ABSTRACT
Mansoa hirsuta (Bignoniaceae) is a native plant from caatinga in Brazilian semiarid. This plant has been locally used as antimicrobial and hypoglycemiant agents, but their action mechanisms and toxicity remain largely unknown. Therefore, we evaluated the composition and antioxidant, cytoprotective and hypoglycemiant effects of raw extract, fractions and compounds from leaves of M. hirsuta. The cytogenotoxic effects of ursolic and oleanolic acids, the main phytotherapic components of this plant, were assessed. The raw extract and fractions presented steroids, saponins, flavonols, flavanonols, flavanones, xanthones, phenols, tannins, anthocyanins, anthocyanidins and flavonoids. The ethyl acetate fraction inhibited efficiently the cascade of lipid peroxidation while the hydroalcoholic fraction was richer in total phenols and more efficient in capturing 2,2-diphenyl-1-picrylhydrazyl (·DPPH) and 2,2'-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS·+) radicals. The isolated fraction of M. hirsuta also inhibited the α-amylase activity. Cytotoxic effects were absent in both raw extract and fractions while ursolic+oleanolic acids were efficient in protecting cells after exposure to hydrogen peroxide. Moreover, this mixture of acid shad no significant interference on the mitotic index and frequency of nuclear and/or chromosomal abnormalities in Allium cepa test. Therefore, M. hirsuta represents a potential source of phytochemicals against inflammatory and oxidative pathologies, including diabetes.
Key words:
antioxidant; caatinga; cytotoxicity; hypoglycemiant; phytochemistry.
INTRODUCTION
Plants have been widely used in traditional medicine to prevent and treat several human pathologies, besides playing a major role in the development of commercial pharmacological compounds such as aspirin, morphine, digoxin, vinblastine and vincristine (Yarnell 2000YARNELL E. 2000. The botanical roots of pharmaceutical discovery. J Altern Complement Med, p. 125-128.). In fact, about 4 to 5% of nearly 400,000 known species of higher plants should present some relevant biological activity, even though refined chemical and pharmacological analyses are scarce for most species (Heywood 2011HEYWOOD V. 2011. Ethnopharmacology, food production, nutrition and biodiversity conservation: towards a sustainable future for indigenous peoples. J Ethnopharmacol 137: 1-15.).
Brazil is recognized as the country with the highest genetic diversity of plants worldwide, comprising about 15 to 20% of vegetal biodiversity (Elisabetsky and Costa-Campos 1996ELISABETSKY E AND COSTA-CAMPOS L. 1996. Medicinal plant genetic resources and international cooperation: the Brazilian perspective. J Ethnopharmacol 51: 110-120., Guerra and Nodari 2006GUERRA MP AND NODARI RO. Biodiversidade: aspectos biológicos, geográficos, legais e éticos. 2006. In: Simões CM, Schenkel EP, Gosmann G, Mello JCP, Mentz LA and Petrovick PR (Org), Farmacognosia: da planta ao medicamento, Editora da Universidade UFRGS/ Editora da UFSC, Porto Alegre, Florianópolis, p. 13-26.). Besides the Amazon forest, other Brazilian regions are rich in both fauna and flora representing biodiversity hotspots, like the Atlantic Forest and Cerrado (Brazilian Savannah). Another unique biome in South America is the Caatinga (dry bushlands) distributed exclusively through semiarid region in northeastern and a small part of southeastern Brazil. Caatinga is characterized by a high number of endemic species of therapeutic value for local populations (Nogueira et al. 2010NOGUEIRA RC, DE CERQUEIRA HF AND SOARES MB. 2010. Patenting bioactive molecules from biodiversity: the Brazilian experience. Expert Opin Ther Pat 2: 145-157., Pereira Júnior et al. 2014PEREIRA JÚNIOR LR, ANDRADE AP, ARAÚJO KD, BARBOSA AS AND BARBOSA FM. 2014. Espécies da caatinga como alternativa para o desenvolvimento de novos fitofármacos. Floresta e Amb 21: 509-520.).
Since most people living in semiarid Brazilian regions are economically and socially vulnerable, the utilization of Caatinga plants usually represents the only treatment option to local communities (De Toledo et al. 2011DE TOLEDO CE ET AL. 2011. Antimicrobial and cytotoxic activities of medicinal plants of the Brazilian cerrado, using Brazilian cachaça as extractor liquid. J Ethnopharmacol 133: 420-425.). As a matter of fact, several therapeutic drugs have been developed from native Brazilian species, like pilocarpine extracted from leaves of Pilocarpus jaborandi, emetine from Psychotria ipecacuanha or Cephaelis ipecacuanha and d-tubocurarine isolated from Chondrodendron tomentosum (Nogueira et al. 2010NOGUEIRA RC, DE CERQUEIRA HF AND SOARES MB. 2010. Patenting bioactive molecules from biodiversity: the Brazilian experience. Expert Opin Ther Pat 2: 145-157.) among others.
Mansoa hirsuta D.C. (Figure 1a) known as cipó-de-alho (Brazil) is a Bignoniaceae plant endemic of semiarid Brazilian region (Lemos and Zappi 2012LEMOS JR AND ZAPPI DC. 2012. Distribuição geográfica mundial de plantas lenhosas da Estação Ecológica de Aiuaba, Ceará, Brasil. R Bras Bioci 10: 446-456.) that stands out by their popularity and potential to the production of phytotherapic drugs and/or functional foods (D.M. Silva, unpublished data). In traditional medicine, the leaves of this species have been used to control diabetes (Chaves and Reinhard 2003CHAVES SM AND REINHARD KJ. 2003. Paleopharmacology and pollen: theory, method, and application. Mem Inst Oswaldo Cruz 98: 207-211., Agra et al. 2008AGRA MF, SILVA KN, BASÍLIO JLD, FRANÇA PF AND BARBOSA-FILHO JM. 2008. Survey of medicinal plants used in the region Northeast of Brazil. Braz J Pharmacogn 18: 472-508.) while the ethanol extract has inhibited the growth of Aspergillus niger and Fusarium oxysporum cultures (Rocha et al. 2004ROCHA AD, OLIVEIRA AB, FILHO JDS, LOMBARDI JÁ ANDBRAGA FC . 2004. Antifungal constituents of Clytostoma ramentaceum and Mansoa hirsuta. Phytother Res 18: 463-467.). The antioxidant activity of M. hirsuta was also demonstrated by Braga et al. (2000BRAGA FC, WAGNER H, LOMBARDI JÁ AND OLIVEIRA AB. 2000. Screening the Brazilian flora for anti-hypertensive plant species for in vitro angiotensin-I converting enzyme inhibiting activity. Phytomedicine7: 245-250.) while isolated proanthocyanidins were capable of inhibiting angiotensin-converting enzyme (ACE) (Campana et al. 2009CAMPANA PR, BRAGA FC AND CORTES SF. 2009. Endothelium-dependent vasorelaxation in rat thoracic aorta by Mansoa hirsuta D.C. Phytomedicine 16: 456-461.).
(a) M. hirsuta D.C. collected in the municipality of Morro do Chapéu, Bahia, northeastern Brazil. (b) Chemical structure of ursolic acid. (c) Chemical structure of oleanolic acid.
In a previous study, D.M. Silva (unpublished data) isolated a mixture (1:1) of ursolic (Figure 1b) and oleanolic (Figure 1c) acids from the ethyl acetate fraction of leaves of M. hirsuta, whose patent has been recorded in the National Institute of Industrial Propriety (INPI) under the number BR102015008180 (Silva et al. 2015SILVA DM, SANT’ANA AEG, CASTRO MMS, QUEIROZ LP, SOARES MB AND COSTA JFO. 2015. Isolamento de triterpenos pentacíclicos: ácido ursólico e oleanólico, e fitoesteróides: estigmasterol e β-sitosterol extraídos das folhas da Mansoa hirsuta D.C. Bignoniaceae, para aplicação em formulações de suplementos, alimentos funcionais e fitoterápicos. BR. Pat 102015008180, 01 abr, 19 p. ). Both acids were efficient in reducing the proliferation of lymphocytes (>99%) besides reducing the formation of nitric oxide by macrophages (in 41.5 to 44.1%) (D.M. Silva, unpublished data).
Because of the biological potential and cultural of M. hirsuta, as well as the lack of detailed pharmacological studies about the utilization of this species in traditional medicine, the goal of the present work was to analyze antioxidant, cytoprotective and hypoglycemiant properties of the raw extract, fractions and compounds isolated from leaves. Moreover, putative cytotoxic, genotoxic and mutagenic side effects of the mixture of ursolic and oleanolic acids from the important plant species from caatinga were investigated to test their safety as potential pharmaceutical drugs.
MATERIALS AND METHODS
REAGENTS AND PLANT MATERIAL
For the comparative analyses based on thin-layer chromatography (TLC), silica gel 60 PF254 (Merck) and pure ethanol (Quemis) were used. The other analytical reagents (Folin-Ciocalteu reagent - FCR, ·DPPH, ABTS, β-carotene, butylated hydroxytoluene - BHT, tween 40, gallic acid, trolox, and amylase) were obtained from Sigma-Aldrich.
Specimens of M. hirsuta were collected in two municipalities (Santo Inácio and Gameleira do Assuruá) in the state of Bahia, northeastern Brazil (11º19'S, 42º40'W). The exsiccates were deposited in the Herbarium at Universidade Estadual de Feira de Santana (number 59456).
PREPARATION OF RAW EXTRACT
The leaves were dried, ground, weighed (15.0 kg) and submitted to hydroalcoholic extraction (50:50) in a percolator at room temperature (27 to 30ºC) for 96 h. The destilation of the solvent under reduced pressure in a rotary evaporator provided 1800 g of concentrated and homogeneous ethanol extract with 12% of yield.
PHYTOCHEMICAL PROSPECTION AND FRACTIONING OF ETHANOL EXTRACT
The phytochemical prospection of the raw ethanol extract (REE) and hydroalcoholic (HAF), alcoholic (AF), ethyl acetate (EAF), chloroform (CF) and hexane (HF) fractions were carried out according to D.M. Silva (unpublished data). A solution of 1 mol/L FeCl3 was used to identify phenols and tannins. Flavonols, flavanonols, flavanones, and xanthones were identified via reaction with granulate magnesium and concentrated HCl. The analysis of steroids and triterpenoids was performed with chloroform, anhydrous Na2SO4 and concentrated H2SO4. The identification of saponins was based on the formation of persistent and abundant foam. The alkaloids were identified by thin-layer chromatography (TLC) and revealed with Dragendorff's reagent.
A total of 250 g of the ethanol extract was fractioned in reverse phase using activated charcoal. After rotoevaporation, five fractions were obtained: hydroalcoholic fraction (HAF) 1:1 (130 g), alcoholic fraction (AF) (35 g), ethyl acetate fraction (EAF) (40 g), chloroform fraction (CF) (41 g), and hexane fraction (HF) (4 g).
The EAF was submitted to column chromatography in silica gel yielding 15 g of EAF, which was again fractioned by column chromatography in silica gel using hexane (C6H14), ethyl acetate (AcOEt) and a mixture of both solvents. This process resulted in 100 fractions with a mean volume of 5 mL each. After comparative analysis in TLC and revelation with anisaldehyde, ceric sulfate and/or iodine vapor, these fractions were separated into seven groups.
The fractions {F-(43 - 60)} (2.05 g) and {F-(74-100)} (1.04 g) were then purified using column chromatography in silica gel, which resulted in the obtaining of two solid amorphous compounds named MHFA1 (60 mg) and MHFA2 (70 mg), respectively. The structural identification of MHFA1 and MHFA2 was carried out by 1H-13C (DEPT 90° and 135°), HSQC, HMBC, COSY and NOESY nuclear magnetic resonance spectroscopy. The results were compared to literature reports for both compounds (Seebacher et al. 2003SEEBACHER W, SIMIC N, WEIS R, SAT R AND KUNERT O. 2003. Complete assignments of 1H and 13C NMR resonances of oleanolic acid, 18α-oleanolicacid, ursolic acid and their 11-oxo derivatives. Magn Reson Chem 41: 636-638., Moghaddam et al. 2007MOGHADDAM FM, FARIMANI MM, SALAHVARZI S AND AMIN G. 2007. Chemical Constituents of Dichloromethane Extract of Cultivated Satureja khuzistanica. Evid Based Alternat Med 4: 95-98., Martins et al. 2013MARTINS D, CARRION LL, RAMOS DF, SALOMÉ KS, DA SILVA PE, BARISON A AND NUNEZ CV. 2013. Triterpenes and the antimycobacterial activity of Duroia macrophylla Huber (Rubiaceae). Biomed Res Int 2013: 1-7.).
QUANTIFICATION OF TOTAL PHENOLS BY FOLIN-CIOCALTEU METHOD
The determination of total phenols in samples was accomplished by Folin-Ciocalteu method with modifications (Singleton et al. 1992SINGLETON VL, ORTHOFER R AND LAMUELA RM. 1992. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzym 299: 152-178.). The samples were diluted in ethanol to a final concentration of 1 mg/mL. Using assay tubes, 125 μL of each sample was mixed with 125 μL of FC Rand 1 mL of distilled water. After 3 min, 125 μL of a saturated solution of Na2CO3 were added and the samples were incubated at 37ºC. After 30 min, the absorbance of samples was measured at 750 nm. The amount of total phenols in samples was determined based on the standard curve of gallic acid (0.5 at 25 μg), being expressed in equivalent μg of gallic acid per milligram of sample (μg EGA/mg). The calibration curve equation of gallic acid was y= 0.366x + 0.0721, R2= 0.9932.
TEST OF CO-OXIDATION OF Β-CAROTENE/LINOLEIC ACID
The β-carotene/linoleic acid system was prepared with 8 mL of β-carotene diluted in chloroform (0.1 mg/mL), 40 µL of linoleic acid and 400 µL of tween 40. After agitation, the chloroform was evaporated and 400 mL of oxygen-saturated water were added up to the absorbance of 0.7 in 470 nm, as estimated using Shimadzu UV mini 1240 UV visible scanning spectrophotometer. The final suspension (3.0 mL) was mixed with 40 µL of sample (1 mg/mL) following immediately by the determination of absorbance. After incubation at 45ºC, the absorbance was established again within 15-min intervals for 2 h. BHT was used as to establish the standard absorbance (Rufino 2007RUFINO MS. Metodologia Científica: Determinação da atividade antioxidante total em frutas pela captura do radical livre DPPH. 2007. Embrapa, Comunicado Técnico 127.). The antioxidant activity was expressed in percentage of oxidation inhibition in relation to 100% of oxidation in the negative (sample-free) control.
TOTAL ANTIOXIDANT ACTIVITY BY ABTS·+ RADICAL SCAVENGING
The antioxidant capacity of raw extract and fractions of M. hirsuta was also evaluated by their ability in scavenging ·DPPH free radical (Brand-Williams et al. 1995, Rufino 2007RUFINO MS. Metodologia Científica: Determinação da atividade antioxidante total em frutas pela captura do radical livre DPPH. 2007. Embrapa, Comunicado Técnico 127.). The extraction and fractions were diluted to a final concentration of 50, 100, 200, and 300 µg/mL. Afterwards, 300 µL of each dilution were incubated in 1700 µL of ethanol solution of ·DPPH (70 µM) for 20 min at 25ºC. The measurement of absorbance was performed at a wavelength of 517 nm using a Gehaka 340G UV spectrophotometer. Ethanol was used as negative control while gallic acid and trolox were selected as positive controls. The percentage of antioxidant activity was plotted on a graph against the concentration of extracts, after adjustment of data by the rectangular hyperbolic equation to obtain the CE50, i.e., the required concentration of sample to reduce the free radical in 50%.
TOTAL ANTIOXIDANT ACTIVITY BY ABTS·+ RADICAL SCAVENGING
The ability in scavenging the ABTS·+ radical was performed as that described by Re et al. (1999RE R, PELLEGRINI N, PROTEGGENTE A, PANNALA A, YANG M AND RICE-EVANS C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med Discipline 26: 1231-1237.) with modifications. Firstly, 87.5 µL of ammonium persulfate (140 mM) was incubated in 5 mL of ABTS (7 mM) for 16 h at 25ºC, protected from light, to produce the ABTS· + radical. The final solution was diluted in ethanol up to absorbance of 0.70 ± 0.02 at 734 nm. The extract and fractions were diluted (2, 6, 12, 5, 25, 50, and 100 µg/mL), and 300 µL of each dilution were incubated in ethanol solution of ABTS· + (1700 µL) for 20 min at 25ºC. The absorbance values were determined at 734 nm. Ethanol was used as negative control while gallic acid and trolox were used as positive controls. The rate of antioxidant activity was plotted on a graph against the extract concentration was described in the previous section.
ASSAY OF CELL VIABILITY
Hamster V79 fibroblasts were grown in Dulbecco's Modified Eagle Medium (DMEM) with 10% of fetal bovine serum, penicillin (100 U/mL) and streptomycin (100 µg/mL) at 37°C in a 5% CO2 atmosphere. The medium was changed when the culture reached 80% of confluence, being spread to other culture bottles.
The cell viability was firstly determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method, which allows evaluating the mitochondrial activity in viable cells (Hansen et al. 1989HANSEN MB, NIELSEN SE AND BERG K. 1989. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 119: 203-210.). The cells (1x104/well) were dropped into 96-well plates and pre-incubated with the extraction and fractions of M. hirsuta at a concentration of 0 to 100 µg/mL at 37°C for 24 h. The MTT was added (0.25 mg/mL) to the medium and cells were incubated at 37°C. After 3 h, the absorbance was measured in a microplate reader at 570 nm (reference wavelength = 630 nm). The residual metabolic activity of cells was calculated as a percentage in relation to the negative control. As positive control, the cells were cultured with 1% Triton X-100.
CYTOPROTECTIVE ACTIVITY
After culturing the fibroblasts as commented above, cell samples (1x104/well) were placed into a 96-well plate and pre-incubated at 37°C with the extract and fractions of M. hirsuta (10 µg/mL). After 24 h, the cells were washed twice in saline phosphate buffer (PBS). The medium was changed, and H2O2 (100 µM) was added, following incubation for 4 h. The residual cell viability was determined as described in MTT method. Ethanol (5%) and tempol (10 µM) were used as negative and positive controls, respectively.
ANTIOXIDANT ACTIVITY IN CELL MODEL
To determine the antioxidant activity of isolated extract and fractions in cells, cultured fibroblasts (1x104/well) were plated, pre-incubated, incubated, washed, and re-incubated with H2O2 as described in the experiment of cytoprotective activity. Afterwards, the cells were washed in PBS, incubated in 2',7'-dichlorofluorescein diacetate (DCFH-DA) at 10 µM for 30 min at 37°C in a dark chamber. The extracellular DCFH-DA was removed after washing the cells twice in PBS. The oxidation of DCFH was monitored by fluorescence (lex=485 nm; lex=520 nm) in a microplate reader in as much as the fluorescent signal that indicates the intracellular redox state. Again, ethanol (5%) and tempol (10 µM) were selected as negative and positive controls, respectively.
TEST OF α-AMYLASE ACTIVITY
The α-amylase inhibitory activity of extract and fractions was determined according to Bernfeld (1955BERNFELD P. 1955. Amylases, α and β. Meth Enzymology 1: 149-158.). For that, swine pancreatic α-amylase (EC 3.2.1.1, type VI, Sigma) was diluted in 4 mL of phosphate buffer (10 mM) up to a concentration of 180 µg/mL. Afterwards, 100 µL of enzyme solution was incubated with 5 µL of sample (200 µL/mL) for 15 min at 37°C. Then, the residual activity of amylase was determined using Bioclin commercial kit. Acarbose (EMS Sigma Pharma) was used as positive control while ethanol was used as negative control.
TOXICOLOGY TESTS OF URSOLIC+OLEANOLIC ACIDS
The toxicity test was based on the Allium cepa system (Leme and Marin-Morales 2009LEME DM AND MARIN-MORALES MA. 2009. Allium cepa test in environmental monitoring: A review on its application. Mutat Res 682: 71-78. ) to verify the toxicity, cytotoxicity, genotoxicity, and mutagenicity at chromosome level of ursolic and oleanolic acids (UOA) fraction from M. hirsuta. In this test, three concentrations (100, 10 and 1 µg/mL) of a mixture of ursolic+oleanolic acids diluted in 0.1% dimethyl sulfoxide (DMSO) were used. Both distilled water and 0.1% DMSO (solvent) were used as negative controls while 4x10-4 M methyl methanesulfonate (MMS) and the herbicide trifluralin (C13H16F3N3O4) at 0.84 ppm were used as positive controls.
Seeds of A. cepa (Vale Ouro - IPA 11), provided by the Instituto Agronômico de Pernambuco - IPA, were cultured in moist Petri plates with distilled water (150 seeds/plate) at 25±5°C. After reaching 1 cm in length, they were transferred to Petri plates containing the test solutions. After, 24 h the roots were measured for toxicity evaluation. The roots were fixed in Carnoy's fixative (ethanol: acetic acid; 3:1) and stored at -20°C up to preparation of slides to evaluate cytotoxicity, genotoxicity and mutagenicity.
The stored roots were washed three times in distilled water (5 min each) and hydrolyzed in 1 N HCl at 60°C for 10 min. Afterwards, the roots were transferred to amber flasks containing Schiff Reactive (Merck) and stored for 2 h in dark chamber. Then, they were washed in distilled water, squashed over a glass slide containing a drop of acetic carmim (2%) and the slides were mounted with Entellan (Merck). A total of 10 slides were analyzed per treatment (500 cells/slide, totaling 5,000 cells/treatment) under optic microscope.
The following parameters were evaluated: mean length of roots (toxicity), mitotic index (cytotoxicity), frequency of chromosomal abnormalities (genotoxicity) and index of chromosomal breakages and micronuclei (mutagenicity at chromosomal level). The results were compared to the negative and positive controls.
STATISTICAL ANALYSIS
All analyses were performed in triplicates and the results were expressed in mean±standard deviation values. The normality of data was verified by Shapiro-Wilk's test (p>0.05). The results from antioxidant tests were analyzed by Kruskal-Wallis test and post-hoc Dunn's test. The correlation was established according to Spearman's correlation coefficient. The results of α-amylase inhibition were analyzed by Mann-Whitney's test. The level of significance for statistical analyses was p<0.05, using the software GraphPad Prism (6.0). For the A. cepa test, the differences among treatments and control were based on Kruskal-Wallis, followed by a posteriori Tukey's test (p<0.05), using the software Statistica (8.0).
RESULTS
The phytochemistry prospection of the raw ethanol extract (REE), hydroalcoholic (HAF), alcoholic (AF), ethyl acetate (EAF), chloroform (CF) and hexane (HF) fractions, and the isolated fraction containing a mixture of ursolic and oleanolic acids (UOA) from M. hirsuta are shown in Table I. The raw extract and fractions presented phenols, tannins, anthocyanins, anthocyanidins, and flavonoids. Steroids and triterpenoids were observed in both REE and EAF while saponins, flavonols, flavanonols, flavanones, and xanthones were identified in REE, HAF, and AF. The chloroform and hexane fractions contained only fatty acids and have not been analyzed in this study.
The MHFA1 and MHFA2 compounds were identified as ursolic and oleanolic acids by spectrophotometry (D.M. Silva, unpublished data) and comparisons to previous reports (Seebacher et al. 2003SEEBACHER W, SIMIC N, WEIS R, SAT R AND KUNERT O. 2003. Complete assignments of 1H and 13C NMR resonances of oleanolic acid, 18α-oleanolicacid, ursolic acid and their 11-oxo derivatives. Magn Reson Chem 41: 636-638., Moghaddam et al. 2007MOGHADDAM FM, FARIMANI MM, SALAHVARZI S AND AMIN G. 2007. Chemical Constituents of Dichloromethane Extract of Cultivated Satureja khuzistanica. Evid Based Alternat Med 4: 95-98., Martins et al. 2013MARTINS D, CARRION LL, RAMOS DF, SALOMÉ KS, DA SILVA PE, BARISON A AND NUNEZ CV. 2013. Triterpenes and the antimycobacterial activity of Duroia macrophylla Huber (Rubiaceae). Biomed Res Int 2013: 1-7.).
The quantification of total phenols in REE and fractions of M. hirsuta are shown in Table II. The raw extract from leaves presented ~ 20 μg EGA/mg. After fractioning, most of phenols were concentrated in the AF (~56μg EGA/mg).
The antioxidant potential of REE, HAF, AF, EAF and UOA fraction of M. hirsuta based on the co-oxidation of β-carotene/linoleic acid (Table II) revealed that EAF had the highest inhibition of oxidation (79.36%), followed by HAF, REE, AF and UOA (45.3%, 43.2%, 39.2, and 21.58%, respectively).
On the other hand, the HAF was more efficient in scavenging ·DPPH radical (CE50=29.1 μg/mL) when compared to the other fractions, even though this value was lower than that presented by the standard trolox (CE50=1.5 μg/mL) and gallic acid (CE50= 1.4 μg/mL) tests. Likewise, HAF presented the highest antioxidant activity (CE50= 5.2 μg/mL) in ABTS·+ test, followed by AF (CE50= 6.8 μg/mL), both comparable to the values obtained with trolox (Table II).
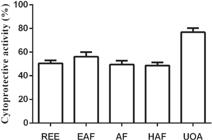
Moreover, the fibroblasts incubated with the extract and fractions of M. hirsuta remained viable even at the highest tested concentrations (100 µg/mL) (Figure 2). In addition, both extract and fractions (10 µg/mL) were also effective in protecting the cells against apoptosis caused by exposure to H2O2 (Figure 3). Thus, the compounds of M. hirsuta were characterized by cytoprotective activity, particularly remarkable for the UOA fraction (76.8%). Accordingly, the reduction of oxidation by H2O2 in fibroblasts by the REE and fractions (10 µg/mL) was higher for the UOA fraction (~83%) (Figure 4). For comparison, tempol (10 µM), a well-recognized antioxidant (Soule et al. 2007SOULE BP, HYODO F, MATSUMOTO K, SIMONE NL, COOK JA, KRISHNA MC AND MITCHELL JB. 2007. The Chemistry and Biology of Nitroxide Compounds. Free Radic Biol Med 42: 1632-1650. ), reduced H2O2-mediated death and ameliorated the oxidative stress in cells in around 53%.
Cell viability (%) after incubation of fibroblasts with raw ethanol extract (REE), hydroalcoholic (HAF), alcoholic (AF), ethyl acetate (EAF) fractions and ursolic and oleanolic acids mixture (UOA) from M. hirsuta at 100 µg/mL for 24 h (37ºC) followed by incubation in MTT. Positive control (C+): Triton X-100 (1%). Negative control (C-): cells from exposure to extract and fractions. *p>0.05, when compared to the negative control.
Cytoprotective activity (%) of the raw ethanol extract (REE), ethyl acetate (EAF), alcoholic (AF), and hydroalcoholic (HAF) fractions and ursolic and oleanolic acids mixture (UOA) at 10 µg/mL from M. hirsuta after exposure to H2O2 (100 µM). The residual viability was determined by assay with MTT.
Antioxidant activity in cell model of the raw ethanol extract (REE), hydroalcoholic (HAF), alcoholic (AF), ethyl acetate (EAF) fractions and ursolic and oleanolic acids mixture (UOA) at 10 µg/mL from M. hirsuta after exposure to H2O2 (100 µM). The cells were incubated with DCFH-DA (10 µM) for 30 min, and the oxidation was monitored by fluorescence signals.
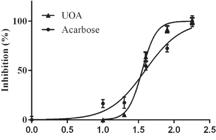
The evaluation of the ability in inhibiting the glycolytic activity of α-amylase by the REE and fractions (at 200 µg/mL) of M. hirsuta revealed that only the isolated UOA fraction was significantly efficient, with inhibition rates of ~72% (Table III). Therefore, a curve comparing concentration versus effect was built only for this fraction (Figure 5). We observed that the mixture of ursolic and oleanolic acids was effective with IC50 values of 29.6±0.2, remarkably lower than those obtained for acarbose (IC50= 37.2 ±5.2), a well-known and clinically available enzyme inhibitor.
Curve of concentration versus inhibition effects over α-amylase by the isolated UOA (ursolic and oleanolic acids) fraction of M. hirsuta when compared to acarbose. The IC50 was determined by the hyperbolic adjustment of data in the software GraphPad (C+: Acarbose; UOA: ursolic and oleanolic acids).
Considering the cytoprotective, hypoglycemiant and antioxidant activities of UOA fraction, the toxicity tests were performed in A. cepa system only for the mixture of ursolic and oleanolic acids in order to verify their potential clinical use. Indeed, the values of the mean root length (MRLV) and mitotic index (MI) of meristem cells exposed to the tested concentrations of UOA fraction from M. hirsuta and 0.1% DMSO had no significant differences in relation to the negative control (Table IV).
After 24 h of exposure to the mixture of both acids, the meristem cells of A. cepa presented the following chromosomal alterations: nuclear buds (NB), micronuclei (MN), chromosomal breakage (CB), chromosomal losses (CL), chromosomal bridges (CBr) and C-metaphase (C-M) (Figure 6). However, the frequency of these abnormalities in the three tested concentrations of UOA and 0.1% DMSO (solvent) was low and similar to the negative control (Table IV). Similarly, no significant increases in the genotoxicity index - IGen (total of chromosomal alterations) or mutagenicity - IMut (MN + CB) were detected when cells were exposed to the different concentrations of these compounds (Table V). On the other hand, as expected, the chromosomal aberrations in meristem cells from positive controls were significantly increased. The MMS induced the formation of MN and CB, characterizing a clastogenic activity, while trifluralin presented aneugenic effects once it increased the number of nuclear buds, chromosomal losses and bridges (Table V).
Chromosomal abnormalities in meristem cells of Allium cepa exposed to the mixture of ursolic and oleanolic acids of Mansoa hirsuta: (a) nuclear bud (100µg/mL); (b) micronucleus (100µg/mL); (c) C-metaphase (100µg/mL); (d) chromosomal loss (10µg/mL); (e) chromosomal bridge (1µg/mL); (f) anaphase with chromosomal loss (10µg/mL).
DISCUSSION
Even though M. hirsuta represents a typical plant species of Caatinga (Albuquerque et al. 2007ALBUQUERQUE UP, MEDEIROS PM, ALMEIDA AL, MONTEIRO JM, NETO EMFL, MELO JGM AND SANTOS JP. 2007. Medicinal plants of the caatinga (semi-arid) vegetation of NE Brazil: a quantitative approach. J Ethnopharmacol 114: 325-354., Nogueira et al. 2010NOGUEIRA RC, DE CERQUEIRA HF AND SOARES MB. 2010. Patenting bioactive molecules from biodiversity: the Brazilian experience. Expert Opin Ther Pat 2: 145-157.) with regional use in folk medicine, chemical and biological studies in this species remain scarce (D.M. Silva, unpublished data, Zoghbi et al. 2009ZOGHBI MGB, OLIVEIRA J AND GUILHON GMSP. 2009. The genus Mansoa (Bignoniaceae): a source of organosulfur compounds. Rev Bras Farmacogn 19: 795-804., Silva et al. 2015SILVA DM, SANT’ANA AEG, CASTRO MMS, QUEIROZ LP, SOARES MB AND COSTA JFO. 2015. Isolamento de triterpenos pentacíclicos: ácido ursólico e oleanólico, e fitoesteróides: estigmasterol e β-sitosterol extraídos das folhas da Mansoa hirsuta D.C. Bignoniaceae, para aplicação em formulações de suplementos, alimentos funcionais e fitoterápicos. BR. Pat 102015008180, 01 abr, 19 p. ). Considering the potential of this species to therapeutic practices, the phytochemistry evaluation of M. hirsuta herein presented is an essential step to develop new drugs and confirm their utility in complimentary medicine.
In fact, the raw extract and fractions obtained from leaves of M. hirsuta presents several secondary metabolites such as saponins, steroids, triterpenoids, phenols, tannins, anthocyanins, anthocyanidins, flavonoids (flavonols, flavanonols, and flavanones) and/or xanthones and their heterosides. These compounds have been commonly reported in other species of Bignoniaceae, confirming the key role of this family of lianas as a source of pharmaceutical bioproducts (Raju et al. 2011RAJU S, KAVIMANI S, UMA MV AND SREERAMULU RK. 2011. Tecomastans (L.) Juss. ex Kunth (Bignoniaceae): ethnobotany, phytochemistry and pharmacology. J Pharm Biomed Sci 8: 1-5., Choudhury et al. 2011CHOUDHURY S, DATTA S, TALUKDAR AD AND CHOUDHURY MD. 2011. Phytochemistry of the family Bignoniaceae - a review. J Sci Technol Biol Environ Sci 7: 145-150.).
Under this perspective, several studies have demonstrated the beneficial effects of antioxidant activities of medicinal plants to control or treat diseases associated to redox stress and inflammation, such as type 2 diabetes mellitus, hypertension and degenerative pathologies (Aruoma 1998ARUOMA OI. 1998. Free radicals, oxidative trace and antioxidants in human health and diseases. Int J Biomed Sci 4: 89-96., Alves et al. 2010ALVES CQ, DAVID JM, DAVID JP, BAHIA MV AND AGUIAR RM. 2010. Métodos para determinação de atividade antioxidante in vitro em substratos orgânicos. Quím Nova 33: 2202-2210., Krishnaiah et al. 2011KRISHNAIAH D, SARBATLY R AND NITHYANANDAM R. 2011. A review of the antioxidant potential of medicinal plant species. Food bioprod process 89: 217-233., Meena et al. 2012MEENA H, PANDEY KH, PANDEY P, ARYA MC AND AHMED Z. 2012. Evaluation of antioxidant activity of two important memory enhancing medicinal plants Baccopa monnieri and Centella asiatica. Indian J Pharmacol 44: 114-117.). Thus, the search for new species that combine antioxidant properties and reduced side effects is recommended, as evaluated for the extraction and fractions isolated from M. hirsuta.
In spite of some limitations, the method of ·DPPH scavenging has been widely used in trials to assess the potential of natural products in reducing free radicals (Masoko and Eloff 2007MASOKO P AND ELOFF J. 2007. Screening of twenty-four South African Combretum and six Terminalia species (Combretaceae) for antioxidant activities. Afr J Tradit Complement Altern Med 4: 231-239., Shah et al. 2010SHAH R, KATHAD H, SHETH R AND SHETH N. 2010. In vitro antioxidant activity of roots of Tephrosia purpurea Linn. Int J Pharm Sci 2: 30-33.). According to this strategy, products with CE50 lower at concentrations lower than 50 μg/mL indicate high antioxidant properties, while values ranging from 50-100 μg/mL, 100-200 μg/mL to above 200 μg/mL indicate moderate, low, and lack of antioxidant activity, respectively (Reynertson et al. 2005REYNERTSON KA, BASILE MJ AND KENNELLY EJ. 2005. Antioxidant potential of seven myrtaceous fruits. Ethnobotany Res Appl 3: 25-35.). Therefore, the HAF and AF are high antioxidants, as expected for polar fractions. Even though belonging to terpenes, the ursolic and oleanolic acids also presented antioxidant potential, what can be explained to their hydroxyl groups. The method based on ABTS· + radical scavenging, which has the advantage to test the activity properties of both hydrophilic and lipophilic compounds (Wojdyło et al. 2007WOJDYŁO A, OSZMIÁNSKI J AND CZEMERYS R. 2007. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem 105: 940-949.), was also employed in the present work. Again, both extract and fractions proved to be efficient antioxidants, with the highest performance observed for HAF and AF (lower CE50 values).
Moreover, the ability of the tested compounds in reducing the peroxidation cascade of the linoleic acid was evaluated using β-carotene/linoleic acid system (Santos et al. 2015SANTOS MFG, MAMEDE RVS, RUFINO MSM, BRITO ES AND ALVES RE. 2015. Amazonian native palm fruits as sources of antioxidant bioactive compounds. Antioxidants 4: 591-602.). As expected, the EAF was the most active fraction (79.4%) since less polar fractions are usually more effective in blocking the peroxidation cascade in apolar systems (Rahimi-Nasrabadi et al. 2013RAHIMI-NASRABADI M, POURMORTAZAVI SM, NAZARIAN S, AHMADI F AND BATOOLI H. 2013. Chemical composition, antioxidant, and antibacterial activities of the essential oil and methanol extracts of Eucalyptus oleosa leaves. Int J Food Prop 16: 1080-1091.). In general, the extract and fractions of M. hirsuta presented distinct (but usually high) antioxidant properties according to each method. These differences are either related to the differentiated compound classes in each fraction and/or to the particularities of each essay with specific reaction mechanisms, revealing that distinct methodologies should be simultaneously employed to determine precisely the antioxidant properties of bioproducts.
Considering that all tested methods revealed antioxidant activities in the extract and fractions of M. hirsuta, the content of total phenols were estimated in each sample since this class of phytochemicals has been related to their antioxidant potential because of their structure and ability in chelating metals and interfering in peroxidation kinetics (Michalak 2006MICHALAK A. 2006. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J of Environ Stud 4: 523-530., Othman et al. 2007OTHMAN A, AMIN ISMAILA A, GHANIA NA AND ADENANB I. 2007. Antioxidant capacity and phenolic content of cocoa beans. Food Chem 100: 1523-1530.). Along with phenolic compounds, other secondary metabolites, such as steroids (Mooradian 1993MOORADIAN AD. 1993. Antioxidant properties of steroids. J. Steroid Biochem Mol Biol 45: 509-511.), terpenes (Graßmann 2005GRAßMANN J. 2005. Terpenoids as plant antioxidants. Vitam Horm 72: 505-535.), and saponins (Gülçin et al. 2004GÜLÇIN I, MSHVILDADZE V, GEPDIREMEN A AND ELIAS R. 2004. Antioxidant activity of saponins isolated from ivy: alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside- F. Plant Med 70: 561-563.) also take account into the antioxidant activity of plant extracts. Indeed, the results from the present study showed no correlation (p>0.05) between phenol content in M. hirsuta and antioxidant potential by the tested methods. Apparently, this behavior indicated that the diversity of chemical compounds of this plant has a synergistic antioxidant effect.
On the other hand, the clinical applicability of natural products requires a balance between their therapeutic benefits and toxic effects over normal cells. Thus, the cytotoxic potential of compounds from M. hirsuta against murine fibroblasts was evaluated, revealing no harmful effects of both extract and fractions in concentrations as high as 100 µg/mL. This apparent low cytotoxicity is a promising result for further clinical studies in this species.
To refine the in vitro essays, the ability of the extract and fractions of M. hirsuta in protecting fibroblasts against the oxidative damages was also analyzed. Interestingly, all samples were able to prevent cell death after exposure to H2O2, with particular efficiency for the mixture of ursolic and oleanolic acids (UOA). This result suggests that the isolated acids of M. hirsuta induce an adaptive cell response, increasing their antioxidant defense mechanisms (Martin-Aragón et al. 2001MARTIN-ARAGÓN S, HERAS B, SANCHEZ-REUS MI AND BENEDI J. 2001. Pharmacological modification of endogenous antioxidant enzymes by ursolic acid on tetrachloride-induced liver damage in rats and primary cultures of rat hepatocytes. Exp Toxicol Pathol 53: 199-206.). Similarly, Ovesná et al. (2006OVESNÁ Z, KOZICS K AND SLAMENOVÁ D. 2006. Protective effects of ursolic acid and oleanolic acid in leukemic cells. Mutat Res 600: 131-137. ) and Tsai and Yin (2008TSAI SJ AND YIN MC. 2008. Antioxidative and anti-inflammatory protection of oleanolic acid and ursolic acid in PC12 cells. J Food Sci 73: 174-178.) reported that both acids could protect cell lineages against the harmful effects of H2O2.
The hypoglycemiant properties of the raw extract and fractions of M. hirsuta was also assessed since the search for plants with antidiabetogenic potential has increased as an alternative to conventional treatment in as much as most commercial drugs have side effects (Uddin et al. 2014UDDIN N, HASAN MR, HOSSAIN MM, SARKER A, HASAN AH, ISLAM AF, CHOWDHURY MM AND RANA MS. 2014. In vitro α-amylase inhibitory activity and in vivo hypoglycemic effect of methanol extract of Citrus macroptera Montr. fruit. Asian Pac J Trop Dis 4: 473-479., Rangika et al. 2015RANGIKA BS, DAYANANDA PD AND PEIRIS DC. 2015. Hypoglycemic and hypolipidemic activities of aqueous extract of flowers from Nycantus arbor-tristis L. in male mice. BMC Complement Altern Med 15: 1-9.). In addition, diabetes mellitus is one of the main issues for the high costs of public health services because of their worldwide incidence combined with high mortality and high morbidity indexes (Alarcon-Aguilar et al. 2000ALARCON-AGUILAR FJ, JIMENEZ-ESTRADA M, REYES-CHILPA R AND ROMAN-RAMOS R. 2000. Hypoglycemic effect of extracts and fractions from Psacalium decompositum in healthy and alloxan-diabetic mice. J Ethnopharmacol 72: 21-27.).
The immunosuppressive activity of the mixture of ursolic and oleanolic acids (D.M. Silva, unpublished data) place these compounds as potential candidates in treating type 2 diabetes mellitus (Sheng and Sun 2011SHENG H AND SUN H. 2011. Synthesis, biology and clinical significance of pentacyclic triterpenes: a multi-target approach to prevention and treatment of metabolic and vascular diseases. Nat Prod Rep 28: 543-593.). Moreover, both acids are able to act as antihyperlipidemic (Somova et al. 2003SOMOVA LO, NADAR A, RAMMANAN P AND SHODE FO. 2003. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 10: 115-121.), hepatoprotective (Liu et al. 1995LIU J, LIU Y AND KLAASSEN CD. 1995. Protective effect of oleanolic acid against chemical-induced acute necrotic liver injury in mice. Zhongguo Yao Li Xue Bao 16: 97-102.) and inhibitors of α-amylase, an enzyme that regulates the absorption of carbohydrates in the intestine (Ali et al. 2006ALI H, HOUGHTON PJ AND SOUMYANATH A. 2006. Alpha-amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol 107: 449-455.). Other beneficial effects against diabetes have been reported for these compounds, particularly the ursolic acid, since they increase the biosynthesis and the secretion of insulin (Castellano et al. 2013CASTELLANO JM, GUINDA A, DELGADO T, RADA M AND CAYUELA JA. 2013. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes 62: 1791-1799.).
In this study, we confirmed for the first time the ability of compounds from M. hirsuta in reducing the α-amylase activity. Since α-amylase plays a major role in the cleavage of starch into oligosaccharides (Sales et al. 2012SALES PM, SOUZA PM, SIMEONI LA, MAGALHÃES PO AND SILVEIRA D. 2012. α-Amylase inhibitors: a review of raw material and isolated compounds from plant source. Eur J Pharm Sci 15: 141-183.), amylase inhibitors have been widely used to control post-prandial hyperglycemia and obesity (Mahomoodally et al. 2012MAHOMOODALLY MF, SUBRATTY AH, GURIB-FAKIM AM, CHOUDHARY I AND KHAN NS. 2012. Traditional medicinal herbs and food plants have the potential to inhibit key carbohydrate hydrolyzing enzymes in vitro and reduce postprandial blood glucose peaks in vivo. Sci World J 2012: 1-9.). The α-amylase inhibition ability observed in M. hirsuta was related to the isolated mixture of ursolic and oleanolic acids, which presented levels of IC50 comparable to that reported in acarbose, a clinically available drug to treatment of type 2 diabetes mellitus (Ke et al. 2014KE E, SHI JC AND MAO XM. 2014. Safety and efficacy of acarbose in the treatment of diabetes in Chinese patients. Ther Clin Risk Manag 10: 505-511.).
Since the mixture of ursolic and oleanolic acids (UOA) isolated from the EAF were the most effective fraction in preventing cell death and reducing intracellular oxidation, besides presenting antioxidant properties in vitro, this fraction seems to be more suitable for clinical use. However, potential toxic, cytotoxic, genotoxic and mutagenic effects over eukaryote cells of this fraction remain unknown. Therefore, these effects were evaluated for the first time using meristem cells of A. cepa. Based on the tested concentrations, the UOA fraction presented no toxicity once the mean length of roots of A. cepa and had no significant effects in the mitotic index (MI) of meristem cells. A reduced value of MI would indicate cell death or interference on the kinetics of cell division, leading to developmental disorders (Rojas et al. 1993ROJAS E, HERRERA LA, SORDO M, GONSEBATT ME, MONTERO R, RODRIGUEZ R AND OSTROSKY-WEGMAN P. 1993. Mitotic index and cell proliferation kinetics for identification of antineoplastic activity. Anti-Cancer Drugs 4: 637-640.). On the other hand, increased MI suggests increased frequencies of cell division that potentially determine growth disorders, including tumors (Leme and Marin-Morales 2009LEME DM AND MARIN-MORALES MA. 2009. Allium cepa test in environmental monitoring: A review on its application. Mutat Res 682: 71-78. ). Therefore, the lack of increasing or decreasing of MI in cells exposed to UOA fraction indicates that these compounds are not cytotoxic in vivo. Besides, the reduced and non-significant frequency of chromosomal abnormalities in meristem cells exposed to UOA fraction when compared to negative control provided additional evidence that these pentacyclic triterpenes from M. hirsuta have no genotoxic or mutagenic effects in the tested concentrations.
The present dataset is particularly interesting since previous studies showed a cytotoxicity of 18.62% at a concentration of 10 µg/mL for the total EAF from leaves of M. hirsuta (D.M. Silva, unpublished data), differing from the results obtained here for isolated mixture of acids. In fact, the phytochemistry of the EAF of M. hirsuta revealed that, besides these triterpenes, other metabolites are also present in this fraction (steroids, phenols, tannins, anthocyanins, anthocyanidins and flavonoids). Therefore, the cytotoxic activity previously observed in the EAF might be related to the presence or interaction of these additional constituents (Maciel et al. 2002MACIEL MAM, PINTO AC AND VEIGA JR VF. 2002. Plantas medicinais: a necessidade de estudos multidisciplinares. Quím Nova 25: 429-438., Shitan 2016SHITAN N. 2016. Secondary metabolites in plants: transport and self-tolerance mechanisms. Biosci Biotechnol Biochem 4: 1-11.), thus revealing the importance of bioassays and toxicity tests of isolated active compounds prior to their clinical utilization and prevention of potential side effects (Rates 2001RATES SMK. 2001. Plants as source of drugs. Toxicon 39: 603-613.).
In conclusion, the present results place the leaves of M. hirsuta as a potential source of bioproducts against oxidative and inflammatory disorders to be particularly used in traditional medicine for local communities along caatinga region. Additionally, the most effective isolated fraction, composed of a mixture of ursolic and oleanolic acids, had no cytotoxic, genotoxic or mutagenic effects in the tested systems, indicating their relative safety what remains to be further investigated under a pharmacodynamics perspective. Finally, this study also highlights the importance of conserving regional biodiversity since they might represent a rich, accessible and underestimated natural pharmacy.
ACKNOWLEDGMENTS
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) (RED038/2014), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (462401/2014-6) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The authors are grateful to Léia Alexandre Alves, Jorge Vitório Gomes das Neves and Tassia Liz A. dos Santos for helping in hypoglycemiant and antioxidant assays, respectively.
REFERENCES
- AGRA MF, SILVA KN, BASÍLIO JLD, FRANÇA PF AND BARBOSA-FILHO JM. 2008. Survey of medicinal plants used in the region Northeast of Brazil. Braz J Pharmacogn 18: 472-508.
- ALARCON-AGUILAR FJ, JIMENEZ-ESTRADA M, REYES-CHILPA R AND ROMAN-RAMOS R. 2000. Hypoglycemic effect of extracts and fractions from Psacalium decompositum in healthy and alloxan-diabetic mice. J Ethnopharmacol 72: 21-27.
- ALBUQUERQUE UP, MEDEIROS PM, ALMEIDA AL, MONTEIRO JM, NETO EMFL, MELO JGM AND SANTOS JP. 2007. Medicinal plants of the caatinga (semi-arid) vegetation of NE Brazil: a quantitative approach. J Ethnopharmacol 114: 325-354.
- ALI H, HOUGHTON PJ AND SOUMYANATH A. 2006. Alpha-amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus J Ethnopharmacol 107: 449-455.
- ALVES CQ, DAVID JM, DAVID JP, BAHIA MV AND AGUIAR RM. 2010. Métodos para determinação de atividade antioxidante in vitro em substratos orgânicos. Quím Nova 33: 2202-2210.
- ARUOMA OI. 1998. Free radicals, oxidative trace and antioxidants in human health and diseases. Int J Biomed Sci 4: 89-96.
- BERNFELD P. 1955. Amylases, α and β. Meth Enzymology 1: 149-158.
- BRAGA FC, WAGNER H, LOMBARDI JÁ AND OLIVEIRA AB. 2000. Screening the Brazilian flora for anti-hypertensive plant species for in vitro angiotensin-I converting enzyme inhibiting activity. Phytomedicine7: 245-250.
- BRAND-WILLIANS W, CUVELIER ME AND BERSET C. 1995. Use of free radical method to evaluate antioxidant activity. Food Sci Technol 28: 25-30.
- CAMPANA PR, BRAGA FC AND CORTES SF. 2009. Endothelium-dependent vasorelaxation in rat thoracic aorta by Mansoa hirsuta D.C. Phytomedicine 16: 456-461.
- CASTELLANO JM, GUINDA A, DELGADO T, RADA M AND CAYUELA JA. 2013. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes 62: 1791-1799.
- CHAVES SM AND REINHARD KJ. 2003. Paleopharmacology and pollen: theory, method, and application. Mem Inst Oswaldo Cruz 98: 207-211.
- CHOUDHURY S, DATTA S, TALUKDAR AD AND CHOUDHURY MD. 2011. Phytochemistry of the family Bignoniaceae - a review. J Sci Technol Biol Environ Sci 7: 145-150.
- DE TOLEDO CE ET AL. 2011. Antimicrobial and cytotoxic activities of medicinal plants of the Brazilian cerrado, using Brazilian cachaça as extractor liquid. J Ethnopharmacol 133: 420-425.
- ELISABETSKY E AND COSTA-CAMPOS L. 1996. Medicinal plant genetic resources and international cooperation: the Brazilian perspective. J Ethnopharmacol 51: 110-120.
- GRAßMANN J. 2005. Terpenoids as plant antioxidants. Vitam Horm 72: 505-535.
- GUERRA MP AND NODARI RO. Biodiversidade: aspectos biológicos, geográficos, legais e éticos. 2006. In: Simões CM, Schenkel EP, Gosmann G, Mello JCP, Mentz LA and Petrovick PR (Org), Farmacognosia: da planta ao medicamento, Editora da Universidade UFRGS/ Editora da UFSC, Porto Alegre, Florianópolis, p. 13-26.
- GÜLÇIN I, MSHVILDADZE V, GEPDIREMEN A AND ELIAS R. 2004. Antioxidant activity of saponins isolated from ivy: alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside- F. Plant Med 70: 561-563.
- HANSEN MB, NIELSEN SE AND BERG K. 1989. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 119: 203-210.
- HEYWOOD V. 2011. Ethnopharmacology, food production, nutrition and biodiversity conservation: towards a sustainable future for indigenous peoples. J Ethnopharmacol 137: 1-15.
- KE E, SHI JC AND MAO XM. 2014. Safety and efficacy of acarbose in the treatment of diabetes in Chinese patients. Ther Clin Risk Manag 10: 505-511.
- KRISHNAIAH D, SARBATLY R AND NITHYANANDAM R. 2011. A review of the antioxidant potential of medicinal plant species. Food bioprod process 89: 217-233.
- LEME DM AND MARIN-MORALES MA. 2009. Allium cepa test in environmental monitoring: A review on its application. Mutat Res 682: 71-78.
- LEMOS JR AND ZAPPI DC. 2012. Distribuição geográfica mundial de plantas lenhosas da Estação Ecológica de Aiuaba, Ceará, Brasil. R Bras Bioci 10: 446-456.
- LIU J, LIU Y AND KLAASSEN CD. 1995. Protective effect of oleanolic acid against chemical-induced acute necrotic liver injury in mice. Zhongguo Yao Li Xue Bao 16: 97-102.
- MACIEL MAM, PINTO AC AND VEIGA JR VF. 2002. Plantas medicinais: a necessidade de estudos multidisciplinares. Quím Nova 25: 429-438.
- MAHOMOODALLY MF, SUBRATTY AH, GURIB-FAKIM AM, CHOUDHARY I AND KHAN NS. 2012. Traditional medicinal herbs and food plants have the potential to inhibit key carbohydrate hydrolyzing enzymes in vitro and reduce postprandial blood glucose peaks in vivo Sci World J 2012: 1-9.
- MARTIN-ARAGÓN S, HERAS B, SANCHEZ-REUS MI AND BENEDI J. 2001. Pharmacological modification of endogenous antioxidant enzymes by ursolic acid on tetrachloride-induced liver damage in rats and primary cultures of rat hepatocytes. Exp Toxicol Pathol 53: 199-206.
- MARTINS D, CARRION LL, RAMOS DF, SALOMÉ KS, DA SILVA PE, BARISON A AND NUNEZ CV. 2013. Triterpenes and the antimycobacterial activity of Duroia macrophylla Huber (Rubiaceae). Biomed Res Int 2013: 1-7.
- MASOKO P AND ELOFF J. 2007. Screening of twenty-four South African Combretum and six Terminalia species (Combretaceae) for antioxidant activities. Afr J Tradit Complement Altern Med 4: 231-239.
- MEENA H, PANDEY KH, PANDEY P, ARYA MC AND AHMED Z. 2012. Evaluation of antioxidant activity of two important memory enhancing medicinal plants Baccopa monnieri and Centella asiatica Indian J Pharmacol 44: 114-117.
- MICHALAK A. 2006. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J of Environ Stud 4: 523-530.
- MOGHADDAM FM, FARIMANI MM, SALAHVARZI S AND AMIN G. 2007. Chemical Constituents of Dichloromethane Extract of Cultivated Satureja khuzistanica Evid Based Alternat Med 4: 95-98.
- MOORADIAN AD. 1993. Antioxidant properties of steroids. J. Steroid Biochem Mol Biol 45: 509-511.
- NOGUEIRA RC, DE CERQUEIRA HF AND SOARES MB. 2010. Patenting bioactive molecules from biodiversity: the Brazilian experience. Expert Opin Ther Pat 2: 145-157.
- OTHMAN A, AMIN ISMAILA A, GHANIA NA AND ADENANB I. 2007. Antioxidant capacity and phenolic content of cocoa beans. Food Chem 100: 1523-1530.
- OVESNÁ Z, KOZICS K AND SLAMENOVÁ D. 2006. Protective effects of ursolic acid and oleanolic acid in leukemic cells. Mutat Res 600: 131-137.
- PEREIRA JÚNIOR LR, ANDRADE AP, ARAÚJO KD, BARBOSA AS AND BARBOSA FM. 2014. Espécies da caatinga como alternativa para o desenvolvimento de novos fitofármacos. Floresta e Amb 21: 509-520.
- RAJU S, KAVIMANI S, UMA MV AND SREERAMULU RK. 2011. Tecomastans (L.) Juss. ex Kunth (Bignoniaceae): ethnobotany, phytochemistry and pharmacology. J Pharm Biomed Sci 8: 1-5.
- RAHIMI-NASRABADI M, POURMORTAZAVI SM, NAZARIAN S, AHMADI F AND BATOOLI H. 2013. Chemical composition, antioxidant, and antibacterial activities of the essential oil and methanol extracts of Eucalyptus oleosa leaves. Int J Food Prop 16: 1080-1091.
- RANGIKA BS, DAYANANDA PD AND PEIRIS DC. 2015. Hypoglycemic and hypolipidemic activities of aqueous extract of flowers from Nycantus arbor-tristis L. in male mice. BMC Complement Altern Med 15: 1-9.
- RATES SMK. 2001. Plants as source of drugs. Toxicon 39: 603-613.
- RE R, PELLEGRINI N, PROTEGGENTE A, PANNALA A, YANG M AND RICE-EVANS C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med Discipline 26: 1231-1237.
- REYNERTSON KA, BASILE MJ AND KENNELLY EJ. 2005. Antioxidant potential of seven myrtaceous fruits. Ethnobotany Res Appl 3: 25-35.
- ROCHA AD, OLIVEIRA AB, FILHO JDS, LOMBARDI JÁ ANDBRAGA FC . 2004. Antifungal constituents of Clytostoma ramentaceum and Mansoa hirsuta Phytother Res 18: 463-467.
- ROJAS E, HERRERA LA, SORDO M, GONSEBATT ME, MONTERO R, RODRIGUEZ R AND OSTROSKY-WEGMAN P. 1993. Mitotic index and cell proliferation kinetics for identification of antineoplastic activity. Anti-Cancer Drugs 4: 637-640.
- RUFINO MS. Metodologia Científica: Determinação da atividade antioxidante total em frutas pela captura do radical livre DPPH. 2007. Embrapa, Comunicado Técnico 127.
- SALES PM, SOUZA PM, SIMEONI LA, MAGALHÃES PO AND SILVEIRA D. 2012. α-Amylase inhibitors: a review of raw material and isolated compounds from plant source. Eur J Pharm Sci 15: 141-183.
- SANTOS MFG, MAMEDE RVS, RUFINO MSM, BRITO ES AND ALVES RE. 2015. Amazonian native palm fruits as sources of antioxidant bioactive compounds. Antioxidants 4: 591-602.
- SEEBACHER W, SIMIC N, WEIS R, SAT R AND KUNERT O. 2003. Complete assignments of 1H and 13C NMR resonances of oleanolic acid, 18α-oleanolicacid, ursolic acid and their 11-oxo derivatives. Magn Reson Chem 41: 636-638.
- SHAH R, KATHAD H, SHETH R AND SHETH N. 2010. In vitro antioxidant activity of roots of Tephrosia purpurea Linn. Int J Pharm Sci 2: 30-33.
- SHENG H AND SUN H. 2011. Synthesis, biology and clinical significance of pentacyclic triterpenes: a multi-target approach to prevention and treatment of metabolic and vascular diseases. Nat Prod Rep 28: 543-593.
- SHITAN N. 2016. Secondary metabolites in plants: transport and self-tolerance mechanisms. Biosci Biotechnol Biochem 4: 1-11.
- SILVA DM, SANT’ANA AEG, CASTRO MMS, QUEIROZ LP, SOARES MB AND COSTA JFO. 2015. Isolamento de triterpenos pentacíclicos: ácido ursólico e oleanólico, e fitoesteróides: estigmasterol e β-sitosterol extraídos das folhas da Mansoa hirsuta D.C. Bignoniaceae, para aplicação em formulações de suplementos, alimentos funcionais e fitoterápicos. BR. Pat 102015008180, 01 abr, 19 p.
- SINGLETON VL, ORTHOFER R AND LAMUELA RM. 1992. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzym 299: 152-178.
- SOMOVA LO, NADAR A, RAMMANAN P AND SHODE FO. 2003. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 10: 115-121.
- SOULE BP, HYODO F, MATSUMOTO K, SIMONE NL, COOK JA, KRISHNA MC AND MITCHELL JB. 2007. The Chemistry and Biology of Nitroxide Compounds. Free Radic Biol Med 42: 1632-1650.
- TSAI SJ AND YIN MC. 2008. Antioxidative and anti-inflammatory protection of oleanolic acid and ursolic acid in PC12 cells. J Food Sci 73: 174-178.
- UDDIN N, HASAN MR, HOSSAIN MM, SARKER A, HASAN AH, ISLAM AF, CHOWDHURY MM AND RANA MS. 2014. In vitro α-amylase inhibitory activity and in vivo hypoglycemic effect of methanol extract of Citrus macroptera Montr. fruit. Asian Pac J Trop Dis 4: 473-479.
- WOJDYŁO A, OSZMIÁNSKI J AND CZEMERYS R. 2007. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem 105: 940-949.
- YARNELL E. 2000. The botanical roots of pharmaceutical discovery. J Altern Complement Med, p. 125-128.
- ZOGHBI MGB, OLIVEIRA J AND GUILHON GMSP. 2009. The genus Mansoa (Bignoniaceae): a source of organosulfur compounds. Rev Bras Farmacogn 19: 795-804.
Publication Dates
-
Publication in this collection
Mar 2017
History
-
Received
05 Sept 2016 -
Accepted
03 Jan 2017