG and Papp Cog. 2000. Decline in a population of the treefrog Phyllodytes luteolus after fire. Herpetol Rev 31(2): 93-95.). Such dependence of bromeliads is in direct relation with species natural history, influencing different aspects of its ecology (Giaretta 1996Frost DR. 2013. Amphibian Species of the World: an Online Reference. Version 5.6 (January 9, 2013). Electronic Database accessible athttp://research.amnh.org/herpetology/amphibia/index.html.American Museum of Natural History, New York.
http://research.amnh.org/herpetology/amp...
, Ferreira et al. 2012, Lantyer-Silva et al. 2014Howard AK, Forester JD,Ruder JM, Parmerlee Jr JS and Powell R. 1999. Natural history of a terrestrial Hispaniolan anole: Anolis barbouri. J Herpetol 4(33): 702-706.).
Phyllodytesluteolus is a bromeligenous anuran species predominantly found in restinga habitats, where they inhabit tank bromeliads (Peixoto 1995, Ferreira et al. 2012). Restingas are sandy, usually open coastal habitats interspersed within the Atlantic forest biome of Brazil.Phyllodytesluteolus occurs along the Brazilian coast from the state of Paraíba southwards to the north portion of the state of Rio de Janeiro (Vrcibradic et al. 2006Toft CA. 1981. Feeding ecology of Panamanian litter anurans: patterns in diet and foraging mode. J Herpetol 15: 139-144., Frost 2013), with the most inland record being from the state of Minas Gerais, where it occurs in some forest remnants (Feio and Caramaschi 2002Feio RNand Caramaschi U. 2002. Contribuição ao conhe cimento da herpetofauna do nordeste do estado de Minas Gerais, Brasil. Phyllomedusa1(2): 105- 111.). In addition, an introduced population has been recorded for the municipality of Rio de Janeiro (Salles and Silva-Soares 2010Sabagh LT, Carvalho-e-SilvaAmpt and RochaCfd. 2012.Diet of the toad Rhinella icterica (Anura: Bufonidae) from Atlantic Forest Highlands of southeastern Brazil. Biota Neotrop 12(4): 258-262.). This species is common inside bromeliads from open areas, and avoids bromeliad axils containing less than 100 ml of water (Teixeira et al. 1997Shine R. 1979. Sexual selection and sexual dimorphism in the Amphibia. Copeia 1979: 297-306.).Phyllodytes luteolus lay up to three eggs in bromeliad axils, where the larvae develop and feed on debris (Peixoto 1995, Giaretta 1996). Papp and Papp (2000)Nimer E. 1979. Climatologia do Brasil. Instituto Brasileiro de Geografia e Estatística, Rio de Janeiro, 421 p. showed a drastic population reduction of P. luteolus after a fire in the Linhares municipality, in the state of Espírito Santo. This fire destroyed more than 99.9% of the bromeliad Vrisea neoglutinosa (Rocha et al. 1996Pianka ER. 1973. The structure of lizard communities. Annu Rev Ecol Syst 4: 53-74.), supporting the species strong dependence on bromeliads.
Inter-population studies regarding trophic and spatial ecology are important to explain species niche breadth, position in local trophic webs, foraging behavior, and metabolic necessities (Duellman and Trueb 1986, Toft 1980Teixeira RL, Zamprogno C, Almeida GIand Schinei der Jap. 1997. Ecological topics of Phyllodytes luteolus (Amphibia: Hylidae) in a Sandy coastal plain of São Mateus, Espírito Santo state. Rev Bras Biol 57(4): 647-654., 1981, Wells 2007Vrcibradic D, Hatano FH,Rocha Cfd andVan Sluys M. 2006. Geographic distribution: Phyllodytes luteolus. Herpetol Rev 37: 489. ). Furthermore, it enables the elucidation of whether these parameters are conservative or variable among localities. In addition, an inter-population study about ecology and behavior of P. luteolus may help to elucidate how individuals correlate with each other in a limited space (the bromeliads) inside a limited habitat (the restinga remnants). The goal of this study was to investigate the diet of three populations of P. luteolus from restinga remnants, and to evaluate the relationship of frogs with the bromeliads. We specifically aimed to assess i) the differences in array of prey types consumed and trophic niche among populations, and among adult males, adult females and juveniles, ii) sexual dimorphism in body and head size in the species, and iii) the bromeliad species used by the individuals.
MATERIALS AND METHODS
Study Areas
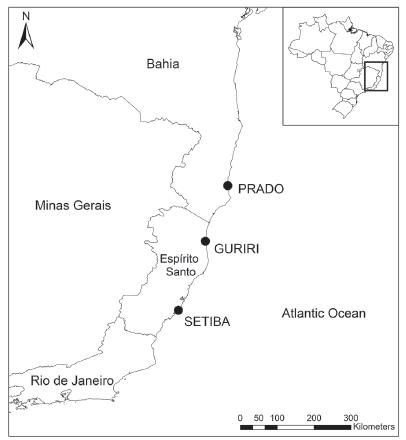
Field work was carried out between November 1999 and March 2000, in three restinga sites along the east coast of Brazil: Setiba district (20° 35' S and 39° 13' W) in the municipality of Guarapari, Guriri district, in the municipality of São Mateus (18° 41' S and 39° 45' W), both in the state of Espírito Santo, and one site in the municipality of Prado (17° 18' S and 39° 13' W), in the state of Bahia (Fig. 1). The mean annual temperature on these regions is around 23 °C and the annual rainfall ranges between 1000 and 1350 mm (Nimer 1979Martins Acjs, Kiefer MC,Siqueira CC, Van Sluys M, Menezes VA and Rocha Cfd. 2010. Ecology of Ischnocnema parva (Anura: Brachycephalidae) at the Atlantic rainforest of Serra da Concórdia, state of Rio de Janeiro, Brazil. Zoologia 27(2): 201-208.). Setiba and Guriri are separated by 210 km, whereas Guriri and Prado are 160 km apart, and Setiba and Prado 380 km apart. The bromeliad species composition varied among localities.
- Map showing restinga habitats wherePhyllodytes luteolus were collected: Guriri (18°41'S and 39°45'W) and Setiba (20°35'S and 39°13'W), in the state of Espírito Santo, and Prado (17°18'S and 39°13'W), in the state of Bahia.
Data Collection and Statistical Analyses
We conducted diurnal and nocturnal surveys inside bromeliads through visual encounter surveys (Crump and Scott 1994Crump ML and Scott Jr NJ. 1994. Visual encounter surveys. In: Heyer WR et al. (Eds), Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians, Washington D.C.:Smithsonian Institution Press, USA, p.84-92. ) (total of 80 sampling hours). Moreover, 200 tank bromeliads of different species were randomly sampled in each area (total of 600). All individuals of P. luteolus found, were collected (IBAMA collection permit No. 096/99). During the collections in the field amphibians were killed with anesthetic overdose (sodium pentobarbital) as recommended by the protocols of the American Society of Ichthyologists and Herpetologists (ASIH), the Herpetologists' League (HL) (1987), the American Veterinary Medical Association (AVMA) (2000) and the Canadian Council on Animal Care (CCAC) (1993), followed by 10% formalin solution for fixation.
We measured snout-vent length (SVL) and jaw width (JW) of the frogs with calipers to the nearest 0.01 mm. We analyzed gonads to determine sex; those individuals whose gonads were not developed were considered juveniles. We analyzed stomach contents using a stereomicroscope, and characterized the diet in terms of number of individual prey items consumed (N), total volume of prey (V, in mm³), and frequency of occurrence (F) of each prey category. Additionally, for each category of prey we calculated an index of importance value (Ix): Ix = [(nx/N) + (vx/V) + (fx/F)]/3, where nx, vx, and fx are number, volume, and frequency of prey item x, respectively, and N, V, and F are summations of the number, volume, and frequency of all prey items (Howard et al. 1999Giaretta AA. 1996. Reproductive specializations of the bromeliad hylid frog Phyllodytes luteolus. J Herpetol 30(1): 96-97.). Prey items were identified to the taxonomic level of order (or family, in the case of Formicidae). Unidentified arthropod remains and plant residues were not considered in total volumetric analysis.
We estimated the volume of each prey using the formula for an ovoid spheroid: V = 4/3π (length/2) (width/2)² (Dunham 1983Dunham AE. 1983. Realized niche overlap, resource abundance and intensity of interspecific competition in lizard ecology. In: Huey RD, Pianka ER and Schoener TW (Eds), Lizards Ecology: Studies of Model Organism,Cambridge: Harvard University Press,USA, p. 261-280.). We used Pianka's overlap index (Pianka 1973Peixoto OL. 1995. Associação de anuros a bromeliáceas na Mata atlântica. Rev Univ Rural. Sér Ciênc da Vida 17(2): 75-83. ) to determine the trophic niche similarity between populations:
where j and k refer to the populations under compa rison and pi is the proportion of prey componenti. We calculated food niche overlap using the proportional values of number and volume of prey consumed. To determine if the frogs from each populations statistically differed in terms of prey consumption, we performed a one-way analysis of similarity (ANOSIM) with Bray-Curtis distance measure (Clarke 1993Clarke KR. 1993. Nonparametric multivariate analyses of changes in community structure. Aust J Ecol 18:117-143. ). We also did a nonmetric multidimensional scaling (NMDS) based on a Bray-Curtis dissimilarity matrix to compare prey consumption by each frog from each population. Mean prey volume was estimated as the mean volume of the three largest prey items consumed per frog (or all items when stomach contained less than three). We tested for differences between populations in the number of prey and in the mean prey volume consumed per frog using one-way analysis of variance (ANOVA), followed by Scheffe's post hoc test (Zar 1999Weygoldt P. 1981. Beobachtungen zur Fortplanzurngs biologie von Phyllodytes luteolus (Wied, 1824) im Terra rium. Salamandra 17(1/2): 1-11.) when the results were significant. We used Simple Regression Analysis between prey number and frog SVL and between mean prey volume and frog JW, to evaluate if the number of prey items or the prey volume were associated to body size and JW.
We identified the bromeliad species occupied by P. luteolus when the frog was first seen. Additio nally, in Setiba and Guriri restingas, we counted the number of frogs per bromeliad, identified the ontogenetic stage (juvenile or adult) and sex, and searched for eggs and/or tadpoles in the bromeliad phytotelmata (i.e. axils and central rosette).
To assess whether there was sexual dimorphism in body size and head width ofP. luteolus, we used one-way ANOVA for differences in SVL and in JW between males and females of each population. We used analysis of covariance (ANCOVA, with SVL as a covariate; Zar 1999) to test for differences in relative jaw width between sexes.
Descriptive statistics are presented throughout the text as mean ± one standard deviation. All data were tested for normality and for homogeneity of variances before performing statistical analyses, and were log-transformed when necessary. We performed ANOVA and ANCOVA analysis with Systat 11 software, and used PAST software for ANOSIM and NMDS analysis.
RESULTS
We analyzed 161 stomach content of P. luteolus from three restinga remnants in Brazil, of which 58 were from Setiba (27 males, 23 females, six juveniles and two unknown), 43 from Guriri (20 males, 10 females, 10 juveniles and three unknown) and 60 from Prado (29 males, 26 females, two juveniles and three unknown). A total of 21 stomachs were empty of which: five were from Setiba (8.6%; two males, three females), 14 from Guriri (32.6%; 10 males, two females, two juveniles), and two were from Prado (3.3%; one male, one juvenile). Thirteen different arthropod categories were found in the stomachs (Table I). Formicidae and Isoptera were the most numerous and frequent prey (Table I). These two taxa accounted for more than 90% of prey consumed in each population, and were the most important (Ix) prey items. Isoptera represented the highest proportional volume in the stomachs (55.2% in Setiba, 53.0% in Guriri; 43.0% in Prado), followed by Formicidae (19.3% in Setiba; 16.4% in Guriri; 38.7% in Prado).
Diet of Phyllodytes luteolus in three restinga remnants along Brazil's coast (Setiba, Guriri and Prado). N = Number of prey items; F = Frequency of occurrence; V = Volume (in mm³), and Ix = Index of Importance Value. U.A.R = Unidentified arthropod remains.
There was no difference in the mean number of prey items consumed by males, females and juveniles of P. luteolus (ANOVA, F2,118 = 1.350, P = 0.263; Table II). The mean volume of prey ingested differed among males, females and juveniles (ANOVA, R2 = 0.091, F2,117 = 5.890, P = 0.004). Juveniles ingested smaller prey compared to males (Scheffe, P = 0.019), and females (Scheffe, P = 0.004). There was no difference in the mean volume of prey consumed between adult males and females (Scheffe, P = 0.639).
Number of prey and prey volume (mm3) per stomach for males, females and juveniles of Phyllodytes luteolus in three restinga remnants along Brazil's coast. SD = Standard deviation.
The number of prey items consumed per frog was not related to frog SVL in any population (Regression Analysis, Setiba: F1,45 = 3.133, P = 0.083, Guriri: F1,45 = 3.133, P = 0.956, and Prado: F1,53 = 0.499, P = 0.483). For Setiba population, the volume of prey was influenced by JW (Regression Analysis, F1,45 = 19.876, R2 = 0.31, P < 0.0001). In Prado population, the volume of prey had a nearly significant influence by JW (F1,45 = 3.778, P = 0.057), but Guriri's population showed no relationship (F1,20 = 1.781, P = 0.197).
There was no difference in the mean number of prey items per stomach among P. luteolus populations (ANOVA, F2,121 = 0.694, P = 0.502). The mean volume of the three largest prey ingested per frog differed among populations (ANOVA, F2,121 = 3.757, P = 0.026). Frogs from Prado consumed larger prey than frogs from Setiba (Scheffe, P = 0.025) but not from Guriri (Scheffe, P = 0.193;Fig. 2). Prey ingested by frogs from Setiba and Guriri did not differ in mean volume (Scheffe, P = 0.947).
- Mean volume (log-transformed) of the three largest prey items ingested per stomach of Phyllodytes luteolus in three remnants restinga along the eastern coast of Brazil (Setiba, Guriri and Prado). The asterisk means that the volume of prey consumed was significantly different between these populations. Sample sizes: Setiba = 47, Guriri = 22 and Prado = 55.
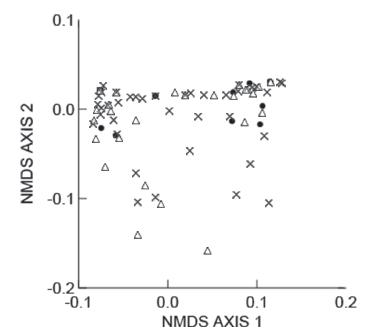
The food niche overlap among populations was high, both in terms of numerical and volumetric proportions of prey items, with values of 0.90 or higher in all cases (Table III). The ANOSIM indicated divergence in prey consumption between populations, but with an R close to zero (R = 0.0470, P = 0.0195). Prado population had the most divergent prey consumption in comparison with the others (ANOSIM, Prado vs. Setiba: R = 0.0567, P = 0.0118; Prado vs. Guriri: R = 0.0895, P = 0.0466). Setiba and Guriri populations had similar prey consumption (ANOSIM, R = - 0.0415, P = 0.8687). The prey consumption between indivi duals (Fig. 3) can be interpreted as similar, with just a few individuals from Prado standing out (stress = 0.1148).
Diet niche overlap for Phyllodytes luteolus in three remnants of restinga along the Brazilian coast based on proportional values of prey items and prey volume.
- Multidimensional scaling analyses of the diet composition in individuals of Phyllodytes luteolus from Setiba (empty triangles), Guriri (filled dots) and Prado ("X"). Stress = 0.1148.
Female were larger (SVL) than males in Prado (ANOVA, R2 = 0.31, F1,55 = 24.801, P < 0.0001), but not in Setiba (ANOVA, F1,46 = 0.033, P = 0.856) nor in Guriri (ANOVA, F1,28 = 2.785, P = 0.106) (Table IV). Across the populations, males had comparatively wider jaws than females, irrespective of body size: Setiba - ANCOVA, R2 = 0.589, F1,1,45 = 47.220, P < 0.0001, Guriri - ANCOVA, R2 = 0.710, F1,1,27 = 63.984, P < 0.0001, Prado - ANCOVA, R2 = 0.922, F1,1,54 = 480.146, P < 0.0001.
Number of individuals (N), range, mean and standard deviation (SD) of snout-vent length (SVL, in mm) and jaw width (JW, in mm) of males, females and juveniles of Phyllodytes luteolus in three restinga remnants along Brazil's coast.
Phyllodytes luteolus was found in five bromeliad species (Table V), where in 135 bromeliads (22.5%) were occupied by at least one P. luteolus. We recorded 102 (25.5%) bromeliads containing individuals of P. luteolus in Setiba and Guriri. In 45 of them (44.1%) we found only one individual of P. luteolusinside the bromeliad, of which 28 (27.4%) were males and 17 (16.6%) were females. Four individuals (one male, one female and two of unknown sex; each one in a different bromeliad) were found with clutches containing a maximum of six eggs. In 57 of the 102 bromeliads containing P. luteolus (55.9%), two to four individuals were found inside the same bromeliad. In none of the cases, males were found sharing a bromeliad, but males co-occurred with females, juveniles and/or tadpoles. In the 14 cases in which tadpoles were present inside bromeliads, there were one to six tadpoles and, in most cases, at least one frog inside the same bromeliad (11 females, four males, one juvenile, and two of unknown sex).
Number and percentage of individuals of Phyllodytes luteolus found in bromeliads in three restinga remnants along Brazil's coast.
DISCUSSION
The three populations of P. luteolus had a diet composed exclusively of arthropods. The diet was composed of a relatively wide variety of prey types, but Formicidae and Isopteras dominated the prey consumed. Similarly, in the restinga of Regência in the state of Espírito Santo, Ferreira et al. (2012) reported on a population of P. luteolus that fed on 19 different prey types, with Formicidae and Isoptera being the most important prey. Due to the high proportion of Formicidae and Isoptera consumed, these authors suggested that P. luteolus had a specialized diet, predominantly composed of colonial arthropods. Because specialization in diet is a result of evolution, we consider Formicidae and Isoptera as prey preferentially consumed. Apparently, P. luteolus has a conservative diet across sites, independently of local peculiarities and differences among sites. It is possible that access to Formicidae and Isoptera might be facilitated in microhabitats that accumulate plant remains, like the interior of some tank bromeliads. Formicidae is commonly associated with bromeliads (Schimd et al. 2010), which may facilitate them being found and subsequent consumption by frogs. Toft (1980, 1981) suggests the use of Jacob's elective index to evaluate if a species has a specialized diet, based on prey abundances in the habitat and in the stomachs. Thus, to better infer if the diet ofP. luteolus is specialized or generalized, it would be necessary to evaluate whether frogs select prey items or if they consume them according to prey availability inside bromeliads. However, in the absence of specific studies on the arthropod fauna inside bromeliads, at the study sites, we were not able to confirm to what extent P. luteolus specializes in colonial insects. The presence of plant remains and sand in the diet of P. luteolus was considered accidental ingestion. The difference of empty stomachs between populations may be due to prey availability at the moment of the fieldwork in each locality.
Such as expected, we observed that juveniles of P. luteolus tended to consume smaller prey compared to adults. Anurans ingest prey with size proportional to their JW, thus consuming larger prey as their jaw size increases (e.g. Lima and Moreira 1993, Marra et al. 2004Maia-Carneiro T, Kiefer MC,Van Sluys M andRocha Cfd. 2013. Feeding habits, microhabitat use, and daily activity period of Rhinella ornata (Anura, Bufonidae) from three Atlantic rainforest remnants in southeastern Brazil. North-West J Zool 9(1): 157-165., Dietl et al. 2009Dietl J,Engels W and Solé M. 2009. Diet and feeding behaviour of the leaf-litter frog Ischnocnema henselii (Anura: Brachycephalidae) in Araucaria rain forests on the Serra Geral of Rio Grande do Sul, Brazil. J Nat Hist 43(23): 1473-1483., Martins et al. 2010). Smaller preys should be less energetically profitable for adults, and the consumption of larger prey should result in a greater amount of energy.
There were some inter-population differences in the mean volume of prey items consumed by P. luteolus. The volume of prey consumed may have differed due to variation in prey availability among the studied restingas. The distance between sites might explain the variation in the volume of prey consumed. For instance, a study with Rhinella ornata (Spix, 1824) in three different localities (which are at least 27 km distance from each one) revealed that the populations differed with regard to the number and volume of prey consumed by individuals, but with a more similar diet between the areas closest to each other and with similar environments (Maia-Carneiro et al. 2013).
Despite considerable distances between populations (> 150 km), trophic niches ofP. luteolus were very similar (at least 90% overlap in all cases). The probality of significance in the ANOSIM demonstrates a significant difference between populations, but the R close to 0 indicates similar prey consumption. The similarity between populations is reinforced by observation of the NMDS outcomes in Figure 3. These ANOSIM results can be due to the divergence of Prado from others sites, or the analyzed factor (prey consumption) was a weak factor. Two explanations of extensive diet overlap between the populations are: i) similar arthropod faunas inhabiting bromeliads in the studied restingas, or ii) an inherent preference for Formicidae and Isoptera is characteristic of P. luteolus. Evaluation of our data together with those available in the literature (Ferreira et al. 2012) suggests that P. luteolus has some degree of conservatism regarding its trophic niche, feeding on similar prey types (mainly Formicidae and Isoptera) independent of local patterns of prey supply in different sites.
Females were larger than males in the Prado population, which is consistent with observations for congeneric species (e.g. P. wuchereri, Caramaschi et al. 2004; P. maculosus,Cruz et al. 2006Cruz Cag, Feio RN andCardoso Mcs. 2006. Description of a new species of Phyllodytes Wagler, 1830 (Anura, Hylidae) from Atlantic Forest of the states of Minas Gerais and Bahia, Brazil. Arq Mus Nac 64: 321-324.). A possibility for the absence of dimorphism in body size in the other two populations may be due to sample size. Overall, females tend to be larger than males in most anuran species (Shine 1979Schineider JA and Teixeira RL. 2001. Relacionamento entre anuros e bromélias da restinga de Regência, Linhares, Espírito Santo, Brasil. Iheringia, Sér Zool, Porto Alegre 91: 41-48.). This difference in body size occurs due to intra-sexual selection that favors larger relative body size of females, which is linked to a capacity to produce larger clutch sizes or larger eggs (Duellman and Trueb 1986, Martins et al. 2010).
Because no more than one adult male was found in the same bromeliad and maleP. luteolus have larger proportional jaw widths than females, we propose that males of this species may have territorial behavior. Eterovick (1999) did not record territorial behavior in P. luteolus, contrary to Weygoldt (1981)Wells KD. 2007. The Ecology and Behavior of Amphibians.Chicago: The University Chicago Press, USA, 1400 p., who observed agonistic interactions among males under laboratory conditions. Teixeira et al. (1997) suggested that intra-specific competition among males (e.g. for females, preys, shelters and/or reproductive sites) occurs in P. luteolus, and Weygoldt (1981) proposed that the low density of adults per bromeliad suggests that males of this species exhibit intra-specific territorial behavior. In the Brazilian restinga of Regência, two studies also found a high percentage of solitaryP. luteolus inside bromeliads (individuals were not categorized by sex) − Schineider and Teixeira (2001)Schmid VS, Schmid S,Steiner J and Zillikens A. 2010. High diversity of ants foraging on extrafloral nectar of bromeliads in the Atlantic rainforest of southern Brazil. Stud Neotrop Fauna E45(1): 39-53.observed that up to 80% of individuals were found alone within a bromeliad depending on the bromeliad species, and Ferreira et al. (2012) found that 65% of examined bromeliads had only one P. luteolus. Sexual dimorphism in JW (males having relatively larger jaws than females) also suggests territorial behavior. Males of P. luteolus use mandibular odontoids during fights (Weygoldt 1981, Duellman and Trueb 1986), and males with larger jaws are presumably more successful during territorial struggles.
Despite the consumption of a relatively wide array of prey, particular food items, such as colonial insects (ants and termites) for Phyllodytes luteolus, might predominantly compose the diet of species. Although may occur intra- and inter-population differences in types and sizes of preys consumed by P. luteolus, in some cases these parameters might be conservative. Sexual dimorphism in size jaw width and body size, with males having proportionally larger jaws than females and with females larger-sized than males, might confer an advantages during territorial interactions in males and favor greater fecundity in females.
ACKNOWLEDGMENTS
We are grateful to G.R. Winck for preparing the map. Special thanks to J.H.F. Mello, K. Welsh, M. Almeida-Gomes and the anonymous reviewers for the contributions to the manuscript. We are greatful to V.N.T. Borges-Jr. for helping with the statistical analysis. We also thank K. Welsh for the English revision. This study was supported by research grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to C.F.D. Rocha (processes 304791/2010-5 and 472287/2012-5) and to M. Van Sluys (processes CNPq 301401/2002-7 and 307773/2008-6); from the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) through "Cientistas do Nosso Estado" Program (process E-26/102.765.2012) to C.F.D. Rocha. T. Motta-Tavares and L.F. Dantas received undergraduate fellowships from CNPq. T. Maia-Carneiro received PhD scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and from FAPERJ. We thank IBAMA for the collection permit (No. 096/99).
REFERENCES
- Almeida-Gomes M et al. 2008. Herpetofauna of an Atlantic rainforest area (Morro São João) in Rio de Janeiro State, Brazil. An Acad Bras Cienc 80: 291-300.
- Caramaschi U, Peixoto OLand Rodrigues MT. 2004. Revalidation and redescription of Phyllodytes wuchereri (Peters, 1873) (Amphibia, Anura, Hylidae). Arq Mus Nac 62: 185-191.
- Clarke KR. 1993. Nonparametric multivariate analyses of changes in community structure. Aust J Ecol 18:117-143.
- Coco L,Borges-Junior Vnt,Fusinatto LA, Kiefer MC, Oliveira JC, Araujo PG, Costa BM, Van Sluys M and Rocha Cfd. 2014. Feeding habits of the leaf litter frog Haddadus binotatus (Anura, Craugastoridae) from two Atlantic Forest areas in southeastern Brazil. An Acad Bras Cienc 86: 239-249.
- Crump ML and Scott Jr NJ. 1994. Visual encounter surveys. In: Heyer WR et al. (Eds), Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians, Washington D.C.:Smithsonian Institution Press, USA, p.84-92.
- Cruz Cag, Feio RN andCardoso Mcs. 2006. Description of a new species of Phyllodytes Wagler, 1830 (Anura, Hylidae) from Atlantic Forest of the states of Minas Gerais and Bahia, Brazil. Arq Mus Nac 64: 321-324.
- Dietl J,Engels W and Solé M. 2009. Diet and feeding behaviour of the leaf-litter frog Ischnocnema henselii (Anura: Brachycephalidae) in Araucaria rain forests on the Serra Geral of Rio Grande do Sul, Brazil. J Nat Hist 43(23): 1473-1483.
- Duellman WE and Trueb L. 1986. Biology of amphibians. 2ª ed., Baltimore and London: McGraw-Hill, 670 p.
- Dunham AE. 1983. Realized niche overlap, resource abundance and intensity of interspecific competition in lizard ecology. In: Huey RD, Pianka ER and Schoener TW (Eds), Lizards Ecology: Studies of Model Organism,Cambridge: Harvard University Press,USA, p. 261-280.
- Duré MI, Kehr AI andSchaefer EF. 2009. Niche over lap and resource partitioning among five sympatric bufonids (Anura, Bufonidae) from northeastern Argentina. Phyllomedusa8(1): 27-39.
- Eterovick PC. 1999. Use and sharing of calling and retreat sites by Phyllodytes luteolus in a modified environment. J Herpetol1(1): 17-22.
- Feio RNand Caramaschi U. 2002. Contribuição ao conhe cimento da herpetofauna do nordeste do estado de Minas Gerais, Brasil. Phyllomedusa1(2): 105- 111.
- Ferreira RF, Schineider Jap and Teixeira RL. 2012. Diet, fecundity and use of bromeliads by Phyllodytes luteolus (Anura: Hylidae) in southeastern Brazil. J Herpetol 46(1): 19-24.
- Frost DR. 2013. Amphibian Species of the World: an Online Reference. Version 5.6 (January 9, 2013). Electronic Database accessible athttp://research.amnh.org/herpetology/amphibia/index.html.American Museum of Natural History, New York.
» http://research.amnh.org/herpetology/amphibia/index.html - Giaretta AA. 1996. Reproductive specializations of the bromeliad hylid frog Phyllodytes luteolus. J Herpetol 30(1): 96-97.
- Howard AK, Forester JD,Ruder JM, Parmerlee Jr JS and Powell R. 1999. Natural history of a terrestrial Hispaniolan anole: Anolis barbouri. J Herpetol 4(33): 702-706.
- Lantyer-SilvaAsf, SoléM and ZinaJ. 2014. Repro ductive biology of a bromeligenous frog endemic to the Atlantic Forest: Asparasphenodon arapapa Pimenta, Napoli and Haddad, 2009 (Anura: Hylidae). An Acad Bras Cienc86: 865-880.
- Lima APand Moreira G. 1993. Effects of prey size and foraging mode on the ontogenetic change in feeding niche of Colostethus stepheni (Anura: Dendrobatidae). Oecologia 95(1): 93-102.
- Maia-Carneiro T, Kiefer MC,Van Sluys M andRocha Cfd. 2013. Feeding habits, microhabitat use, and daily activity period of Rhinella ornata (Anura, Bufonidae) from three Atlantic rainforest remnants in southeastern Brazil. North-West J Zool 9(1): 157-165.
- Marra RV, Van Sluys M and Rocha Cdf. 2004. Food habits of Eleutherodactylus parvus (Anura: Leptodac tylidae) at Atlantic rainforest area, Southeastern Brazil. Herpetol Rev 35(2): 135-137.
- Martins Acjs, Kiefer MC,Siqueira CC, Van Sluys M, Menezes VA and Rocha Cfd. 2010. Ecology of Ischnocnema parva (Anura: Brachycephalidae) at the Atlantic rainforest of Serra da Concórdia, state of Rio de Janeiro, Brazil. Zoologia 27(2): 201-208.
- Nimer E. 1979. Climatologia do Brasil. Instituto Brasileiro de Geografia e Estatística, Rio de Janeiro, 421 p.
- Papp MG and Papp Cog. 2000. Decline in a population of the treefrog Phyllodytes luteolus after fire. Herpetol Rev 31(2): 93-95.
- Peixoto OL. 1995. Associação de anuros a bromeliáceas na Mata atlântica. Rev Univ Rural. Sér Ciênc da Vida 17(2): 75-83.
- Pianka ER. 1973. The structure of lizard communities. Annu Rev Ecol Syst 4: 53-74.
- Rocha Cfd, Van Sluys M, Ornellas AB, Siqueira AE,Conde Cfv, Bittencourt EB, Oliveira Mgn, Barros MC and Magalhães Sap. 1996. The effects of fire on natural populations of Vrisea neoglutinosa in a relict restinga of Espírito Santo state. Bromélia 3: 16-26.
- Sabagh LT, Carvalho-e-SilvaAmpt and RochaCfd. 2012.Diet of the toad Rhinella icterica (Anura: Bufonidae) from Atlantic Forest Highlands of southeastern Brazil. Biota Neotrop 12(4): 258-262.
- Salles Rdol and Silva-Soares T. 2010. Phyllodytes luteolus (Anura, Hylidae) as an alien species in the Rio de Janeiro municipality, state of Rio de Janeiro, Southeastern Brazil. Herpetol Notes 3: 257-258.
- Schmid VS, Schmid S,Steiner J and Zillikens A. 2010. High diversity of ants foraging on extrafloral nectar of bromeliads in the Atlantic rainforest of southern Brazil. Stud Neotrop Fauna E45(1): 39-53.
- Schineider JA and Teixeira RL. 2001. Relacionamento entre anuros e bromélias da restinga de Regência, Linhares, Espírito Santo, Brasil. Iheringia, Sér Zool, Porto Alegre 91: 41-48.
- Shine R. 1979. Sexual selection and sexual dimorphism in the Amphibia. Copeia 1979: 297-306.
- Teixeira RL, Zamprogno C, Almeida GIand Schinei der Jap. 1997. Ecological topics of Phyllodytes luteolus (Amphibia: Hylidae) in a Sandy coastal plain of São Mateus, Espírito Santo state. Rev Bras Biol 57(4): 647-654.
- Toft CA. 1980. Seasonal variation in populations of Panama nian litter frogs and their prey: a comparison of wetter and drier sites. Oecologia 47: 34-38.
- Toft CA. 1981. Feeding ecology of Panamanian litter anurans: patterns in diet and foraging mode. J Herpetol 15: 139-144.
- Vrcibradic D, Hatano FH,Rocha Cfd andVan Sluys M. 2006. Geographic distribution: Phyllodytes luteolus. Herpetol Rev 37: 489.
- Wells KD. 2007. The Ecology and Behavior of Amphibians.Chicago: The University Chicago Press, USA, 1400 p.
- Weygoldt P. 1981. Beobachtungen zur Fortplanzurngs biologie von Phyllodytes luteolus (Wied, 1824) im Terra rium. Salamandra 17(1/2): 1-11.
- Zar JH. 1999. Biostatistical Analysis, 4th ed., New Jersey: Prentice Hall, USA, 663 p.
Publication Dates
-
Publication in this collection
05 Feb 2016 -
Date of issue
Mar 2016
History
-
Received
22 July 2014 -
Accepted
16 Jan 2015