Abstracts
Dichelops melacanthus was studied under controlled conditions (60 ± 10% RH and 14/10 h L/D photoperiod), and three constant temperatures (19, 25, and 31 ± 2 °C). Fresh pods of MON 87701 × MON 89788 soybeans and its near non-Bt isoline (A5547) were supplied to nymphs and adults. The biology of T. podisi was studied in the same controlled RH conditions, but only at the standard temperature of 25 ± 2 °C. Overall, the development of D. melacanthus was better at higher temperatures, which accelerated the development of the stink bug without affecting adult biological parameters. No influence of Bt-soybeans on the biology of the pest was observed in any temperature studied, which shows that D. melacanthus is not affected by this transgenic soybean. The egg parasitoid T. podisi also was not harmed when it parasitized eggs of the pest fed with MON 87701 × MON 89788 soybeans, with similar results to those obtained in non-Bt isogenic soybeans. Thus, this study demonstrates that D. melacanthus is favored at high temperatures (31 ± 2 °C), and that neither did MON 87701 × MON 89788 soybean pods affect the development of the pest nor its parasitoid T. podisi.
egg parasitoids; global warming; natural enemy; non-target organisms
Dichelops melacanthus foi avaliado em condições con troladas (60 ± 10% UR e fotoperíodo de 14/10 h L/E) em três temperaturas constantes (19, 25, e 31 ± 2 °C). Vagens frescas da soja MON 87701 x MON 89788 e de sua isolinha não-Bt (A5547) foram oferecidas a ninfas e adul tos. A biologia de T. podisi foi estudada nessas mesmas condições controladas de UR, mas apenas na temperatura padrão de 25 ± 2 °C. Em geral, o desenvolvimento de D. mela canthus foi melhor nas temperaturas mais altas, as quais aceleraram o desenvolvimento do percevejo, mas sem afetar os parâmetros biológicos da fase adulta. Não houve influência da soja-Bt na biologia da praga em nenhuma das temperaturas estudadas, o que mostra que D. melacanthus não é afetado pela soja transgênica. O parasitoide de ovos T. podisi também não foi impactado quando parasitou ovos da praga alimentada com soja MON 87701 x MON 89788, com resultados semelhantes àqueles obtidos na soja isogênica não-Bt. Portanto, este estudo comprova que D. melacanthus é favorecido por temperaturas mais altas (31 ± 2 °C), e que soja-Bt MON 87701 x MON 89788 não afeta o desenvolvimento desta praga assim como de seu parasitoide T. podisi.
parasitoide de ovos; aquecimento glo bal; inimigo natural; organismos não-alvos; soja trans gênica
INTRODUCTION
The soybean [Glycine max (L.) Merril] is one of the most important crops worldwide, with an average annual production of around 260 million tons in an area of about 103 million ha (Faostat 2013Faostat - Food and Agriculture Organization of the United Nations. 2013. http://faostat3.fao.org/home/index.html.
http://faostat3.fao.org/home/index.html...
). However, the occurrence of pests has become one of the main factors that has hindered obtaining good yields of this grain, mainly defoliating insects (Panizzi and Corrêa-Ferreira 1997Panizzi AR. 1997. Wild hosts of pentatomids: ecological and role in their pest status on crops. Annu Rev Entomol 42: 99-122., Bueno et al. 2011Bueno Rcof, Bueno AF, Moscardi F, Parra Jrp and Hoffmann-Campo CB. 2011. Lepidopteran larva consumption of soybean foliage: basis for developing multiple- species economic thresholds for pest management decisions. Pest Manag Sci 67: 170-174.) and sucking insects (Panizzi 1997, Smith et al. 2009Smith JF, Luttrell RG and Greene JK. 2009. Seasonal abundance, species composition, and population dynamics of stink bugs in production fields of early and late soybean in south Arkansas. J Econ Entomol 102: 229-236., Musser et al. 2009Musser FR, Stewart SD and Catchot Jr AL. 2009. 2008 soybean insect losses for Mississippi and Tennessee. Midsouth Entomol 2: 42-46.).
Currently there is evident population growth of the green belly stink bug Dichelops melacanthus (Dallas) (Hemiptera: Pentatomidae), in soybean growing areas of Brazil, especially after the adoption of a no-tillage system in most producing regions of the country (Chocorosqui and Panizzi 2004Chocorosqui VR and Panizzi AR. 2002. Influência da temperatura na biologia de ninfas de Dichelops melacanthus (Dallas, 1851) (Heteroptera: pentatomidae). Semina 23: 217-220. ). This species attacks the soybean crop, but now also occurs in winter crops such as wheat (Manfredi-Coimbra et al. 2005Manfredi-Coimbra S, Silva JJ, Chocorosqui VR and Panizzi AR. 2005. Danos do percevejo barriga-verde Dichelops melacanthus (Dallas) (Heteroptera: Pentatomidae) em trigo. Cienc Rural 35: 1243-1247.). The influence of temperature on the life cycle of insects has been demonstrated in several studies (Hochachka and Sommero 1984Hochachka PW and Sommero GN. 1984. Temperature adaptation. In: Hochachka PW and Sommero GN (Eds), Biochemical Adaptation. Princeton University Press, Princeton, NJ, p. 355-449., Praslicka and Huszár 2004Praslicka J and Huszár J. 2004. Influence of temperature and host plants on the development and fecundity of the spider mite Tetranychus urticae (Acarina: Tetranychidae). Plant Prot Science 40: 141-144., Silva et al. 2011Silva DM, Hoffmann-Campo CB, Bueno AF, Bueno Rcof, De Oliveira Mcn and Moscardi F. 2011. Biological characteristics of Anticarsia gemmatalis (Lepidoptera: Noctuidae) for three consecutive generations under different temperatures: understanding the possible impact of global warming on a soybean pest. Bull Entomol Res 102: 285-292.). In this regard, Chocorosqui and Panizzi (2002) conducted a preliminary study on the impact of temperature on the nymphal stage of D. melacanthus, and found that the nymphs did quite poorly in low temperatures. However, the literature lacks detailed data on the impact of temperature on the life cycle of this stink bug, so this is crucial information that would make it practical to determine the regions and times when the pest could benefit from climate changes.
Within the context of integrated pest manage ment, one of the tools that have been long adopted by producers is the cultivation of plants resistant to insects. The process of gene insertion with the bacterium Bacillus thuringiensis Berlinier (Bt) in the plant has been developed primarily for the control of lepidopterans, as in the case of Bt cotton and Bt corn. This bacterium expresses an insecticidal protein (Cry) that contacts the gut of susceptible insects, debilitating them immediately after ingestion (Santos et al. 2009Santos K, Neves P, Meneguim AM, Santos RB and Santos WJ. 2009. Selection and characterization of the Bacillus thunringiensis strains toxic to Spodoptera eridania (Cramer), Spodoptera cosmioides (Walker) and Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). Biol Control 50: 157-163.). MON 87701 × MON 89788 soybeans express the insecticidal cry1Ac insecticide gene, which is resistant to the main crop defoliators, Anticarsia gemmatalis Hübner, 1818 (Lepidoptera: Eribidae) and the soybean looper Chrysodeixis (= Pseudoplusia) includens (Walker, 1857) (Lepidoptera: Noctuidae) (Bernardi et al. 2012Bernardi O, Malvestiti GS, Dourado PM, Oliveira WS, Martinelli S, Berger GU, Head GP and Omoto C. 2012. Assessment of the high -dose concept and level of control provided by MON 87701 x MON 89788 soybean against Anticarsia gemmatalis and Pseudoplusia includens (Lepidoptera: Noctuidae) in Brazil. Pest Manag Sci 68: 1083-1091.).
Among the advantages of the cultivation of Bt plants are the reduction in insecticide use and lower environmental impact compared to the use of insecticides (Kouser and Qaim 2011Kouser S and Qaim M. 2011. Impact of Bt cotton on pesti cide poisoning in smallholder agriculture: A panel data analysis. Ecol Econ 70: 2105-2113., Lu et al. 2012Lu Y, Wu K, Jiang Y, Guo Y and Desneux L. 2012. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nat 487: 362-367.). However, it is still unclear what impact this technology can have on non-target pests, since the information in the literature is scarce and conflicting. For example, Kim et al. (2012)Kim YH, Hwang CE, Kim TS, Lee JH and Lee S. 2012. Assessment of potential impacts due to unintentionally released Bt maize plants on non-target aphid Rhopa losiphum padi (Hemiptera: Aphididae). J Asia Pac Entomol 15: 443-446. reported in a laboratory study that the aphid Rhopalosiphum padi (L.) (Hemiptera: Aphididae) has higher fecundity when developed on Bt corn. This benefit for the biology of the pest can change the scenario of reduced insecticide use on crops, because of the need to use pesticides to control pests that were formerly secondary on the crop (Li et al. 2011Li G, Feng H, McNeil JN, Liu B, Chen P and Qiu F. 2011. Impacts of transgenic Bt cotton on a non-target pest, Apolygus lucorum (Meyer-Dür) (Hemiptera: Miridae), in northern China. Crop Protect 30: 1573-1578.). In Asia, after the large-scale adoption of Bt cotton farming, the population growth of the mirid Apolygus locurum (Meyer-Dur) (Hemiptera: Miridae) has been observed. These studies indicate that the expansion of Bt plant cultivation can benefit non-target pests through changes in the physico-chemical composition of plants (Latham et al. 2006Latham JR, Wilson AK and Steinbrecher RA. 2006. The Mutational Consequences of Plant Transformation. J Biomed Biotechnol 2006: 1-7.), where the main beneficiaries may be sucking insects sucking, such as D. melacanthus.
Among the important natural enemies of this bug are the egg parasitoid Telenomus podisi (Ashmed) (Hymenoptera: Platygastridae) (Corrêa-Ferreira and Moscardi 1994, Laumann et al. 2010Laumann RA, Moraes Mcb, Silva JP, Vieira Amc, Silveira S and Borges M. 2010. Vespas parasitoides de ovos como inimigos naturais do percevejo neotropical Dichelops melacanthus. Pesq Agropec Bras 45: 442-449.). This parasitoid is distributed in almost all Brazilian agroecosystems, but its efficiency can be affected by many factors. Thus, some studies have shown that the cultivation of Bt plants can alter the nutritional status of the host and consequently reduce the performance of natural enemies (Zhang et al. 2006Zhang SY, Fie BY, Cui J and Li DM. 2006. Biology of Campoletis chlorideae (Uchida) (Hym., Ichneumonidae) developing in Bt-treated, Bt-resistant Helicoverpa armigera (Hübner) (Lep., Noctuidae) larvae. J Appl Entomol 130: 268-274., Sanders et al. 2007Sanders CJ, Pell JK, Poppy GM, Raybould A, Garcia-Alonso M and Schuler TH. 2007. Host-plant mediated effects of transgenic maize on the insect parasitoid Campoletis sonorensis (Hymenoptera: Ichneumonidae). Biol Control 40: 362-369.), while other studies have not demonstrated such a relationship (Manachini and Lozzia 2002Manachini B and Lozzia GC. 2002. First results into the effects of Bt corn crop on nematofauna. Boll Zool Agric Bachic 34: 85-96., Fernandes et al. 2007Fernandes FS, Ramalho FS, Malaquias JB, Nascimento Junior JL, Correia ET and Zanuncio JC. 2012. Within-plant distribution of cotton aphid (Hemip tera: Aphididae) in cotton cultivars with colored fibers. An Acad Bras Cien 84: 707-719.), suggesting that different results may be obtained depending on the taxon studied. Thus, the aim of this study was to assess the biology of D. melacanthus at different temperatures when fed MON 87701 × MON 89788 soybean pods and possible impacts of this technology on the egg parasitoid T. podisi.
MATERIALS AND METHODS
Rearing And Multiplication of D. melacanthus and T. podisi
Eggs of D. melacanthus for the study came from the rearing laboratory of the Agronomic Institute of Paraná (located in Londrina, Paraná), where it has been established for 19 years. The parasitoids were derived from the rearing of the Embrapa Soybean Laboratory, where it has been reared for over 27 generations in the laboratory. Both populations were raised under controlled laboratory conditions (25 ± 2 °C, 60 ± 10% RH, and a photoperiod of 14:10 h [L:D]).
Soybean Cultivation and Preparation of the Material Used in Bioassays
The soybean cultivar was developed and made available by the company Monsanto Brazil Ltda. The near isoline A5547 (non-Bt soybean, maturation group 5.5) and MON 87701 × MON 89788 expressing the Cry1Ac protein (Bt soybean) were used. The material was sown in sterile soil in plastic pots (8 L) maintained in a greenhouse. Five seeds per pot were distributed in a total of 25 pots per breeding line. In terms of cultural practices, there was weekly control of powdery mildew with the application of a product based on sulfur (S) (Kumulus(r)) at a dose of 0.5 g L-1. At the V2 phenological stage of soybeans (Fehr and Caviness 1977Fehr WR and Caviness CE. 1977. Stages of soybean de velopment. Ames: University of Sciense and Technology. Special Report 80: 11.), plants were fertilized with a chemical fertilizer with the formulation 0-20-20 (N-P-K), according to the technical recommendations of the region. The humidity of the vessels was monitored daily and drip irrigation of plants occurred whenever necessary.
The pods were collected from the R6 phenological stage (Fehr and Caviness 1977) when they were already full. Before being offered to the stink bugs, the pods went through a process of cleaning, in which they were immersed for 15 min in a solution of water and 5% sodium hypochlorite. After this period, the pods were removed from the solution, rinsed, and dried for 2 h before the material was offered to insects.
Biology of D. melacanthus FED non-Bt and Bt Soybean Pods at Different Temperatures
The testing was conducted in a completely rando mized design in a 3 × 2 factorial [three temperatures vs. two soybean isolines (Bt and non-Bt)] with seven replicates of 10 nymphs per treatment. The temperatures to which the specimens were submitted were 19, 25, and 31 ± 2 °C under controlled relative humidity (60 ± 10%) and 14/10 h L/D photoperiod conditions. The sources of food offered were the soybean MON 87701 × MON 89788, which expresses the Cry1Ac protein (Bt soybean), and its non-Bt isoline A5547 (non-Bt soybean).
The nymphs were placed in Petri(r) dishes (Ø = 6 cm), lined with filter paper. The individualization occurred from the second instar, as in the first, the nymphs had herd behavior and did not feed. Each individual was offered a soybean pod (each two days) and a plastic Eppendorf(r) tube containing water and capped with cotton. Plates were moistened daily to aid in maintaining the relative humidity (RH%) of the plates. Every day we reviewed the nymphal stage and recorded dead individuals in all treatments.
Within 24 h after the last molt, the adult stink bugs were weighed (g) with the aid of an analytical balance and separated by sex. Later, stink bugs were put into Gerbox(r) boxes that had perforated lids to allow air to enter and were lined with filter paper. Additionally, within each box a cap with cotton soaked in water was made available for adults. There were two females and one male per Gerbox(r), and every 48 h their filter paper and food were changed.
The biological parameters evaluated were the weight (g), sex ratio, fecundity, and egg viability (%). To assess the viability of the eggs, these were taken from each Gerbox(r), packaged in petri dishes (Ø = 6 cm), and kept under the same conditions of temperature, humidity, and photoperiod as adults. As the nymphs emerged, they were counted to calculate viability.
Biology of T. podisi Developed in Eggs of D. melacanthus Reared With Bt and non-Bt Soybean Pods
The eggs of D. melacanthus used to study the bio logy of T. podisi were obtained from rearing with the same methodology used in the study of the biology of the stink bugs, except for the use of large plastic boxes of 25 × 30 × 30 cm. The eggs collected from the boxes were stored in liquid nitrogen at -196 °C, which does not affect the quality of these eggs for a period of 360 d (Corrêa-Ferreira and Oliveira 1998).
A mean of 25 eggs from each treatment were separated and glued to non-toxic white cardboard glue mats (0.8 cm × 5 cm). The experiment was carried out in a completely randomized design with 35 replications. In each repetition, we used a newly emerged T. podisi female (between 24 h and 48 h) that had previously mated and individualized in Duran tubes (6 cm × 1 cm Ø) containing a droplet of honey to feed the parasitoid. The cards were individually introduced into the tubes and immediately sealed with plastic wrap. Parasitism was allowed for 24 h, after which time the cards were removed and transferred to separate flat-bottom glass tubes (8 cm × 2 cm Ø) until adult emergence. Females also remained acclimatized under the same conditions in which they developed in the chambers. We evaluated the longevity of parental females (days), the egg-to-adult period (days), parasitism, parasitism viability (% emergence), and sex ratio.
Statistical Analysis
Data were analyzed for normality (Shapiro and Wilk 1965Shapiro SS and Wilk MB. 1965. An analysis of variance test for normality. Biometrika 52: 591-611.) and homogeneity of variance of treatments (Burr and Foster 1972Burr IW and Foster LA. 1972. A test for equality of vari ances. Mimeo Series No. 282. University of Purdue, West Lafayette, 26 p.), and if necessary transformed to perform ANOVA. The treatment means were then compared by a Tukey test at the 5% probability level (SAS Institute 2001Sas Institute. 2001. User's Guide: Statistics, Version 6e. Cary, 2001.201p.).
RESULTS
Biology of D. melacanthus Fed non-Bt and Bt Soybean Pods at Different Temperatures
The results demonstrated that the increase in temperature accelerated the nymphal development of D. melacanthus at all instars of the nymphal stage of the species (Table I). At 31 °C, the nymphal stage of D. melacanthus took 16.73 d, while at 25 °C it was 27.50 d (F = 195.8, DF = 1, P = <0.01). When the insects were subjected to a temperature of 19 °C, development was highly degraded, and only one individual reached the adult stage. Overall, the highest mortality rate was always observed in the second instar of the stink bug, demonstrating that the food is not very appropriate for D. melacanthus development (Table II).
Although nymphal viability was relatively low at both temperatures, it was superior when the insects were subjected to a temperature of 31 °C (approximately 30%), while that at a 25 °C average was 17.85% (F = 294.74, DF= 1, P = <0.01). On the other hand, MON 87701 × MON 89788 soybeans did not affect the nymphal biology at any temperature, thus indicating that they were not detrimental to the development of D. melacanthus (Tables I and II).
Due to the high mortality of nymphs at 19 °C, no data on the biological parameters of adults were obtained from that treatment. Overall, the adults that emerged at 31 °C showed a greater weight than those at 25 °C, but the other biological characteristics, such as sex ratio, fecundity, and egg viability, were similar at the two temperatures (Fig. 1). As observed for nymphal stage D. melacanthus, the adults fed on MON 87701 × MON 89788 soy beans had biological parameters similar to those fed on non-Bt soybeans (Fig. 2), confirming that the transgenic soybean does not affect the biology of the pest.
- Biological characteristics of the adult phase Dichelops melacanthus developed with soybean pods submit ted at different constant temperatures. Means were compared by Tukey test (* = P ≤ 0.05). (PS. At the temperature of 19 °C hatched only 1 adult individual, not allowing to perform statistical analysis).
- Biological characteristics of the adult of Dichelops melacanthus developted on Bt and non-Bt soybean pods. Means were compared by Tukey test.
Biology of T. podisi Developed in Eggs of D. melacanthus Reared With Bt and non-Bt Soybean Pods
The development of the parasitoid T. podisi was not harmed when it parasitized eggs of D. melacanthus reared on MON 87701 × MON 89788 soybeans (Table III). Parental females lived about 12 d in both treatments, and had a parasitism rate exceeding 80%, indicating good nutritional quality of the host. Confirming this statement, the viability parameters of parasitism and sex ratio were also similar between treatments (Table III).
DISCUSSION
Our study proves that the stink bug D. melacanthus develops better in higher temperatures, explaining the higher occurrence of this species in the warmer regions of Brazil. Even when the insects were subjected to 31 °C, the biological parameters of adult females were not affected. Among nymphs that developed at 19 °C, almost all individuals died in the third instar without performing ecdysis, confirming that the species is better adapted to higher temperatures. These results corroborate Chocorosqui and Panizzi (2002), where the authors found that at low temperatures (15-20 °C) there was a high mortality of the pest, while the best performance was at 25 °C. However, the maximum temperature studied by the authors was 25 °C, and the study was limited only to the impact on the nymphal stage of the stink bug.
In our study examining the different stages of D. melacanthus, temperatures up to 31 °C shortened the juvenile phase of the stink bug, but did not affect the biological parameters of adults, indicating that in areas or times of higher temperatures, this species can cause major damage to crops, due to a possible increase in the number of generations of the pest. The fecundity of females that emerged at 31 °C was 104.63 eggs, with a viability of 65.22%. These results did not differ for females from 25 °C, which had fecundity and viability of 86.33 eggs and 60.18%, respectively. These results are underpinned by Chocorosqui and Panizzi (2008), who claim that fertility can vary from 74.1 to 131.4 eggs/female with viability between 55.8 and 74.9%.
The importance of the influence of temperature on the biology and biodynamics of pests has been increasingly studied (Nondillo et al. 2008Nondillo A, Redaelli LR, Botton M, Pinent Smj and Gitz R. 2008. Exigências térmicas e estimativa do número de gerações anuais de Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) em morangueiro. Neotrop Entomol 37: 646-650., Silva et al. 2011, Kendrick and Benstead 2013Kendrick MR and Benstead JP. 2013. Temperature and nutrient availability interact to mediate growth and body stoichiometry in a detritivorous stream insect. Freshwater Biol 58: 1820-1830.). In Brazil, some insects have recently gained greater importance, and it is believed that the occurrence of higher temperatures has favored some insect pests. For example, currently, the brown stink bug E. heros is the main problem in areas cultivated with soybeans, and this species has become highly adapted to high temperature conditions (Cividanes and Parra 1994Cividanes FJ and Parra Jrp. 1994. Biologia em diferentes temperaturas e exigências térmicas de percevejos pragas da soja: II. Euschistus heros (Fabr.) (Heteroptera: Pentatomidae). Pesq Agropec Bras 29: 1841-1846.). According to Cividanes and Parra (1994), the highest oviposition of this species is in the range of 26 to 28 °C, but it also has good growth at 30 °C. Currently, the soybean looper C. includens is reported as a key pest of soybeans, and its occurrence is often associated with periods of higher temperatures and lower rainfall. According to Mason and Mack (1984)Mason LJ and Mack TP. 1984. Influence of temperature on oviposition and adult female longevity for the soybean looper, Pseudoplusia includens (Walker) (Lepidoptera Noctuidae). Environ Entomol 13: 379-383., the maximum fecundity of moths of this species occurs between 26 and 32 °C, which explain the greater abundance of this pest in the warmer regions of Brazil.
Despite the climatic significance of the dynamics of insects, the nutritional quality of the host plant is crucial for the establishment of a pest. In this sense, our results showed that soybean pods are probably not suitable for the development of green belly stink bugs, due to the high mortality rate observed in general. Similar studies have also indicated a high mortality of D. melacanthus when only fed with soybean pods (Panizzi et al. 2007, Chocorosqui and Panizzi 2008), achieving mortality rates of around 63% (Chocorosqui and Panizzi 2008). This high mortality is observed even when the insect feeds on other food sources, such as an artificial diet (Panizzi et al. 2007) or seeds of wheat or maize seedlings (Chocorosqui and Panizzi 2008). Therefore, the survival of D. melacanthus in nature is not guaranteed by only one food source, but probably by a diversity of host plants (Silva et al. 2013), which may explain the current status of this pest, which is still of secondary importance in soybeans.
The nymphal stage of D. melacanthus at 25 °C took about 25 d to complete, independent of the genetic material of soybeans. These values are similar to those obtained by Chocorosqui and Panizzi (2008), where this stink bug took between 25 and 28 d to reach the adult stage while feeding on non-Bt soybean pods. When the insects reached the adult stage, the parameters of sex ratio, fecundity, and egg viability did not differ between the treatments, thus proving the ineffectiveness of MON 87701 × MON 89788 soybeans on the biology of D. melacanthus.
Thus, these observations demonstrate that MON 87701 × MON 89788 soybeans have no im pact on D. melacanthus. However, although the sucking insects do not come in direct contact with the insecticidal protein, the safety of Bt plants on sucking insects cannot be generalized because there are other factors that can alter the biology of non-target pests. Accordingly, Kim et al. (2012) reported that the development of the aphid Rhopalosiphum padi (L.) (Hemiptera: Aphididae) on Bt maize surprisingly increased its fecundity. The authors also found a high concentration of insecticidal protein (Cry1F) in insects, proving the ingestion of Bt. While the conducting vessels of plants do not carry the insecticidal protein, insects can accidentally ingest protein when they are inserting their stylus on the leaf area of the host. Therefore, it is possible that D. melacanthus has ingested the Bt protein Cry1Ac from soybeans, but is unlikely that buildup occurred in its tissues, since the biological parameters of the pest were not affected.
Although under laboratory conditions the stink bug has not shown differences in their biology, in field conditions there are many contradictory results, where the non-target pest may or may not be influenced by Bt (Pons et al. 2005Pons X, Lumbierres B, López C and Albajes R. 2005. Abundance of non-target pests in transgenic Bt-maize: A farm scale study. Eur J Entomol 102: 73-79., Mann et al. 2010Mann RS, Gill RS, Dhawan AK and Shera PS. 2010. Relative abundance and damage by target and non-target insects on Bollgard and Bollgard II cotton cultivars. Crop Prot 29: 793-801., Li et al. 2011). This was evidenced in the work of Dhillon and Shama (2013)Dhillon MK and Sharma HC. 2013. Comparative studies on the effects of Bt-transgenic and non-transgenic cotton on arthropod diversity, seed cotton yield and bollworms control. J Environ Biol 34: 67-73., in which the authors reported a higher incidence of the whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) on Bt cotton, while the population of Amrasca biguttula (Ishida) (Hemiptera: Cicadellidae) was negatively influenced by the transgenic plant. In another study, Fernandes et al. (2012) observed the opposite behavior, with a lower incidence of aphids in Bt cotton. Therefore, these divergent results demonstrate that this relationship is very specific, depending on the plant and insect species studied.
The gene cry1Ac expressed in Bt soybeans is also present in some Bt cotton (Bollgard I(r)), which is nowadays cultivated on a large-scale worldwide. Some research has been performed with this transgenic cotton, finding no influence of this technology on non-target insects (Torres and Ruberson 2006Torres JB and Ruberson JR. 2006. Interactions of Bt-cotton and the omnivorous big-eyed bug Geocoris puncti pes (Say), a key predator in cotton Welds. Biol Control 39: 47-57., Sujii et al. 2013Sujii ER, Togn Phb, Ribeiro PA, Bernardes TA, Milane Pvgn, Paula DP, Pires Css and Fontes Emg. 2013. Field Evaluation of Bt Cotton Crop Impact on Nontarget Pests: Cotton Aphid and Boll Weevil. Neotrop Entomol 42: 102-111.), similar to the results of our study. However, for the cultivation of Bt maize, a few different results have been observed, including reported population increases in the leafhoppers Dalbulus maidis (DeLong & Wolcott) (Hemiptera: Cicadellidae) (Virla et al. 2010Virla EG, Casuso M and Frias EA. 2010. A preliminary study on the effects of a transgenic corn event on the non-target pest Dalbulus maidis (Hemiptera: Cicadellidae). Crop Prot 29: 635-638.) and Zyginidia scutellaris (Herrich - Schäffer) (Pons et al. 2005). One hypothesis raised by the authors is that the pest may have benefited from the high quality of the uninjured leaves of the plant, due to the lower occurrence of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) (Virla et al. 2010). In the case of Bt soybean crops, the expected reduction in leaf injury caused by A. gemmatalis and C. includens, particularly, may also favor the higher abundance of other pests such as sucking pests like stink bugs. However, this hypothesis needs to be investigated under field conditions.
In the field, the quality of the host plant pest may interfere with the rate of parasitism (Koppel et al. 2009Koppel AL, Herbert DA, Kuhar TP and Kamminga K. 2009. Survey of stink bug (Hemiptera: Pentatomidae) egg parasitoids in wheat, soybean, and vegetable crops in Southeast Virginia. Environ Entomol 38: 375-379.), probably due to being able to change the nutritional quality of host eggs. In our study, this was not observed, and the biology of T. podisi was not affected when developed in eggs of D. melacanthus fed MON 87701 × MON 89788 soybeans. This indicates that the nutritional quality of the eggs of the pest does not affect the development of its natural enemy. This was confirmed by the high parasitism rate (above 80% in both treatments), resembling that on eggs of E. heros, its preferred host (Pacheco and Corrêa-Ferreira 1998Pacheco Djp and Côrrea-Ferreira BS. 1998. Potencial reprodutivo e longevidade do parasitóide Telenomus podisi Ashmead, em ovos de diferentes espécies de percevejos. An Soc Entomol Brasil 27: 585-591.). These results indicate that this parasitoid can be used to mitigate the impact of stink bugs on MON 87701 × MON 89788 soybeans, since the efficiency of this natural enemy will not be impaired.
The innocuous effect of Bt on natural ene mies has been shown in other research. Chen et al. (2008)Chen M, Zhao JZ, Collins HL, Earle ED, Cao J and Shelton AM. 2008. A critical assessment of the effects of Bt transgenic plants on parasitoids. Plos One 3: e2284. showed that the parasitoid Diadegma insu laris (Cresson) (Hymenoptera: Braconidae) is not affected by the Bt protein, regardless of the form in which it is offered to the natural enemy. Similarly, egg parasitoids of Lepidoptera belonging to the genus Trichogramma are also not affected when fed with pollen from Bt corn and cotton in their diet (Geng et al. 2006Geng JH, Shen ZR, Song K and Zheng L. 2006. Effect of pollen of regular cotton and transgenic Bt-CpTI cotton on the survival and reproduction of the parasitoid wasp Trichogramma chilonis (Hymenoptera: Trichogrammatidae) in the laboratory. Environ Entomol 35: 1661-1668., Wang et al. 2007Wang ZY, Wu Y, He KL and Bai SX. 2007. Effects of transgenic Bt maize pollen on longevity and fecundity of Trichogramma ostriniae in laboratory conditions. Bull Insectol 60: 49-55.). However, other studies have found adverse results on natural ene mies. For example, Gao et al. (2010)Gao MQ, Hou SP, Pu DQ, Shi M, Ye GY and Chen XX. 2010. Multi generation effects of Bt rice on Anagrus nilaparvatae a parasitoid of the nontarget pest Nilapavarta lugens. Environ Entomol 39: 2039-2044. found that the development time of the first generations of Anagrus nilaparvatae (Pang and Wang) (Hyme noptera: Mymaridae) in eggs of the non-target pest Nilapavarta lugens (Stal) (Hemiptera: Delphacidae) were affected when the pest was fed Bt rice. In a review by Lovei and Arpaia (2005)Lovei GL and Arpaia S. 2005. The impact of transgenic plants on natural enemies: a critical review of laboratory studies. Entomol Exp Appl 144(1): 1-14., 40% of studies found some negative impact on natural enemy biology caused directly or indirectly by Bt plants. Nevertheless, the authors stress that there is still little research investigating this interaction, mainly because most of the studies were performed with a small group of natural enemies.
Finally, our work demonstrated that the stink bug D. melacanthus has better development at elevated temperatures, and in this condition the pest may cause further damage to cultivated plants. Thus, it is necessary to monitor the temperature over the years and in different regions with soybean crops to determine whether the pest will not be affected by warmer temperatures at these sites. Additionally, we can clearly observe that our study has an important contribution in proving that D. melacanthus and its parasitoid T. podisi are not affected by soybean MON 87701 × MON 89788 technology. Further field studies are needed where it is possible to study the bioecology of the pest, especially considering that there is less competition for food because of the population reduction of the target lepidopteran with this technology, thus changing the biodynamics of pests in crops.
ACKNOWLEDGMENTS
Authors wish to thank Embrapa Soja, the sponsor agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support.
- Bernardi O, Malvestiti GS, Dourado PM, Oliveira WS, Martinelli S, Berger GU, Head GP and Omoto C. 2012. Assessment of the high -dose concept and level of control provided by MON 87701 x MON 89788 soybean against Anticarsia gemmatalis and Pseudoplusia includens (Lepidoptera: Noctuidae) in Brazil. Pest Manag Sci 68: 1083-1091.
- Bueno Rcof, Bueno AF, Moscardi F, Parra Jrp and Hoffmann-Campo CB. 2011. Lepidopteran larva consumption of soybean foliage: basis for developing multiple- species economic thresholds for pest management decisions. Pest Manag Sci 67: 170-174.
- Burr IW and Foster LA. 1972. A test for equality of vari ances. Mimeo Series No. 282. University of Purdue, West Lafayette, 26 p.
- Chen M, Zhao JZ, Collins HL, Earle ED, Cao J and Shelton AM. 2008. A critical assessment of the effects of Bt transgenic plants on parasitoids. Plos One 3: e2284.
- Chocorosqui VR and Panizzi AR. 2002. Influência da temperatura na biologia de ninfas de Dichelops melacanthus (Dallas, 1851) (Heteroptera: pentatomidae). Semina 23: 217-220.
- Chocorosqui VR and Panizzi AR. 2004. Impact of cultivation systems on Dichelops melacanthus (Dallas) (Heteroptera: Pentatomidae) populations and damage and its chemical control on wheat. Neotrop Entomol 33: 487-492.
- Chocorosqui VR and Panizzi AR. 2008. Nymph and adult biology of Dichelops melacanthus (Dallas) (Heteroptera: Pentatomidae) feeding on cultivated and non-cultivated host plants. Neotrop Entomol 37: 356-360.
- Cividanes FJ and Parra Jrp. 1994. Biologia em diferentes temperaturas e exigências térmicas de percevejos pragas da soja: II. Euschistus heros (Fabr.) (Heteroptera: Pentatomidae). Pesq Agropec Bras 29: 1841-1846.
- Corrêa-Ferreira BS and Moscardi F. 1994. Seasonal occurrence and host spectrum of egg parasitoids associated with soybean stink bugs. Biol Control 5: 196-202.
- Corrêa-Ferreira BS and Oliveira Mcn. 1998. Viability of Nezara viridula (L.) eggs for parasitism by Trissolcus basalis (Woll.), under different storage techiniques in liquid Nitrogen. An Soc Entomol Brasil 27: 101-107.
- Dhillon MK and Sharma HC. 2013. Comparative studies on the effects of Bt-transgenic and non-transgenic cotton on arthropod diversity, seed cotton yield and bollworms control. J Environ Biol 34: 67-73.
- Faostat - Food and Agriculture Organization of the United Nations. 2013. http://faostat3.fao.org/home/index.html
» http://faostat3.fao.org/home/index.html - Fehr WR and Caviness CE. 1977. Stages of soybean de velopment. Ames: University of Sciense and Technology. Special Report 80: 11.
- Fernandes FS, Ramalho FS, Malaquias JB, Nascimento Junior JL, Correia ET and Zanuncio JC. 2012. Within-plant distribution of cotton aphid (Hemip tera: Aphididae) in cotton cultivars with colored fibers. An Acad Bras Cien 84: 707-719.
- Fernandes OA, Faria M, Martinelli S, Schmidt F, Carvalho VF and Moro G. 2007. Short-term assessment of Bt maize on non-target arthropods in Brazil. Sci Agric 64: 249-255.
- Gao MQ, Hou SP, Pu DQ, Shi M, Ye GY and Chen XX. 2010. Multi generation effects of Bt rice on Anagrus nilaparvatae a parasitoid of the nontarget pest Nilapavarta lugens. Environ Entomol 39: 2039-2044.
- Geng JH, Shen ZR, Song K and Zheng L. 2006. Effect of pollen of regular cotton and transgenic Bt-CpTI cotton on the survival and reproduction of the parasitoid wasp Trichogramma chilonis (Hymenoptera: Trichogrammatidae) in the laboratory. Environ Entomol 35: 1661-1668.
- Hochachka PW and Sommero GN. 1984. Temperature adaptation. In: Hochachka PW and Sommero GN (Eds), Biochemical Adaptation. Princeton University Press, Princeton, NJ, p. 355-449.
- Kendrick MR and Benstead JP. 2013. Temperature and nutrient availability interact to mediate growth and body stoichiometry in a detritivorous stream insect. Freshwater Biol 58: 1820-1830.
- Kim YH, Hwang CE, Kim TS, Lee JH and Lee S. 2012. Assessment of potential impacts due to unintentionally released Bt maize plants on non-target aphid Rhopa losiphum padi (Hemiptera: Aphididae). J Asia Pac Entomol 15: 443-446.
- Koppel AL, Herbert DA, Kuhar TP and Kamminga K. 2009. Survey of stink bug (Hemiptera: Pentatomidae) egg parasitoids in wheat, soybean, and vegetable crops in Southeast Virginia. Environ Entomol 38: 375-379.
- Kouser S and Qaim M. 2011. Impact of Bt cotton on pesti cide poisoning in smallholder agriculture: A panel data analysis. Ecol Econ 70: 2105-2113.
- Latham JR, Wilson AK and Steinbrecher RA. 2006. The Mutational Consequences of Plant Transformation. J Biomed Biotechnol 2006: 1-7.
- Laumann RA, Moraes Mcb, Silva JP, Vieira Amc, Silveira S and Borges M. 2010. Vespas parasitoides de ovos como inimigos naturais do percevejo neotropical Dichelops melacanthus. Pesq Agropec Bras 45: 442-449.
- Li G, Feng H, McNeil JN, Liu B, Chen P and Qiu F. 2011. Impacts of transgenic Bt cotton on a non-target pest, Apolygus lucorum (Meyer-Dür) (Hemiptera: Miridae), in northern China. Crop Protect 30: 1573-1578.
- Lovei GL and Arpaia S. 2005. The impact of transgenic plants on natural enemies: a critical review of laboratory studies. Entomol Exp Appl 144(1): 1-14.
- Lu Y, Wu K, Jiang Y, Guo Y and Desneux L. 2012. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nat 487: 362-367.
- Manachini B and Lozzia GC. 2002. First results into the effects of Bt corn crop on nematofauna. Boll Zool Agric Bachic 34: 85-96.
- Manfredi-Coimbra S, Silva JJ, Chocorosqui VR and Panizzi AR. 2005. Danos do percevejo barriga-verde Dichelops melacanthus (Dallas) (Heteroptera: Pentatomidae) em trigo. Cienc Rural 35: 1243-1247.
- Mann RS, Gill RS, Dhawan AK and Shera PS. 2010. Relative abundance and damage by target and non-target insects on Bollgard and Bollgard II cotton cultivars. Crop Prot 29: 793-801.
- Mason LJ and Mack TP. 1984. Influence of temperature on oviposition and adult female longevity for the soybean looper, Pseudoplusia includens (Walker) (Lepidoptera Noctuidae). Environ Entomol 13: 379-383.
- Musser FR, Stewart SD and Catchot Jr AL. 2009. 2008 soybean insect losses for Mississippi and Tennessee. Midsouth Entomol 2: 42-46.
- Nondillo A, Redaelli LR, Botton M, Pinent Smj and Gitz R. 2008. Exigências térmicas e estimativa do número de gerações anuais de Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) em morangueiro. Neotrop Entomol 37: 646-650.
- Pacheco Djp and Côrrea-Ferreira BS. 1998. Potencial reprodutivo e longevidade do parasitóide Telenomus podisi Ashmead, em ovos de diferentes espécies de percevejos. An Soc Entomol Brasil 27: 585-591.
- Panizzi AR. 1997. Wild hosts of pentatomids: ecological and role in their pest status on crops. Annu Rev Entomol 42: 99-122.
- Panizzi AR and Corrêa-Ferreira BS. 1997. Dynamics in the insect fauna adaptation to soybean in the tropics. Trends Entomol 1: 71-88.
- Panizzi AR, Duo Ljs, Bortolato NM and Siqueira F. 2007. Nymph developmental time and survivorship, adult longevity, reproduction and body weight of Dichelops melacanthus (Dallas) feeding on natural and artificial diets. Rev Bras Entomol 51: 484-488.
- Pons X, Lumbierres B, López C and Albajes R. 2005. Abundance of non-target pests in transgenic Bt-maize: A farm scale study. Eur J Entomol 102: 73-79.
- Praslicka J and Huszár J. 2004. Influence of temperature and host plants on the development and fecundity of the spider mite Tetranychus urticae (Acarina: Tetranychidae). Plant Prot Science 40: 141-144.
- Sanders CJ, Pell JK, Poppy GM, Raybould A, Garcia-Alonso M and Schuler TH. 2007. Host-plant mediated effects of transgenic maize on the insect parasitoid Campoletis sonorensis (Hymenoptera: Ichneumonidae). Biol Control 40: 362-369.
- Santos K, Neves P, Meneguim AM, Santos RB and Santos WJ. 2009. Selection and characterization of the Bacillus thunringiensis strains toxic to Spodoptera eridania (Cramer), Spodoptera cosmioides (Walker) and Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). Biol Control 50: 157-163.
- Sas Institute. 2001. User's Guide: Statistics, Version 6e. Cary, 2001.201p.
- Shapiro SS and Wilk MB. 1965. An analysis of variance test for normality. Biometrika 52: 591-611.
- Silva DM, Hoffmann-Campo CB, Bueno AF, Bueno Rcof, De Oliveira Mcn and Moscardi F. 2011. Biological characteristics of Anticarsia gemmatalis (Lepidoptera: Noctuidae) for three consecutive generations under different temperatures: understanding the possible impact of global warming on a soybean pest. Bull Entomol Res 102: 285-292.
- Silva JJ, Ventura MU, Silva Fac and Panizzi AR. 2013. Population Dynamics of Dichelops melacanthus (Dallas) (Heteroptera: Pentatomidae) on Host Plants. Neotrop Entomol 42: 141-145.
- Smith JF, Luttrell RG and Greene JK. 2009. Seasonal abundance, species composition, and population dynamics of stink bugs in production fields of early and late soybean in south Arkansas. J Econ Entomol 102: 229-236.
- Sujii ER, Togn Phb, Ribeiro PA, Bernardes TA, Milane Pvgn, Paula DP, Pires Css and Fontes Emg. 2013. Field Evaluation of Bt Cotton Crop Impact on Nontarget Pests: Cotton Aphid and Boll Weevil. Neotrop Entomol 42: 102-111.
- Torres JB and Ruberson JR. 2006. Interactions of Bt-cotton and the omnivorous big-eyed bug Geocoris puncti pes (Say), a key predator in cotton Welds. Biol Control 39: 47-57.
- Virla EG, Casuso M and Frias EA. 2010. A preliminary study on the effects of a transgenic corn event on the non-target pest Dalbulus maidis (Hemiptera: Cicadellidae). Crop Prot 29: 635-638.
- Wang ZY, Wu Y, He KL and Bai SX. 2007. Effects of transgenic Bt maize pollen on longevity and fecundity of Trichogramma ostriniae in laboratory conditions. Bull Insectol 60: 49-55.
- Zhang SY, Fie BY, Cui J and Li DM. 2006. Biology of Campoletis chlorideae (Uchida) (Hym., Ichneumonidae) developing in Bt-treated, Bt-resistant Helicoverpa armigera (Hübner) (Lep., Noctuidae) larvae. J Appl Entomol 130: 268-274.
Publication Dates
-
Publication in this collection
31 May 2016 -
Date of issue
Apr-Jun 2016
History
-
Received
07 May 2015 -
Accepted
19 June 2015




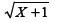 ).
).