ABSTRACT
Surveys in the coastal sandy plains (restingas) of Rio de Janeiro have shown a great richness of galls. We investigated the galling insects in two preserved restingas areas of Rio de Janeiro state: Parque Estadual da Costa do Sol and Reserva Particular do Patrimônio Natural Fazenda Caruara. The collections were done each two months, from June 2011 to May 2012. We investigated 38 points during 45 minutes each per collection. The galls were taken to the laboratory for rearing the insects. A total number of 151 insect galls were found in 82 plant species distributed into 34 botanic families. Most of the galls occurred on leaves and the plant families with the highest richness of galls were Myrtaceae and Fabaceae. All the six insect orders with galling species were found in this survey, where Cecidomyiidae (Diptera) was the main galler group. Hymenoptera and Thysanoptera were found as parasitoids and inquilines in 29 galls. The richness of galls in the surveyed areas reveals the importance of restinga for the composition and diversity of gall-inducing insect fauna.
Key words:
galling insects; sandy coastal plain; cecidomyiid; arthropod-plant interaction
INTRODUCTION
Galls are abnormal modifications in plant tissue induced by insects or mites (Raman 2007RAMAN A. 2007. Insect-induced plant galls of India: unresolved questions. Curr Sci 92: 748-757.). Among the insects, six orders have been recorded by having gall-inducing species, Diptera, Hymenoptera, Lepidoptera, Coleoptera, Hemiptera, and Thysanoptera. The family Cecidomyiidae (Diptera) is the main taxa of galling insect within the arthropods (Gagné and Jaschhof 2014GAGNÉ RJ and JASCHHOF M. 2014. A Catalog of the Cecidomyiidae (Diptera) of the World. 3rd ed., Digital version 2.).
Brazil shows a rich fauna of galling insects, but the taxonomic knowledge of them is poorly known. Cerrado, Atlantic Forest, Amazon Forest, Caatinga, and Pantanal are among the studied biomes and they have shown a high number of insect galls. Some studies have correlated this rich fauna to the flora of these areas, mainly the most diverse ones, such as Amazon, Atlantic Forest, and Cerrado (Maia 2001MAIA VC. 2001. The gall midges (Diptera, Cecidomyiidae) from three restingas of Rio de Janeiro State, Brazil. Rev Bras Zool 18: 583-629., Araújo 2013ARAÚJO WS. 2013. Different relationships between galling and non-galling herbivore richness and plant species richness: a meta-analysis. Anthropod-Plant Interact 7: 373-377., Santos et al. 2013SANTOS AW, SCARELI-SANTOS C, GUILHERME FAG and CUEVA-REYES P. 2013. Comparing galling insects richness among Neotropical savanas: effects of plant richness, vegetation structure and super host presence. Biodivers Conserv 22: 1080-1094.).
The surveys of galls in Brazil are concentrated in the southeastern region, and these contributions have been corroborating with the high diversity of insect galls in this region. Most of the records are related to surveys in sandy coastal plains, also called restinga (Maia 2001MAIA VC. 2001. The gall midges (Diptera, Cecidomyiidae) from three restingas of Rio de Janeiro State, Brazil. Rev Bras Zool 18: 583-629., Oliveira and Maia 2005OLIVEIRA JC and MAIA VC. 2005. Ocorrência e caracterização de galhas de insetos na restinga de Grumari (Rio de Janeiro, RJ, Brasil). Arqu Mus Nac 63: 669-675., Maia and Oliveira 2010, Rodrigues et al. 2014RODRIGUES AR, MAIA VC and COURI MS. 2014. Insect galls of restinga areas of Ilha da Marambaia, Rio de Janeiro, Brazil. Rev Bras Ent 58: 173-197 .).
Restinga areas were investigated in Espírito Santo, São Paulo, and Rio de Janeiro states, being the last one the most studied (Maia 2013MAIA VC. 2013. Galhas de insetos em restingas da região sudeste do Brasil com novos registros. Biota Neotrop 13: 183-209., Maia et al. 2014). The Atlantic forest, biome where the restinga vegetation is inserted, is known by the high plant diversity and endemism level. Considering the high specificity degree of this guild to their host plants, we expect a high diversity of these insects even in areas where there are some available data.
To the present contribution we investigated the galling insects associated with coastal shrub vegetation in two preserved areas of Rio de Janeiro state.
MATERIAL AND METHODS
Surveys were conducted in two preserved areas: Parque Estadual da Costa do Sol (PECS) and Reserva Particular do Patrimônio Natural Fazenda Caruara (RPPNFC). The PECS was created in 2011 and has about 9,800 ha, being the surveys done in the municipalities of Saquarema, Araruama, Arraial do Cabo, and Cabo Frio. The RPPNFC has 3,800 ha of area and is a private reserve located in the municipality of São João da Barra.
The collections were made each two months, from June 2011 to May 2012. 38 points were chosen and distributed along the restinga vegetation from each Municipality, proportionally to the restinga area (Fig. 1). In each point we investigated the plants during 45 minutes. All the galls were photographed and separated in morphotypes following the classification proposed by Isaias et al. (2013ISAIAS RMS, CARNEIRO RGS, OLIVEIRA DC and SANTOS JC. 2013. Illustrated and Annotated Checklist of Brazilian Gall Morphotypes. Neotrop Entomol 42: 230-239.), where the authors standardized the most common galls shapes found in Brazil. The host plants were pressed, dried and sent for identification to botanist of Museu Nacional, Universidade Federal do Rio de Janeiro (MNRJ). The plant species names were updated using the Flora do Brasil website (Flora do Brasil 2015).
Location of sites of collection in restinga vegetation from Rio de Janeiro state, Brazil. Abreviations: ARA: Araruama, ARRA: Arraial do Cabo, CF: Cabo Frio, SAQ: Saquarema, SJB: São João da Barra, RJ: Rio de Janeiro state.
The galls were taken to the Diptera laboratory of Museu Nacional, for rearing the insects and obtain the adults for identification. Each gall morphotype was kept individually in plastic pots layered at the bottom with damp cotton and covered by fine screening. The pots were checked daily for adult emergence (gallers, parasitoids, inquilines and predators). Immature insects were obtained by dissecting some galls. All specimens were preserved in 70% ethanol. The Cecidomyiidae specimens were mounted in slides following Gagné (1994GAGNÉ RJ. 1994. The Gall Midges of the Neotropical Region. Ithaca, Cornell University Press.), except by using butyl acetate instead of creosote oil. These specimens were identified using taxonomic keys (Gagné 1994) and comparisons with original descriptions. All insect and plant specimens were deposited in the Entomological collection and Herbarium of Museu Nacional, Rio de Janeiro (MNRJ), respectively.
RESULTS
A total number of 151 insect galls were found in 82 plant species distributed into 34 botanic families (Table SI TABLE SI host plants, galls morphology, inducers and investigated localities of Parque estadual Costa do Sol (araruama, arraial do Cabo, Cabo Frio, and Saquarema) and of RPPn Fazenda Caruara, Grussaí (São João da Barra), Rio de Janeiro, Brazil, from June 2011 to May 2012. abbreviations: aRa: araruama, aRRa: arraial do Cabo, CF: Cabo Frio, SaQ: Saquarema, SJB: São João da Barra. , Figs. 2-121). Galls were recorded in seven plant organs, most of them occurred on leaves (64%, n = 97), but also on buds (9%, n = 14), stems (21%, n = 32), flowers (3%, n = 4), fruits (2%, n = 3), and tendril (1%, n = 1).
Galling insects from Parque Estadual da Costa do Sol and RPPN Fazenda Caruara (RJ, Brazil). 2, Lithraea brasiliensis, marginal roll. 3-4, 3, Oxypetalum banksii, globoid bud gall, 4, fusiform leaf vein. 5, Eupatorium punctulatum, fusiform stem gall. 6, Mikania hoehnei, stem fusiform gall. 7-8, Vernonia rufogrisea, 7, stem gall, 8, petiole gall. 9, Asteraceae sp., globoid leaf gall. 10-11, Fridericia conjugata, 10, conical leaf gall, 11, fusiform gall. 12, Euploca polyphylla, stem gall. 13-15, Varronia curassavica, 13, leaf vein gall, 14, lenticular leaf vein gall, 15, stem gall. 16, Tournefortia villosa, stem gall. 17-19, Protium heptaphyllum, 17, marginal roll, 18, globoid leaf gall, 19, lenticular gall. 20, Hylocereus setaceus, stem gall. 21-22, Maytenus obtusifolia, 21, fruit gall, 22, lenticular leaf gall. 23-24, Couepia ovalifolia, 23, marginal roll, 24, globoid leaf gall. 25, Clusia fluminensis, lenticular leaf gall.
Galling insects from Parque Estadual da Costa do Sol and RPPN Fazenda Caruara (RJ, Brazil). 26, Clusia hilariana, lenticular leaf gall. 27-29, Erythroxylum ovalifolium, 27, lenticular leaf gall, 28, conical bud gall, 29, marginal roll. 30, Croton compressus, stem gall. 31, Dalechampia micromeria, marginal roll. 32, Microstachys corniculata, marginal roll. 33-35, Pera glabrata, 33, stem gall, 34, lenticular leaf gall, 35, marginal roll. 36, Andira legalis, marginal roll. 37, Andira sp., globoid leaf gall. 38, Centrosema virginianum, leaf vein gall. 39, Dalbergia ecastaphyllum, globoid leaf gall. 40, Indigofera sabulicola, lenticular leaf gall. 41-42, Inga laurina, 41, globoid bud gall, 42, leaf vein gall. 43-44, Inga maritima, 43, leaf vein gall, 44, globoid leaf gall. 45-46, Machaerium lanceolatum, 45, globoid bud gall, 46, lenticular leaf gall. 47, Senegalia lacerans, bud gall. 48, Swartzia apetala, lenticular leaf gall. 49, Humiria balsamifera, stem gall.
Galling insects from Parque Estadual da Costa do Sol and RPPN Fazenda Caruara (RJ, Brazil). 50, Hyptis sp., leaf vein gall. 51, Ocotea pulchella, globoid leaf gall. 52-53, Ocotea notata, 52, stem gall, 53, lenticular leaf gall. 54, Malpighiaceae sp., fruit gall. 55, Stigmaphyllon paralias, stem gall. 56, Miconia cinnamomifolia, leaf vein gall. 57-61, Eugenia astringens, 57, marginal roll, 58, conical leaf gall, 59, clavate gall, 60, lenticular leaf gall, 61, globoid leaf gall. 62-70, Eugenia copacabanensis, 62, bud gall, 63, conical leaf gall, 64, conical gall, 65, spiral leaf gall, 66, leaf fold, 67, marginal roll, 68, leaf vein gall, 69, rosette gall, 70, stem gall. 71, Eugenia punicifolia, stem gall. 72, Eugenia selloi, marginal roll. 73, Eugenia uniflora, lenticular leaf gall.
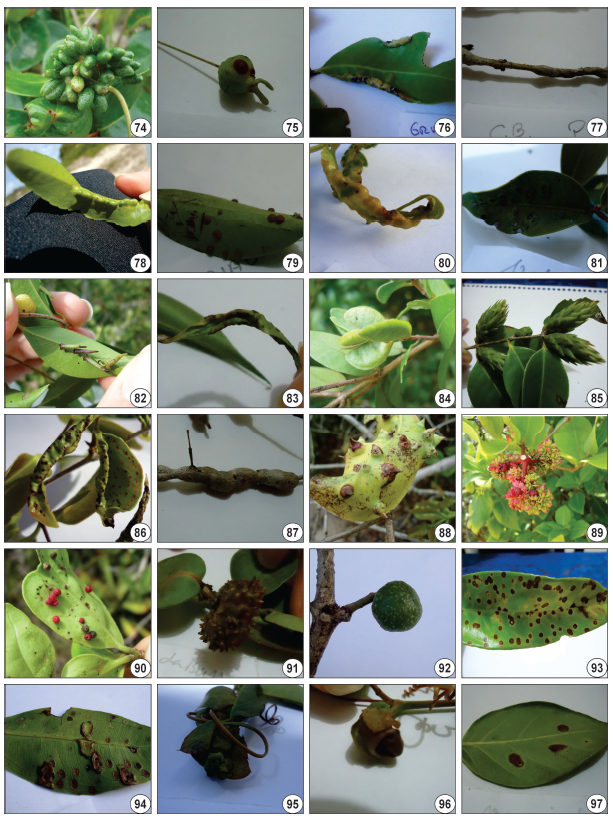
Galling insects from Parque Estadual da Costa do Sol and RPPN Fazenda Caruara (RJ, Brazil). 74-75, Eugenia uniflora, 74, conical leaf gall, 75, fruit gall. 76-77, Eugenia sp., 76, marginal roll, 77, stem gall. 78-81, Myrcia ovata, 78, conical leaf gall, 79, globoid leaf gall, 80, marginal roll, 81, lenticular leaf gall. 82-83, Myrciaria floribunda, 82, cylindrical leaf gall, 83, marginal roll. 84, Myrciaria tenella, young leaf fold. 85-87, Neomitranthes obscura, 85, pine-like gall, 86, leaf fold, 87, stem gall. 88, Psidium cattleianum, conical leaf gall. 89-92, Guapira opposita, 89, rosette gall, 90, globoid leaf gall, 91-92, bud gall. 93, Ouratea cuspidata, lenticular leaf gall. 94, Ouratea sp., lenticular leaf gall. 95, Passiflora alliaceae, bud gall. 96, Passiflora mucronata, leaf fold gall. 97, Coccoloba rigida, lenticular leaf gall.
Galling insects from Parque Estadual da Costa do Sol and RPPN Fazenda Caruara (RJ, Brazil). 98, Coccoloba rigida, stem gall. 99, Myrsine parvifolia, stem gall. 100, Scutia arenicola, stem gall. 101, Diodella apiculata, stem gall. 102, Enhydra sessilis, bud gall. 103, Ladenbergia hexandra, lenticular leaf gall. 104-105, Psychotria carthagenensis, 104, globoid leaf gall, 105, stem gall. 106, Spermacocae verticilata, stem gall. 107, Paullinia racemosa, conical leaf gall. 108-110, Paullinia weinmanniaefolia, 108, conical leaf gall, 109, tendril gall, 110, stem gall. 111-112, Chrysophyllum lucentifolium, 111, lenticular leaf gall, 112, leaf vein gall. 113-114, Manilkara subsericea, 113, globoid leaf gall, 114, stem gall. 115, Sideroxylum obtusifolium, lenticular leaf gall. 116-117, Smilax rufescens, 116, leaf vein gall, 117, stem gall. 118-120, Lantana fucata, 118, globoid leaf gall, 119, cylindrical leaf gall, 120, stem gall. 121, Phoradendron quadrangulare, globoid leaf gall.
The galls also presented varied shapes, most of them were fusiform (27%, n = 41), followed by globoid (20%, n = 31), lenticular (18%, n = 28), conical (9%, n = 14), and marginal roll (10%, n = 16). Other shapes as cylindrical, clavate, leaf fold, and rosette galls corresponded, each one, to less than 5% of the amount.
The super host families were Myrtaceae with 36 gall morphotypes on 11 species followed by Fabaceae with 14 galls on 11 species and Rubiaceae with nine galls on seven species. Eugenia L. (Myrtaceae) was the super host genera, with a total number of 21 gall morphotypes, which corresponded to 60% of the galls found on Myrtaceae.
Eugenia copacabanensis Kiaersk (Myrtaceae) was the species with the highest gall richness, with nine gall morphotypes.
The restinga area with the richest galling species was Arraial do Cabo with 88 species, followed by São João da Barra (n = 66), Araruama (n = 64), Cabo Frio (n = 57), and Saquarema (n = 49).
All the insect orders known for comprising gall inducers were found in the investigated restingas. Diptera was represented by three families, Agromyzidae (Japanagromyza inferna Spencer, 1973, n=1), Cecidomyiidae (n=95), and Tephritidae (Procecidocchares sp., n=1). Among the Hemiptera galls (n=9), one species was identified, Calophya terebinthifolii Burckhardt & Basset, 2000 (Psyllidae) inducing lenticular galls on leaves of Schinnus terebenthifolius Raddi (Anacardiaceae). Lepidoptera (n=7), Coleoptera (Curculionidae, n=1), Thysanoptera (n=4), and Hymenoptera (n=1) totalled less than 10% of the galls.
Forty five Cecidomyiidae were determined at species level, and they are distributed into 22 genera. The remaining specimens were identified at genus level or higher categories, totalizing 29 genera, because the material collected was insufficient for a more accurate identification. The most representative genera were Dasineura Rondani, 1840 with seven species, Lopesia Rübsaamen, 1908 with six species, Stephomyia Tavares, 1916, with four species, Asphondylia Loew, 1850, Bruggmannia Tavares, 1906, Clinodiplosis Kieffer, 1894 (each with three species). 70% of the remaining genera were represented only by one species (n=19).
Sixteen Cecidomyiidae species were found in all investigated areas: Asphondylia cordiae Möhn, 1975, Bruggmannia robusta Maia & Couri, 1992, B. elongata Maia & Couri, 1992, Clinodiplosis profusa Maia, 2001, Dasineura byrsonimae Maia, 2011, D. globosa Maia, 1995, D. copacabanensis Maia, 1993, Lopesia erythroxyli Rodrigues & Maia, 2010, L. singularis Maia, 2001, Mayteniella distincta Maia, 2001, Neolasioptera eugeniae Maia, 1993, Paulliniamyia ampla Maia, 2001, Schismatodiplosis lantanae Rübsaamen, 1907, Smilasioptera candelariae Möhn, 1975, Stephomyia rotundifoliorum Maia, 1993, and Youngomyia pouteriae Maia, 2001. Some species were recorded only in one area, as Dactylodiplosis heptaphylli Maia, 2004, Lopesia similis Maia, 2004, Neolasioptera cerei Rübsaamen, 1905, Bruggmanniella maytenuse Maia & Couri, 1992, Pisphondylia braziliensis Couri & Maia, 1992, Asphondylia communis Maia & Couri, 1992, in São João da Barra; Clusiamyia granulosa Maia, 2001, Stephomyia tetralobae Maia, 1993, Clinodiplosis costai Maia, 2005, in Arraial do Cabo; Myrciamyia maricaensis Maia, 1995 in Cabo Frio.
The associated fauna was composed by parasitoids and inquilines. Parasitoids were represented by six Hymenoptera families found in 27 gall morphotypes: Braconidae, Elasmidae, Eulophidae, Eupelmidae, Eurytomidae, and Torymidae (Table I). Dimeromicrus cecidomyiae Ashmead, 1887 (Torymidae) was found in galls of Paulliniamyia ampla on Paullinia weinmanniaefolia Mart. (Sapindaceae). The inquilines were represented by Thysanoptera and were found in globoid galls on buds of Senegalia lacerans (Benth.) Seigler & Ebinger (Fabaceae), and marginal roll on Eugenia astringens Cambess (Myrtaceae).
Hymenoptera families associated with Cecidomyiidae galls in restingas of the Parque Estadual da Costa do Sol (Saquarema, Araruama, Arraial do Cabo and Cabo Frio) and Reserva Particular do Patrimônio Natural Fazenda Caruara (São João da Barra) Rio de Janeiro state, Brazil.
All the gall records in Saquarema, Araruama and São João da Barra are new to these municipilaties. The new galls recorded from Rio de Janeiro state are: marginal roll on Lithraea brasiliensis Marchand, stem gall on Eupatorium punctulatum DC., stem gall on Euploca polyphylla (Lehm.) J.I.M.Melo & Semir DC., galls on stem of Tournefortia villosa Salzm. Ex DC., marginal roll on Dalechampia micromeria Baill., lenticular on leaves of Andira fraxinifolia Benth., bud galls on Inga laurina Willd., lenticular leaf galls on Indigofera sabulicola Benth., leaf gall on Machaerium lanceolatum (Vell.) J.F. Macbr., bud gall on S. lacerans, leaf gall on Swartzia apetala Raddi, stem gall on Humiria balsamifera (Aubl.) J.St.-Hill., leaf gall on Ocotea pulchella (Nees & Mart.) Mez; leaf vein gall on Miconia cinnamomifolia (DC.) Naudim, conical leaf gall and rosette gall on Eugenia copacabanensis Kiaersk, marginal roll on Eugenia selloi B. D. Jacks., conical leaf vein gall and marginal roll on Myrcia ovata Cambess., cylindrical gall on Myrciaria floribunda (H. West ex Willd.) O. Berg., young leaf fold on Myrciaria tenella (DC.) O. Berg, bud gall on Guapira opposita (Vell.) Reitz., bud gall on Passiflora alliaceae Barb. Rodr., stem and leaf galls on Coccoloba rigida Meisn., stem gall on Diodella apiculata (Willd. ex Roem. & Schult.) Delprete; bud gall on Enhydra sessilis (Sw.) DC., lenticular leaf gall on Ladenbergia hexandra (Pohl) Klotzsch, leaf gall on Psychotria carthagenensis Jacq., stem and flower galls on Spermacocae verticilata L., leaf gall on Paullinia racemosa Wawra, leaf and leaf vein galls on Chrysophyllum lucentifolium Cronquist, globoid and cylindrical gall on Lantana fucata Lindl., globoid leaf gall on Phoradendron quadrangulare (Kunt) Griseb.
DISCUSSION
In restinga areas a total number of 480 gall morphotypes in 229 plant species and 60 botanic families have been recorded in São Paulo, Espírito Santo, and Rio de Janeiro states (Maia et al. 2014MAIA VC, CARVALHO-FERNANDES SP, RODRIGUES AR and ASCENDINO SS. 2014. Galls in the Brazilian Coastal Vegetation. In: FERNANDES GW and SANTOS JC (Eds), Neotropical Insect Galls. 1st ed., Springer, v. 1, p. 295-361 .). Most of the gall inducers are identified only on family level or superior categories. Cecidomyiidae is the best known family among the gall inducers in Brazil, but the gall midge taxonomy is far to reach all the species that probably may exist.
Cecidomyiidae is a family with many different feeding habits, but about 75% of the species are plant feeders. The majority are gall-inducing insects, being this family the most important taxon among this herbivore guild. About 60% of the galls occurring in restingas from Southeast of Brazil are induced by Cecidomyiidae (Maia 2013MAIA VC. 2013. Galhas de insetos em restingas da região sudeste do Brasil com novos registros. Biota Neotrop 13: 183-209., Gagné and Jaschhof 2014GAGNÉ RJ and JASCHHOF M. 2014. A Catalog of the Cecidomyiidae (Diptera) of the World. 3rd ed., Digital version 2., Maia et al. 2014).
Tephritidae is the second family of Diptera in importance among the galling species, being the cecidogenous represented by about 5% of all described species, most of them associated with Asteraceae (Freidberg 1998FREIDBERG A. 1998. Tephritidae galls and gall Tephritidae revisited, with special emphasis on myoptine galls. In: CSÓKA G ET AL. (Eds), The Biology of Gall-Inducing Arthropods. USDA Forest Service, North Central Research Statoon, USA, p. 36-43.). This is the second record of Tephritidae galls in restingas from Rio de Janeiro, the other previous species was associated with another Asteraceae species, Vernonia rufrogisea St.-Hill. in Ilha da Marambaia (Mangaratiba, Rio de Janeiro state), and another record on Vernonia beyrichii Less. from Bertioga, São Paulo (Maia et al. 2008MAIA VC, MAGENTA MAG and MARTINS SE. 2008. Ocorrência e caracterização de galhas de insetos em áreas de restinga de Bertioga (São Paulo, Brasil). Biota Neotrop 8: 167-197., Rodrigues et al. 2014RODRIGUES AR, MAIA VC and COURI MS. 2014. Insect galls of restinga areas of Ilha da Marambaia, Rio de Janeiro, Brazil. Rev Bras Ent 58: 173-197 .).
Agromyzidae are mainly known as leaf miners, but so far the galling species are known from seven genera. They usually induce galls on Salicaceae, Asteraceae and Fabaceae (Spencer 1990SPENCER KA. 1990. Host specialization in the world Agromyzidae (Diptera). Kluwer Academic Publishers Dordrecht, The Netherlands.). The gall of Japanagromyza inferna was previously recorded in restingas from Arraial do Cabo, Marambaia, and Saquarema (Rio de Janeiro state) associated with the same host plant found in this work (Sousa and Couri 2014SOUSA VR and COURI MC. 2014. Redescription of Japanagromyza inferna Spencer, first recorded from Brazil, and a key to the Neotropical species of Japanagromyza Sasakawa (Diptera, Agromyzidae). Zookeys 374: 45-55.).
Galls of Lepidoptera, Coleoptera, Hemiptera, Hymenoptera and Thysanoptera were previously recorded in Brazilian restingas. They comprise about 10% of all galls found in coastal shrub vegetation, with Hemiptera and Lepidoptera the most abundant (Maia et al. 2014MAIA VC, CARVALHO-FERNANDES SP, RODRIGUES AR and ASCENDINO SS. 2014. Galls in the Brazilian Coastal Vegetation. In: FERNANDES GW and SANTOS JC (Eds), Neotropical Insect Galls. 1st ed., Springer, v. 1, p. 295-361 .).
Leaves are the main affected organ by the galling insects (Mani 1964MANI MS. 1964. Ecology of plant galls. W. Junk, The Hague.) probably because this is the most abundant organ and, in most of the case, in restingas vegetation is available during all the year, unlike other dry forests, as caatinga in Brazil, where trees lose their leaves during the dry season. Leaf galls in restingas correspond to about 60% of all galls, but as recorded in this survey, galls are also present in other organs, such as stems, buds, fruits, and flowers (Maia 2013MAIA VC. 2013. Galhas de insetos em restingas da região sudeste do Brasil com novos registros. Biota Neotrop 13: 183-209., Maia et al. 2014).
Despite in this inventory the most common gall shape found was fusiform, globoid galls are the most common shape in the Neotropical region (Isaias et al. 2013ISAIAS RMS, CARNEIRO RGS, OLIVEIRA DC and SANTOS JC. 2013. Illustrated and Annotated Checklist of Brazilian Gall Morphotypes. Neotrop Entomol 42: 230-239.), the same pattern is observed in various ecosystems in Brazil, such as Cerrado, Amazon forest and also in restingas (Carvalho-Fernandes et al. 2012, Santos et al. 2012SANTOS JC, ALMEIDA-CORTEZ JS and FERNANDES GW. 2012. Gall-inducing insects from Atlantic forest of Pernambuco, northeastern Brazil. Biota Neotrop 12: 197-213., Maia 2013MAIA VC. 2013. Galhas de insetos em restingas da região sudeste do Brasil com novos registros. Biota Neotrop 13: 183-209., Maia et al. 2014).
The two most galled plant families recorded in this study are well reported in restingas as the super host families. Myrtaceae can be considered the main host family in coastal shrub vegetation in Southeastern Brazil, due to its great representativeness in all insect galls inventories. Other families, such as Fabaceae, Asteraceae, and Nyctaginaceae are also pointed as important hosts, nonetheless they vary in their importance according to the survey, probably because the diversity of vegetation physiognomies presented in the distinct areas (Bregonci et al. 2010BREGONCI JM, POLYCARPO PV and MAIA VC. 2010. Galhas de insetos do Parque Estadual Paulo César Vinha (Guarapari, ES, Brasil). Biota Neotrop 10: 265-274., Maia 2001MAIA VC. 2001. The gall midges (Diptera, Cecidomyiidae) from three restingas of Rio de Janeiro State, Brazil. Rev Bras Zool 18: 583-629., Maia et al. 2008, Maia and Oliveira 2010, Rodrigues et al. 2014RODRIGUES AR, MAIA VC and COURI MS. 2014. Insect galls of restinga areas of Ilha da Marambaia, Rio de Janeiro, Brazil. Rev Bras Ent 58: 173-197 .). In other Brazilian biomes, such as Araucaria Forest, Cerrado, Amazon Forest, Caatinga, and Caatinga-Cerrado transition these families also present the role of main gall hosts (Coelho et al. 2009COELHO MS, ALMADA ED, FERNANDES GW, CARNEIRO MAA, SANTOS RM, QUINTINO AV and SANCHEZ-AZOFEIFA A. 2009. Gall inducing arthropods from a seasonally dry tropical forest in Serra do Cipó, Brazil. Rev Bras Ent 53: 404-414., Almada and Fernandes 2011ALMADA ED and FERNANDES GW. 2011. Insetos indutores de galhas em floresta de terra firme e em reflorestamentos com espécies nativas na Amazônia Oriental, Pará, Brasil. Bol Mus Para Emílio Goeldi 6: 163-196., Santos et al. 2011SANTOS JC, ALMEIDA-CORTEZ JS and FERNANDES GW. 2011. Richness of gall-inducing insects in the tropical dry forest (caatinga) of Pernambuco. Rev Bras Ent 55: 45-54., Costa et al. 2014COSTA EC, CARVALHO-FERNANDES SP and SANTOS-SILVA J. 2014. Galhas de insetos em área de transição Caatinga-Cerrado no Nordeste brasileiro. Sitientibus Ser Ci Biol 14: 1-9., Toma and Mendonça Jr 2013TOMA TSP and MENDONÇA JR MS. 2013. Gall-inducing insects of an Araucaria Forest in southern Brazil. Rev Bras Ent 57: 225-233.).
The identified gall midges of the present study comprise about 35% of species and 50% of genera that are known from Rio de Janeiro state. The recorded richest genera are also among the most speciose in the state (Maia and Barros 2009MAIA VC and BARROS GPS. 2009. Espécies de Cecidomyiidae (Diptera) registradas no estado do Rio de Janeiro, Brasil. Arq Mus Nac 67: 211-220.).
Parasitoidism is the main mortality factor affecting the survival rate of cedidomyiids. The Hymenoptera families represented in this study are frequent in galls in restingas of Rio de Janeiro, and Eulophidae and Eupelmidae are among the most common families associated with many galls in a great variety of plant families and species (Maia and Azevedo 2009MAIA VC and BARROS GPS. 2009. Espécies de Cecidomyiidae (Diptera) registradas no estado do Rio de Janeiro, Brasil. Arq Mus Nac 67: 211-220.).
The restingas of the State of Rio de Janeiro can be considered the most studied when we compare with other Brazilian restingas. Even so, we still have a great diversity to be discovered, as shown with the new records presented here and a high number of new species to be named in these areas.
ACKNOWLEDGMENTS
To Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the scholarship for the first author (PROTAX Proc. 140344/2011-0). To Juliana Fernandes (MNRJ) for the Tephritidae identification, Viviane Sousa (MNRJ) for the Agromyzidae identification, Dr. Maria Antonieta Pereira de Azevedo (MNRJ) for the Hymenoptera identification, and Dr. Ricardo Silva (UFCE), Dr. Débora Medeiros (MNRJ), Dr. Marcelo Souza (UFRJ), and Dr. Roberto Esteves (UERJ) for the plant species identification.
REFERENCES
- ALMADA ED and FERNANDES GW. 2011. Insetos indutores de galhas em floresta de terra firme e em reflorestamentos com espécies nativas na Amazônia Oriental, Pará, Brasil. Bol Mus Para Emílio Goeldi 6: 163-196.
- ARAÚJO WS. 2013. Different relationships between galling and non-galling herbivore richness and plant species richness: a meta-analysis. Anthropod-Plant Interact 7: 373-377.
- BREGONCI JM, POLYCARPO PV and MAIA VC. 2010. Galhas de insetos do Parque Estadual Paulo César Vinha (Guarapari, ES, Brasil). Biota Neotrop 10: 265-274.
- CARVALHO-FERNANDES SP, ALMEIDA-CORTEZ JS and FERREIRA ALN. 2012. Riqueza de galhas entomógenas em áreas antropizadas e preservadas de Caatinga. Revista Árvore 36: 269-277.
- COELHO MS, ALMADA ED, FERNANDES GW, CARNEIRO MAA, SANTOS RM, QUINTINO AV and SANCHEZ-AZOFEIFA A. 2009. Gall inducing arthropods from a seasonally dry tropical forest in Serra do Cipó, Brazil. Rev Bras Ent 53: 404-414.
- COSTA EC, CARVALHO-FERNANDES SP and SANTOS-SILVA J. 2014. Galhas de insetos em área de transição Caatinga-Cerrado no Nordeste brasileiro. Sitientibus Ser Ci Biol 14: 1-9.
- FLORA DO BRASIL. 2015. Jardim Botânico do Rio de Janeiro. Disponível em: <http://floradobrasil.jbrj.gov.br/>. Accessed in: 10.mar.2015
» http://floradobrasil.jbrj.gov.br/ - FREIDBERG A. 1998. Tephritidae galls and gall Tephritidae revisited, with special emphasis on myoptine galls. In: CSÓKA G ET AL. (Eds), The Biology of Gall-Inducing Arthropods. USDA Forest Service, North Central Research Statoon, USA, p. 36-43.
- GAGNÉ RJ. 1994. The Gall Midges of the Neotropical Region. Ithaca, Cornell University Press.
- GAGNÉ RJ and JASCHHOF M. 2014. A Catalog of the Cecidomyiidae (Diptera) of the World. 3rd ed., Digital version 2.
- ISAIAS RMS, CARNEIRO RGS, OLIVEIRA DC and SANTOS JC. 2013. Illustrated and Annotated Checklist of Brazilian Gall Morphotypes. Neotrop Entomol 42: 230-239.
- MAIA VC. 2001. The gall midges (Diptera, Cecidomyiidae) from three restingas of Rio de Janeiro State, Brazil. Rev Bras Zool 18: 583-629.
- MAIA VC. 2013. Galhas de insetos em restingas da região sudeste do Brasil com novos registros. Biota Neotrop 13: 183-209.
- MAIA VC and AZEVEDO MAP. 2009. Micro-himenópteros associados com galhas de Cecidomyiidae (Diptera) em Restingas do Estado do Rio de Janeiro (Brasil). Biota Neotrop 9: 1-14.
- MAIA VC and BARROS GPS. 2009. Espécies de Cecidomyiidae (Diptera) registradas no estado do Rio de Janeiro, Brasil. Arq Mus Nac 67: 211-220.
- MAIA VC, CARVALHO-FERNANDES SP, RODRIGUES AR and ASCENDINO SS. 2014. Galls in the Brazilian Coastal Vegetation. In: FERNANDES GW and SANTOS JC (Eds), Neotropical Insect Galls. 1st ed., Springer, v. 1, p. 295-361 .
- MAIA VC, MAGENTA MAG and MARTINS SE. 2008. Ocorrência e caracterização de galhas de insetos em áreas de restinga de Bertioga (São Paulo, Brasil). Biota Neotrop 8: 167-197.
- MAIA VC and OLIVEIRA JC. 2010. Galhas de insetos da Reserva Biológica da Praia do Sul (Ilha Grande, Angra dos Reis, RJ). Biota Neotrop 10: 227-238.
- MANI MS. 1964. Ecology of plant galls. W. Junk, The Hague.
- OLIVEIRA JC and MAIA VC. 2005. Ocorrência e caracterização de galhas de insetos na restinga de Grumari (Rio de Janeiro, RJ, Brasil). Arqu Mus Nac 63: 669-675.
- RAMAN A. 2007. Insect-induced plant galls of India: unresolved questions. Curr Sci 92: 748-757.
- RODRIGUES AR, MAIA VC and COURI MS. 2014. Insect galls of restinga areas of Ilha da Marambaia, Rio de Janeiro, Brazil. Rev Bras Ent 58: 173-197 .
- SANTOS AW, SCARELI-SANTOS C, GUILHERME FAG and CUEVA-REYES P. 2013. Comparing galling insects richness among Neotropical savanas: effects of plant richness, vegetation structure and super host presence. Biodivers Conserv 22: 1080-1094.
- SANTOS JC, ALMEIDA-CORTEZ JS and FERNANDES GW. 2011. Richness of gall-inducing insects in the tropical dry forest (caatinga) of Pernambuco. Rev Bras Ent 55: 45-54.
- SANTOS JC, ALMEIDA-CORTEZ JS and FERNANDES GW. 2012. Gall-inducing insects from Atlantic forest of Pernambuco, northeastern Brazil. Biota Neotrop 12: 197-213.
- SOUSA VR and COURI MC. 2014. Redescription of Japanagromyza inferna Spencer, first recorded from Brazil, and a key to the Neotropical species of Japanagromyza Sasakawa (Diptera, Agromyzidae). Zookeys 374: 45-55.
- SPENCER KA. 1990. Host specialization in the world Agromyzidae (Diptera). Kluwer Academic Publishers Dordrecht, The Netherlands.
- TOMA TSP and MENDONÇA JR MS. 2013. Gall-inducing insects of an Araucaria Forest in southern Brazil. Rev Bras Ent 57: 225-233.
Publication Dates
-
Publication in this collection
Sept 2016
History
-
Received
01 Oct 2015 -
Accepted
18 Mar 2016