ABSTRACT
This study aims to identify special metabolites in polar extracts from Urochloa humidicola (synonym Brachiaria humidicola) that have allelopathic effects and induce secondary photosensitization in ruminants. The compounds were isolated and identified via chromatographic and spectroscopic techniques. The compounds 4-hydroxy-3-methoxy-benzoic acid, trans-4-hydroxycinnamic acid, and p-hydroxy-benzoic acid; the flavonols isorhamnetin-3-O-β-d-glucopyranoside and methyl quercetin-3-O-β-d-glucuronate; and kaempferitrin, quercetin-3-O-α-l-rhamnopyranoside, and tricin were identified in the extract from the leaves of Urochloa humidicola. Two furostanic saponins, namely, dioscin and 3-O-α-l-rhamnopyranosyl-(1-4)-[α-l-rhamnopyranosyl-(1-2)]-β-d-glucopyranosyl-penogenin, as well as catechin-7-O-β-d-glucopyranoside were identified in the methanolic extract obtained from the roots of this plant. This species features a range of metabolites that may be toxic for animals if used in food and may interfere with the growth medium, thereby inhibiting the development of other species.
Key words:
Brachiaria humidicola; flavonoids; steroidal saponins; Urochloa
INTRODUCTION
The Urochloa genus belongs to the Poaceae family, Paniceae tribe, also known as Brachiaria (Morrone and Zuloaga 1992MORRONE O AND ZULOAGA FO. 1992. Revision de las espécies sudamericanas nativas e introducidas de los gêneros Brachiaria y Urochloa (Poaceae: Panicoidaea: Paniceae). Darwiniana 4(1/4): 43-109., Veldkamp 1996VELDKAMP JF. 1996. Brachiaria, Urochloa (Gramineae-Paniceae) in Malesia. Blumea - Biodiversity, Evolution and Biogeography of Plants 41: 413-437.). The species of this genus have adapted to different soil types (Lapointe 1993LAPOINTE SL. 1993. Manejo de los plagas clave para forrajes de las sabanas neotropicales. Past Trop 15(3): 1-9.) and are used as dead matter for protecting the agricultural soil system. Among Urochloa species, U. decumbens, U. brizantha, and U. humidicola are the most frequently used as animal feed in Brazil.
The frequent use of these species in animal production need a detailed understanding of their potential dangers. In particular, their secondary metabolites include compounds that adversely affect animal health, such as photosensitization in ruminants and horses (Tokarnia et al. 2012TOKARNIA CH, BRITO MF, BARBOSA JD, PEIXOTO PV AND DOBEREINER J. 2012. Plantas Tóxicas do Brasil para Animais de Produção. 2ª ed., Editora Helianthus, Rio de Janeiro, Brasil, p. 323-333. ). These problems are attributed to the steroidal saponins synthesized by fodder, especially protodioscin (25-R and 25-S) (Brum et al. 2009BRUM KB, HARAGUCHI M, GARUTTI MB, NÓBREGA FN AND ROSA B. 2009. Fioravanti MCS. Steroidal saponin concentrations in Brachiaria decumbens and B. brizantha at different developmental stages. Cienc Rural 39(1): 279-281.). An antinutritional effect has also been reported, which is attributed to the reduction in food intake and digestibility of animals due to the presence of metabolites such as terpenoids and flavonoids (Silva et al. 2012SILVA NDS, SILVA HDS, MARCKS E, ANDRADE G AND SOUSA DE JR. 2012. Fatores antinutricionais em plantas forrageiras. Antinutritional factors in forages. RVADS 7(4): 1-7.).
In terms of allelopathic effects, these species also have adverse effects on cropping systems; for example, the cinnamic acid derivative metabolized by Brachiaria species inhibits seed germination in Euphorbia heterophylla and Bidens pilosa (Oliveira et al. 2014OLIVEIRA RS, RIOS FA, CONSTANTIN J, ISHII-IWAMOTO EL, GEMELLI A AND MARTINI PE. 2014. Grass straw mulching to suppress emergence and early growth weeds. Planta Daninha 32: 11-17.). However, there may be both negative as well as positive allelopathic effects of different species depending upon crops consortiums. In this context, Rodrigues et al. (2012RODRIGUES APDC, LAURA VA, PEREIRA SR AND DEISS C. 2012. Alelopatia de duas espécies de braquiária em sementes de três espécies de estilosantes. Cienc Rural 42(10): 1758-1763.) cited negative allelopathic effects of the extracts from U. brizantha and U. decumbens on the seed germination of Stylosanthes guianensis and of U. decumbens on the germination of Stylosanthes capitata; they also reported a positive allelopathic effect of U. brizantha on the seeds of Stylosanthes macrocephala. The exudates of U. humidicola roots inhibit nitrification by Nitrosomonas europaea bacteria because of the action of two compounds identified in a phytochemical study of this exudate: p-coumaric and ferulic acids. These acids can permeate the cell membrane of these bacteria and inhibit the action of enzymes responsible for their nitrification (Gopalakrishnan et al. 2007GOPALAKRISHNAN S, SUBBARAO GV, NAKAHARA K, YOSHIHASHI T, ITO O, MAEDA I, ONO H AND YOSHIDA M. 2007. Nitrification Inhibitors from the root tissues of Brachiaria humidicola, a tropical grass. J Agric Food Chem 55(4): 1385-1388. , Subbarao et al. 2006SUBBARAO GV, ISHIKAWA T, ITO O, NAKAHARA K, WANG HY AND BERRY WL. 2006. A bioluminescence assay to detect nitrification inhibitors released from plant roots: a case study with Brachiaria humidicola. Plant Soil 288: 101-112.). To the best of our knowledge, no study has investigated the isolation and identification of metabolites in the extracts from U. humidicola.
Terpenoids and flavonoids in fodder are known to have qualitative effects, such as antinutritional effects resulting from a reduction in consumption and digestibility (Silva et al. 2012SILVA NDS, SILVA HDS, MARCKS E, ANDRADE G AND SOUSA DE JR. 2012. Fatores antinutricionais em plantas forrageiras. Antinutritional factors in forages. RVADS 7(4): 1-7.). However, flavonoids such as tricin, quercetin-3-O-α-l-rhamnoside, and isorhamnetin 3-O-β-d-glucoside are cited as beneficial, as they have anti-inflammatory and antioxidant activities (Luyenn et al. 2014LUYENN BTT, TAI BH, THAO NP, CHA JY, LÈE HY, LEE YM AND KIM YH. 2014. Anti-inflammatory components of Chrysanthemum indicum flowers. Bioorg Med Chem Lett 25(2): 266-269., 2015, Riethmüller et al. 2015RIETHMÜLLER E, TÓTH G, ALBERTI Á, VÉGH K, BURLINI I, KÖNCZÖL Á, BALOGH GT AND KÉRY Á. 2015. First characterisation of flavonoid- and diarylheptanoid-type antioxidant phenolics in Corylus maxima by HPLC-DAD-ESI-MS. J Pharm Biomed Anal 107: 159-167., Kim et al. 2009KIM YA, KONG C, UM YR, LIM S, YEA SS AND SEO Y. 2009. Evaluation of Salicornia herbacea as a Potential Antioxidant and Anti-Inflammatory Agent. J Med Food 12(3): 661-668.) as well as antifungal activity, which are associated with increased feed efficiency (Aderogba et al. 2013ADEROGBA M, NDHLALA A, RENGASAMY K AND VAN STADEN J. 2013. Antimicrobial and Selected In Vitro Enzyme Inhibitory Effects of Leaf Extracts, Flavonols and Indole Alkaloids Isolated from Croton menyharthii. Mol 18(10): 12633-12644. ). The saponin dioscin has also been reported to promote beneficial effects, particularly against liver fibrosis (Zhang et al. 2015ZHANG X, HAN X, YIN L, XU L, QI Y, XU Y, SUN H, LIN Y, LIU K AND PENG J. 2015. Potent effects of dioscin against liver fibrosis. Sci Rep 5: 9713. ), beyond its anti-inflammatory (Wu et al. 2015WU S, XU H, PENG J, WANG C, JIN Y, LIU K, SUN H AND QIN J. 2015. Potent anti-inflammatory effect of dioscin mediated by suppression of TNF-α-induced VCAM-1, ICAM-1and EL expression via the NF-κB pathway. Biochimie 110: 62-72. ), antitumoral (Kaskiw et al. 2009Kaskiw MJ, Tassotto ML, Mok M, Tokar SL, Pycko R, Th'ng J and Jiang Z-H. 2009. Structural analogues of diosgenyl saponins: Synthesis and anticancer activity. Bioorg Med Chem 17(22): 7670-7679.), and antioxidant (Gao et al. 2012GAO L, LI F, YAO S AND QIN S. 2012. The Anti-oxidative and Anti-inflammatory Effects of Dioscin from the Roots of Paris polyphylla Smith. International Symposium on Information Technology in Medicine and Education, p. 786-789.) activities. Additionally, Jayanegara et al. (2014JAYANEGARA A, WINA E, TAKAHASHI J. 2014. Meta-analysis on Methane Mitigating Properties of Saponin-rich Sources in the Rumen: Influence of Addition Levels and Plant Sources. Asian Australas J Anim Sci 27(10): 1426-1435.) reported beneficial effects of secondary plant metabolites, particularly in the case of saponins in livestock systems due to the reduction of ruminal methane, which increases animal productivity and provides environmental benefits. These benefits have been yet not explored in animal production, nor has the possibility that metabolites other than cinnamic acid derivatives or a combination of other compounds are responsible for the allelopathic activities. To address this gap, this manuscript presents the first phytochemical study of the extracts from this plant, entailing the isolation of the metabolites in the leaves and roots of U. humidicola, to clarify the possible positive and negative effects of its use in animal feed and crop production.
EXPERIMENTAL
PLANT MATERIAL
The roots and leaves of U. humidicola were collected in April 2013 in an area already established at the Goat Sector of the Animal Science Institute of the Universidade Federal Rural do Rio de Janeiro (UFRRJ) in the municipality of Seropédica-RJ (latitude: 22°44′38″S, longitude: 43°42′27″W; altitude: 26 m). It was identified by Dr. Delci de Deus Nepomuceno, Animal Science Institute, and a voucher specimen (No RBR-38719) is deposited at the Herbarium of Biology Institute, UFRRJ.
EQUIPMENTS
One- and two-dimensional 1H and 13C nuclear magnetic resonance (NMR) spectra were obtained on Bruker NMR spectrometer [400 and 500 MHz (1H), 100 and 125 MHz (13C)] using tetramethylsilane (TMS) as an internal standard for chemical shift reference. Some compounds were identified using a GC-MS unit (model GC-MS- QP-2010 Plus, from Shimadzu, Japan) equipped with HP-5 fused silica capillary column (30mm x 0.25mm i.d x film thickness 0.25 µm) with a quadrupole mass analyzer and electron impact ionization at 70 eV. Electrospray ionization-high-resolution mass spectrometry (EI-HRMS) spectra were recorded on a quadrupole/time-of-flight instrument (microOTOF II, Bruker Daltonics, Billerica, MA). High-performance liquid chromatography (HPLC) analyses were performed using an instrument equipped with pump LC-10AS, SPD-10A detector, CBM-20A-Comunications Module (Shimadzu), and Rheodyne injector with loop of 500 µL. All other equipment commonly used for the preparation and fractionation of extracts belongs to the Laboratory of Natural Products Chemistry at UFRRJ.
EXTRACTION AND ISOLATION
The botanical material was dried at room temperature without exposure to sunlight. The 2.1 kg of leaves and 1.81 kg of roots obtained were then ground separately in a hammer mill at the Laboratory of Animal Nutrition Science, Animal Science Institute, UFRRJ. The milled material was subjected to extraction by maceration using hexane, methanol, and methanol/water (8:2) as solvents. The extractions were performed thoroughly at 7-day intervals, and each solution was concentrated on a rotary evaporator under vacuum. This process yielded six extracts: hexane leaves (UHFH), methanol leaves (UHFM), methanol: water leaves (UHFMH2O), hexane roots (UHRH), methanol roots (UHRM), and methanol: water roots (UHRMH2O). The hydromethanolic and methanolic extracts were solubilized in methanol: water (8:2) subjected to liquid-liquid partitioning by organic solvents of increasing polarity: hexane, dichloromethane, ethyl acetate, and butanol. The ethyl acetate fractions obtained from the methanol (UHFM-Ac) and hydromethanolic (UHFMH2O-Ac) extract from the leaves and the methanol (UHRM-Ac) extracts from the roots were selected for chromatographic fractionation.
First, 6.079 g of UHFMH2O-Ac was subjected to chromatographic fractionation on 70-230 mesh silica gel (224.00 g) using dichloromethane, ethyl acetate, and methanol as the mobile phase, in a gradient of increasing polarity. In total, 320 fraction of 100 ml were collected. The fractions were pooled according to their thin-layer chromatography (TLC) profiles. The group containing fractions 51-56 (0.1342 g), which were obtained with dichloromethane:ethyl acetate (8:2) as the mobile phase, was analyzed by 13C and 1H NMR and GC-MS. These analyses allowed the identification of 4-hydroxy-3-methoxy-benzoic acid (1), trans-4-hydroxycinnamic acid (2), and 4-hydroxy-benzoic acid (3). The group containing fractions 80-86 (0.1024 g), which were obtained with dichloromethane: ethyl acetate (6: 4), was purified on a silica gel column. The purified fractions were then analyzed by 13C and 1H NMR to identify isorhamnetin-3-O-β-d-glucopyranoside (4) and methyl-quercetin-3-O-β-d-glucuronate (5). The group containing fractions 133-136 (0.4668 g), which were obtained with ethyl acetate: methanol (8:2), were combined and chromatographed on a Sephadex LH-20 column with methanol elution. This process produced 34 fractions, and kaempferitrin (6) was obtained.
The ethyl acetate fraction UHFM-Ac (1.59 g) was chromatographed on 70-230 mesh silica gel column (54.50 g) with dichloromethane, ethyl acetate, and methanol elution in a gradient of increasing polarity. In total, 152 fractions of 25 ml were collected. Fractions were combined on the basis of TLC analysis. The group of fractions 22-26 (0.0821 g), obtained with ethyl acetate as eluent, was eluted on Sephadex LH-20 column using methanol as eluent. The analysis of the fractions led to the identification of tricin (8). Fractions 97-99 (0.1240 g), obtained with ethyl acetate and methanol (4:6), were subjected to additional chromatographic fractionation on a silica gel column, and the analysis of the fraction led to the identification of quercetin-3-O-α-l-rhamnopyranoside (7).
The fraction UHRM-Ac (5.74 g) was subjected to fractionation on 70-230 mesh silica gel column (190.00 g) by elution with ethyl acetate and methanol in a gradient of increasing polarity. In total, 227 fractions of 100 ml were collected. The fractions were pooled according to their chromatographic profiles observed by TLC analysis. The group of fractions 120-124 (0.3082 g), obtained with ethyl acetate: methanol (6:4), was subjected to fractionation by HPLC. This chromatographic fractionations were composed of water as solvent (A) and acetonitrile as solvent (B) in a 4:6 ratio. The mobile phase was filtered before use and delivered isocratically at a flow rate of 4 ml min-1. The analysis was conducted at room temperature using a Phenomenex C18 semi-preparative column (250 mm × 10 mm i.d., 5 µm), and the analytes were monitored at 205 nm. In addition to some unidentified components, saponins represented by peaks at 3.084 and 5.801 min were isolated, analyzed by 1H and 13C NMR and HRMS, and identified as the steroidal saponins dioscin (9) and 3-O-α-l-rhamnopyranosyl (1-4)-[α-l-rhamnopyranosyl-(1-2)]-β-d-glucopyranosides penogenin (10), respectively. The group of fractions 129-133 (0.5340 g), eluted with ethyl acetate and methanol (6:4), was subjected to fractionation on a Sephadex LH-20 column using methanol as the eluent. This fractionation produced 47 fractions, among which fractions 36-40 (0.0197 g) were recombined and filtered on (230-400 mesh) flash silica column using ethyl acetate:methanol (8:2) as the eluent. The NMR analysis of fractions 1-3 (0.025 g) then enabled the identification of catechin-7-O-β-d-glucopyranoside (11).
RESULTS AND DISCUSSION
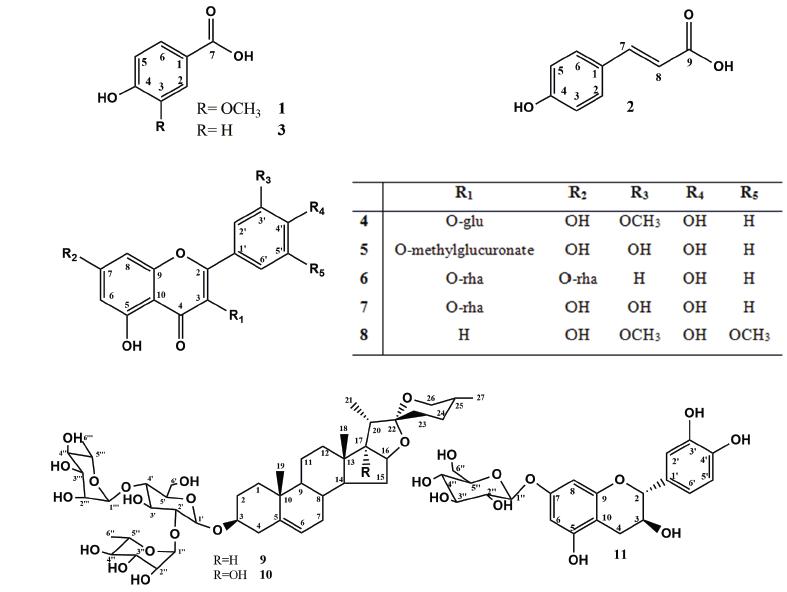
The identification of compounds 1, 2, and 3 (Figure 1) in mixture was accomplished by interpretation of the mass spectra and the 1H and 13C NMR spectra, including such two-dimensional experiments as heteronuclear single-quantum coherence (HSQC), heteronuclear multiple bond correlation (HMBC), and 1H-1H correlation spectroscopy (COSY). Analysis of the 1H NMR and 1H-1H COSY spectra allowed the identification of signals compatible with trans-carboxylic acid (2), para-substituted aromatic rings in 2 and 3, and an ABC system in 1. The doublets at δH 7.48, and 6.31 (J = 16 Hz), represent hydrogens 7 and 8, respectively, of compound 2. The singlet at δH 7.52, and the doublets at δH 6.80 (J = 8.5 Hz) of the hydrogens assigned to the 2, 6 and 3, 5 positions in compound 2; the signals at δH 6.84 and 7.43 assigned to the hydrogens of the p-substituted aromatic ring of 3; and the remaining signals at δH 7.78 (d, J = 8.5Hz), 7.43 (s), and 6.82 (d, J = 8.5 Hz) represent the hydrogens of the aromatic ring of compound 1. Analysis of the 13C-NMR and two-dimensional (HSQC and HMBC) spectra and comparison with the literature data confirmed the proposed structures of 4-hydroxy-3-methoxy-benzoic acid for 1 (Pouchert and Behnke 1993aPOUCHERT CJ AND BEHNKE J. 1993a. The Aldrich Library of 13C and 1H FT NMR spectra, 1st ed., Milwaukee: Aldrich Chemical Company, 1115 p.), trans-4-hydroxycinnamic acid for 2 (Wang et al. 2011WANG XX, HE JM, WANG CL, ZHANG RP, HE WY, GUO SX, SUN RX AND ABLIZ Z. 2011. Simultaneous Structural Identification of Natural Products in Fractions of Crude Extract of the Rare Endangered Plant Anoectochilus roxburghii Using 1H NMR/RRLC-MS Parallel Dynamic Spectroscopy. Int J Mol Sci 12: 2556-2571.), and p-hydroxybenzoic acid for 3 (Pouchert and Behnke 1993b). GC-MS analysis of the fraction containing these compounds confirmed the proposed structures and even the presence of a methoxyl group in 1.
Compounds 4-8 (Figure 1) were identified as flavonoids on the basis of their 1H and 13C NMR spectra, which are consistent with the molecular skeleton of flavonols. Compounds 4 and 5 were identified in mixture from the 1H NMR spectrum, which presented signals at δH 6.21 (d, J = 2.2 Hz), corresponding to H-6 (both 4 and 5), and δH 6.42 (d, J = 2.2 Hz) and 6.45 (d, J = 2.2 Hz), corresponding to H-8 of 4 and 5, respectively. Additional signals at δH 7.51 (d, J = 2.2 Hz, H-2′), 7.57 (dd, J = 8.5 Hz and 2.2 Hz, H-6′), and 6.85 (d, J = 8.5 Hz, H-5′) were attributed to the protons of ring B of compound 4. The signals at δH 7.52 (d, J = 2.0 Hz), 6.93 (d, J = 8.5 Hz), and 7.59 (dd, J = 8.0 and 2.0 Hz) were proposed to correspond to the protons H-2′, H-5′, and H-6′ of ring B in 5, respectively. Additional analysis of the 13C and 2D NMR spectra confirmed the proposed flavonoid skeleton. These analyses allowed the identification of the signals of two sugar moieties that were linked in each flavonol. Two doublets at δH 5.59 (J = 7.25 Hz) and 5.49 (J = 7.25 Hz) were assigned to the hydrogens at the anomeric carbons of the β-d-glycopyranosyl unit. These proposed assignments were confirmed by additional signals observed in the HSQC spectrum. The location of two methoxyl groups and the sugar moieties were determined from the HMBC and nuclear Overhauser effect (NOE) spectra. These analyses allowed the location of the sugar unit at C-3 of each flavonoid, a methoxyl group at C-3′ of 4, and a methyl ester in the sugar moiety of 5. These assignments were confirmed by the absence of CH2-6″ and the connection of this group to a carboxyl group. The 1H and 13C chemical shifts were in agreement with the literature data reported for isorhamnetin-3-O-β-d-glucopyranoside (4) (Yuan et al. 2013YUAN H, ZHOU X, MENG L, QUIN F AND ZHOU G. 2013. Chemical constituents from Commelina communis. China J of Chin Mater Med 38: 3304-3308.) and methyl-quercetin-3-O-β-d-glucuronate (5) (Hilbert et al. 2015HILBERT G ET AL. 2015. Flavonol profiles in berries of wild Vitis accessions using liquid chromatography coupled to mass spectrometry and nuclear magnetic resonance spectrometry. Food Chem 169: 49-58.).
The 1H NMR spectrum of flavonoid 7 presented signals at δH 7.85 (d, J = 8.5 Hz) corresponding to H-2′,6′ of ring B, and δH 6.95 (d, J = 8.5 Hz), corresponding to H-3′,5′ of ring B. The doublets at δH 6.47 (d, J =1.8 Hz) and 6.72 (d, J =1.85 Hz) were assigned to hydrogens H-6 and H-8, respectively. The presence of the sugar units was confirmed by broad singlets at δH 5.58 (brs) and 5.40 (brs), which are characteristic of hydrogen at the anomeric carbon of rhamnose. This assignment was confirmed by the doublets at δH 1.27 ppm (J =5.95 Hz) and 0.96 ppm (J =5.65 Hz). The location of the sugars in the structure was determined by HMBC analysis, which revealed long-range coupling between δH and δC, 5.58/162.58 (H-1″/C-7) and 5.40/135.47 (H-1‴/C-3). The mass spectrum recorded in negative mode showed a signal at m/z 577.15 [M-H], consistent with the molecular formula C27H29O14, as well as signals at m/z 431.09 [(M-H)-146] and 285.03 [(M-H)-292]. These data allowed the identification of compound 6 as kaempferitrin (Pizzolatti et al. 2003PIZZOLATTI MG, JUNIOR AC, SZPOGANICZ B, SOUZA E, BRAZ-FILHO R AND SCHRIPSEMA J. 2003. Flavonoides glicosilados das folhas e flores de Bauhinia fortificata (Leguminosae). Quim Nova 26: 466-469.).
The 1H NMR spectrum showed signals corresponding to five hydrogens in aromatic systems, which were attributed to the A and B rings of quercetin as the basic skeleton of compound 7. The chemical shifts observed in the 13C NMR spectrum confirmed this assignment. The signal at δH 5.37 (s), characteristic of a proton at an anomeric carbon, along with the observed signal at δH 0.97 (d, J = 7.0 Hz) in the 1H NMR spectrum led us to propose that a rhamnose unit was part of the structure. The position of the sugar was determined from the HMBC spectrum, which revealed a coupling between the hydrogen represented by the singlet at δH 5.37 (H-1″) and C-3 (δC 134.82). Further analysis of the 1H and 13C NMR spectra and comparison with the literature led to the identification of compound 7 as quercetin 3-O-β-d-rhamnoside (Ozgem et al. 2010OZGEM U, SEVINDIK H, KAZAZ C, YIGIT D, KANDEMIR A, SECEN H AND CALIS I. 2010. A new sulfated α-ionone glycoside from Sonchus erzincanicus. Mol 15: 2593-25,99.). Compound 8 was obtained as a yellow crystalline solid, soluble in methanol. Its 1H NMR spectrum showed only five signals: one singlet integrating to two hydrogens at δH 7.40, which was attributed to the H-2′,6′; two doublets at δH 6.27 (d, J = 2.2 Hz) and 6.57 (d, J = 2.2 Hz) for the H-6 and H-8 of ring A of a flavonoid; one singlet at δH 3.98 integrating to six hydrogens related to two methoxyls; and a singlet at δH 6.75, which was assigned to the H-3 of a flavonoid. The 2D NMR spectra allowed the location of the methoxyl at C-3′ and C-5′. These analyses and comparison with the literature led to the identification of compound 8 as tricin (Zielinska et al. 2008ZIELINSKA A, PARADOWSKA K, JAKOWSKI J AND WAWER I. 2008. 13C CP MAS NMR and GIAO-CHF/DFT calculation of flavonoids: Morin, kaempferol, tricin, genistein, formononetin and 3,7-dihydroxyflavone. J Mol Struct 873: 109-116.).
Compounds 9 and 10 (Figure 1) were identified as the furostanic steroidal saponins. Both presented three carbohydrate units, glucose linked directly to C-3 of the aglycone and two rhamnoses linked to the glucose at C-2′ and C-4′. These saponins differ only in a hydroxyl group found at C-17 in compound 9. The positive-ion-mode HRMS spectrum of 9 showed peaks at m/z 891.4695 [M + Na], 869.4893 [M + H], 723.4309 [M-146], 577.3731 [M-146-146], and 415.3181 [M-146-146-162], and that of 10 showed peaks at m/z 907.4653 [M + Na], 885.4843 [M + H], 739.6032 [M-146], 593.3676 [M-146-146], and 431.3143 [M-146-146-162]. This difference of 16 units indicates the presence of an additional hydroxyl in 10.
The 13C NMR spectra of 9 and 10 both with signals of 45 carbons, including 4 and 5 quaternary carbons in compounds 9 and 10, respectively; 24 and 23 methyne carbons in compounds 9 and 10, respectively; and 11 methylene and 6 methyl carbons in both compounds. The chemical shift of the quaternary carbon at δC 110 of a spiro system is characteristic of the tetrahydrofuran and pyran rings in the structure. The relative stereochemistry of C-25 was identified as (R) by comparison of the chemical shifts of methyl-27 and CH2-23 (Pires et al. 2002PIRES VS, TAKETA ATC, GOSMANN G AND SCHENKEL EP. 2002. Saponins and Sapogenins from Brachiaria decumbens Stapf. J Braz Chem Soc 13(2): 135-139., Espejo et al. 1982ESPEJO O, LAVOT JC, JUNG H AND GIRAL F. 1982. Spirostanic diosgenin precursors from Dioscorea composita tubers. Phytochemistry 21(2): 413-416.). The presence of three methyne carbons with chemical shifts near 100 ppm was compatible with the presence of pyranosyl units, on what differences in the mass spectra confirmed the existence of two rhamnose and one glucose units in each compound.
The presence of a hydroxyl linked to the C-17 carbon in compound 10 affects the C-16 and C-17 chemical shifts in the 13C NMR spectra; these signals shift downfield to approximately 90 ppm. Detailed analysis of the 1H and 13C NMR spectra besides comparison with the literature data led to the identification of 9 as dioscin (Pires et al. 2002PIRES VS, TAKETA ATC, GOSMANN G AND SCHENKEL EP. 2002. Saponins and Sapogenins from Brachiaria decumbens Stapf. J Braz Chem Soc 13(2): 135-139.), and 10 as 3-O-α-l-rhamnopyranosyl-(1-4)-[α-l-rhamnopyranosyl-(1-2)]-β-d-glucopyranosides penogenin (Espejo et al. 1982ESPEJO O, LAVOT JC, JUNG H AND GIRAL F. 1982. Spirostanic diosgenin precursors from Dioscorea composita tubers. Phytochemistry 21(2): 413-416., Feng et al. 2007FENG B, KANG L, MA B, QUAN B, ZHOU W, WANG Y, ZHAO Y, LIU Y AND WANG S. 2007. The substrate specificity of a glucoamylase with steroidal saponin-rhamnosidade activity from Curvularia lunata. Tetrahedron 63: 6796-6812.).
The 1H and 13C NMR spectra of compound 11 showed characteristic signals of a flavanol, with saturation in the C ring. Analysis of the 1D and 2D 1H and 13C NMR spectra and comparison with the values for catechin led to identifying it as flavane. The additional signals were compatible with a structure containing glucose, and HMBC analysis indicated that it is located at C-7. These analyses led to the identification of compound 11 (Figure 1) as catechin-7-O-β-d-glucopyranoside (Benavides et al. 2006BENAVIDES A, MONTORO P, BASSARELLO C, PIACENTE S AND PIZZA C. 2006 Cathecin derivaties in Jatropha macrantha stems: Characterisation and LC/ESI/MS/MS quali-quantitative analysis. J Pharm Biomed Anal 40: 639-647., Agrawal 1989AGRAWAL PK. 1989. Carbon-13 MNR of Flavonoids. Central Institute of Medicinal and Aromatic Plants, Lucknow- India: Elsevier: 474-478.).
CONCLUSIONS
Twelve substances, including flavonoids, have been reported in this species for the first time in this work. The saponin diosgenin, which was identified in the extracts from the roots of U. humidicola, has been isolated from the leaves U. decumbens, synonym Brachiaria decumbens (Pires et al. 2002PIRES VS, TAKETA ATC, GOSMANN G AND SCHENKEL EP. 2002. Saponins and Sapogenins from Brachiaria decumbens Stapf. J Braz Chem Soc 13(2): 135-139.). This is the first identification of the steroidal saponinin penogenina the Urochloa genus.
The identification of p-coumaric acid and other benzoil acid derivatives is consistent with the literature witch reports that phenolic compounds, such as those present in U. humidicola, promote allelopathic action, and prevent pasture invasion plants in monoculture systems, independent of organic acids (Souza Filho et al. 2005, Kobayashi and Noguchi 2015KOBAYASHI A AND NOGUCHI HK. 2015. The seasonal variations of allelopathic activity and allelopathic substances in Brachiaria brizantha. Bot Stud 56: 25.). However, for pasture system consortiums, the presence of these substances has disadvantages, as the resulting inefficiency of the systems hinders the establishment of different plant species (Rodrigues et al. 2012RODRIGUES APDC, LAURA VA, PEREIRA SR AND DEISS C. 2012. Alelopatia de duas espécies de braquiária em sementes de três espécies de estilosantes. Cienc Rural 42(10): 1758-1763.). The identified constituents improve our understanding of the diversity of metabolites produced by these species. In the literature, metabolites responsible only for the photosensitization and/or allelopathic effects of Urochloa genus have been reported. In contrast, the saponins and flavonoids identified in this study have not been associated with these effects, which merit careful consideration. The identified flavonoids have biological activities and can therefore endow ruminants with beneficial functions, increasing their nutritional value.
ACKNOWLEDGMENTS
The authors thank to Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and to Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior (CAPES) for scholarship and financial support, Dr. Norberto Peporini Lopes (NPPNS- Núcleo de Pesquisa em Produtos Naturais e Sintéticos) for the HRMS.
REFERENCES
- ADEROGBA M, NDHLALA A, RENGASAMY K AND VAN STADEN J. 2013. Antimicrobial and Selected In Vitro Enzyme Inhibitory Effects of Leaf Extracts, Flavonols and Indole Alkaloids Isolated from Croton menyharthii. Mol 18(10): 12633-12644.
- AGRAWAL PK. 1989. Carbon-13 MNR of Flavonoids. Central Institute of Medicinal and Aromatic Plants, Lucknow- India: Elsevier: 474-478.
- BENAVIDES A, MONTORO P, BASSARELLO C, PIACENTE S AND PIZZA C. 2006 Cathecin derivaties in Jatropha macrantha stems: Characterisation and LC/ESI/MS/MS quali-quantitative analysis. J Pharm Biomed Anal 40: 639-647.
- BRUM KB, HARAGUCHI M, GARUTTI MB, NÓBREGA FN AND ROSA B. 2009. Fioravanti MCS. Steroidal saponin concentrations in Brachiaria decumbens and B. brizantha at different developmental stages. Cienc Rural 39(1): 279-281.
- ESPEJO O, LAVOT JC, JUNG H AND GIRAL F. 1982. Spirostanic diosgenin precursors from Dioscorea composita tubers. Phytochemistry 21(2): 413-416.
- FENG B, KANG L, MA B, QUAN B, ZHOU W, WANG Y, ZHAO Y, LIU Y AND WANG S. 2007. The substrate specificity of a glucoamylase with steroidal saponin-rhamnosidade activity from Curvularia lunata. Tetrahedron 63: 6796-6812.
- GAO L, LI F, YAO S AND QIN S. 2012. The Anti-oxidative and Anti-inflammatory Effects of Dioscin from the Roots of Paris polyphylla Smith. International Symposium on Information Technology in Medicine and Education, p. 786-789.
- GOPALAKRISHNAN S, SUBBARAO GV, NAKAHARA K, YOSHIHASHI T, ITO O, MAEDA I, ONO H AND YOSHIDA M. 2007. Nitrification Inhibitors from the root tissues of Brachiaria humidicola, a tropical grass. J Agric Food Chem 55(4): 1385-1388.
- HILBERT G ET AL. 2015. Flavonol profiles in berries of wild Vitis accessions using liquid chromatography coupled to mass spectrometry and nuclear magnetic resonance spectrometry. Food Chem 169: 49-58.
- JAYANEGARA A, WINA E, TAKAHASHI J. 2014. Meta-analysis on Methane Mitigating Properties of Saponin-rich Sources in the Rumen: Influence of Addition Levels and Plant Sources. Asian Australas J Anim Sci 27(10): 1426-1435.
- Kaskiw MJ, Tassotto ML, Mok M, Tokar SL, Pycko R, Th'ng J and Jiang Z-H. 2009. Structural analogues of diosgenyl saponins: Synthesis and anticancer activity. Bioorg Med Chem 17(22): 7670-7679.
- KIM YA, KONG C, UM YR, LIM S, YEA SS AND SEO Y. 2009. Evaluation of Salicornia herbacea as a Potential Antioxidant and Anti-Inflammatory Agent. J Med Food 12(3): 661-668.
- KOBAYASHI A AND NOGUCHI HK. 2015. The seasonal variations of allelopathic activity and allelopathic substances in Brachiaria brizantha. Bot Stud 56: 25.
- LAPOINTE SL. 1993. Manejo de los plagas clave para forrajes de las sabanas neotropicales. Past Trop 15(3): 1-9.
- LUYENN BTT, LEE YM, LEE SH, JANG HD AND KIM YH. 2015. The Anti-Osteoporosis and Antioxidant Activities of Chemical Constituents from Chrysanthemum indicum Flowers. Phytother Res 29: 540-548.
- LUYENN BTT, TAI BH, THAO NP, CHA JY, LÈE HY, LEE YM AND KIM YH. 2014. Anti-inflammatory components of Chrysanthemum indicum flowers. Bioorg Med Chem Lett 25(2): 266-269.
- MORRONE O AND ZULOAGA FO. 1992. Revision de las espécies sudamericanas nativas e introducidas de los gêneros Brachiaria y Urochloa (Poaceae: Panicoidaea: Paniceae). Darwiniana 4(1/4): 43-109.
- OLIVEIRA RS, RIOS FA, CONSTANTIN J, ISHII-IWAMOTO EL, GEMELLI A AND MARTINI PE. 2014. Grass straw mulching to suppress emergence and early growth weeds. Planta Daninha 32: 11-17.
- OZGEM U, SEVINDIK H, KAZAZ C, YIGIT D, KANDEMIR A, SECEN H AND CALIS I. 2010. A new sulfated α-ionone glycoside from Sonchus erzincanicus. Mol 15: 2593-25,99.
- PIRES VS, TAKETA ATC, GOSMANN G AND SCHENKEL EP. 2002. Saponins and Sapogenins from Brachiaria decumbens Stapf. J Braz Chem Soc 13(2): 135-139.
- PIZZOLATTI MG, JUNIOR AC, SZPOGANICZ B, SOUZA E, BRAZ-FILHO R AND SCHRIPSEMA J. 2003. Flavonoides glicosilados das folhas e flores de Bauhinia fortificata (Leguminosae). Quim Nova 26: 466-469.
- POUCHERT CJ AND BEHNKE J. 1993a. The Aldrich Library of 13C and 1H FT NMR spectra, 1st ed., Milwaukee: Aldrich Chemical Company, 1115 p.
- POUCHERT CJ AND BEHNKE J. 1993b. The Aldrich Library of 13C and 1H FT NMR spectra, 1st ed., Milwaukee: Aldrich Chemical Company, 1084 p.
- RIETHMÜLLER E, TÓTH G, ALBERTI Á, VÉGH K, BURLINI I, KÖNCZÖL Á, BALOGH GT AND KÉRY Á. 2015. First characterisation of flavonoid- and diarylheptanoid-type antioxidant phenolics in Corylus maxima by HPLC-DAD-ESI-MS. J Pharm Biomed Anal 107: 159-167.
- RODRIGUES APDC, LAURA VA, PEREIRA SR AND DEISS C. 2012. Alelopatia de duas espécies de braquiária em sementes de três espécies de estilosantes. Cienc Rural 42(10): 1758-1763.
- SILVA NDS, SILVA HDS, MARCKS E, ANDRADE G AND SOUSA DE JR. 2012. Fatores antinutricionais em plantas forrageiras. Antinutritional factors in forages. RVADS 7(4): 1-7.
- SOUZA FILHO APS, PEREIRA AAG AND BAYMA JCA. 2005. Aleloquímico produzido pela gramínea forrageira Brachiaria humidicola. Planta Daninha 23: 25-32.
- SUBBARAO GV, ISHIKAWA T, ITO O, NAKAHARA K, WANG HY AND BERRY WL. 2006. A bioluminescence assay to detect nitrification inhibitors released from plant roots: a case study with Brachiaria humidicola. Plant Soil 288: 101-112.
- TOKARNIA CH, BRITO MF, BARBOSA JD, PEIXOTO PV AND DOBEREINER J. 2012. Plantas Tóxicas do Brasil para Animais de Produção. 2ª ed., Editora Helianthus, Rio de Janeiro, Brasil, p. 323-333.
- VELDKAMP JF. 1996. Brachiaria, Urochloa (Gramineae-Paniceae) in Malesia. Blumea - Biodiversity, Evolution and Biogeography of Plants 41: 413-437.
- WANG XX, HE JM, WANG CL, ZHANG RP, HE WY, GUO SX, SUN RX AND ABLIZ Z. 2011. Simultaneous Structural Identification of Natural Products in Fractions of Crude Extract of the Rare Endangered Plant Anoectochilus roxburghii Using 1H NMR/RRLC-MS Parallel Dynamic Spectroscopy. Int J Mol Sci 12: 2556-2571.
- WU S, XU H, PENG J, WANG C, JIN Y, LIU K, SUN H AND QIN J. 2015. Potent anti-inflammatory effect of dioscin mediated by suppression of TNF-α-induced VCAM-1, ICAM-1and EL expression via the NF-κB pathway. Biochimie 110: 62-72.
- YUAN H, ZHOU X, MENG L, QUIN F AND ZHOU G. 2013. Chemical constituents from Commelina communis. China J of Chin Mater Med 38: 3304-3308.
- ZHANG X, HAN X, YIN L, XU L, QI Y, XU Y, SUN H, LIN Y, LIU K AND PENG J. 2015. Potent effects of dioscin against liver fibrosis. Sci Rep 5: 9713.
- ZIELINSKA A, PARADOWSKA K, JAKOWSKI J AND WAWER I. 2008. 13C CP MAS NMR and GIAO-CHF/DFT calculation of flavonoids: Morin, kaempferol, tricin, genistein, formononetin and 3,7-dihydroxyflavone. J Mol Struct 873: 109-116.
Publication Dates
-
Publication in this collection
Apr-Jun 2017
History
-
Received
10 Mar 2016 -
Accepted
12 Nov 2016