ABSTRACT
Ischemia is responsible for many metabolic abnormalities in the heart, causing changes in organ function. One of modifications occurring in the ischemic cell is changing from aerobic to anaerobic metabolism. This change causes the predominance of the use of carbohydrates as an energy substrate instead of lipids. In this case, the glycogen is essential to the maintenance of heart energy intake, being an important reserve to resist the stress caused by hypoxia, using glycolysis and lactic acid fermentation. In order to study the glucose anaerobic pathways utilization and understand the metabolic adaptations, New Zealand white rabbits were subjected to ischemia caused by Inflow occlusion technique. The animals were monitored during surgery by pH and lactate levels. Transcription analysis of the pyruvate kinase, lactate dehydrogenase and phosphoenolpyruvate carboxykinase enzymes were performed by qRT-PCR, and glycogen quantification was determined enzymatically. Pyruvate kinase transcription increased during ischemia, followed by glycogen consumption content. The gluconeogenesis increased in control and ischemia moments, suggesting a relationship between gluconeogenesis and glycogen metabolism. This result shows the significant contribution of these substrates in the organ energy supply and demonstrates the capacity of the heart to adapt the metabolism after this injury, sustaining the homeostasis during short-term myocardial ischemia.
Key words:
gene expression; glycogen; ischemia; myocardium; rabbit
INTRODUCTION
Myocardial ischemia occurs when blood flow to the heart muscle is reduced by a partial or total blocking. In this event, there is a reducing in the supply of oxygen, glucose and others nutrients to the heart, increasing the metabolic demands of the tissue (Akinmoladun et al. 2016AKINMOLADUN AC, OLOWE JA, KOMOLAFE K, OGUNDELE J AND OLALEYE MT. 2016. Antioxidant activity and protective effects of cocoa and kola nut mistletoe (Globimetula cupulata) against ischemia/reperfusion injury in Langendorff-perfused rat hearts. J Food Drug Anal 24(2): 417-426.). In moderate reduction of coronary flow (between 20-60%), there was a decrease of 10% to 50% of myocardial oxygen consumption, as well as a reduction of fatty acids and an increase of anaerobic glucose, glycogen depletion and lactate production. However, this damage may be reversible, depending on the metabolic demands and the time of reduction of coronary flow (Ferrari et al. 1998FERRARI R, PEPI P, FERRARI F, NESTA F, BENIGNO M AND VISIOLI O. 1998. Metabolic derangement in ischemic heart disease and its therapeutic control. Am J Cardiol 82(5A): 2K-13K.), because what determines the ischemic damage to the heart are the severity, the duration, the temporal sequence of ischemia, physical and metabolic state, myocardial glycogen content and free fatty acids (Vanoverschelde et al. 1994VANOVERSCHELDE JL, JANIER MF, BAKKE JE, MARSHALL DR AND BERGMANN SR. 1994. Rate of glycolysis during ischemia determines extent of ischemic injury and functional recovery after reperfusion. Am J Physiol 267(5 Pt 2): H1785-94.). Each of these factors can be modified to reduce the extent of the deleterious effects of ischemia (Opie 2004OPIE LH. 2004. Heart physiology: from cell to circulation. 4th ed., Lippincott: Williams and Wilkins, 640 p.).
In the application of Inflow occlusion technique, ischemia occurs during the occlusion of cranial and caudal vena cava vein, and azygos vein, without interruption of coronary flow, which occurs in acute myocardial infarction (Fossum 2005FOSSUM TW. 2005. Cirurgia de Pequenos Animais. 2a ed., São Paulo: Roca, 1335 p., Singh et al. 2006SINGH J, DHALIWAL RS, BISWAL S AND SWAMI N. 2006. Inflow occlusion in the era of modern cardiac surgery. J Thorac Cardiovasc Surg 132(5): 1246.). When blood flow is interrupted, a number of metabolic and enzymatic processes are affected (Stopiglia et al. 2001STOPIGLIA AJ, FREITAS RR, IRINO ET, POGLIANI FC, SIMÕES EA, KWASNICKA KL, FANTONI DT, JATENE FB. 2001. Avaliação clínica da parada circulatória total em cães (Canis familiaris). Acta Cir Bras 16(4): 211-217., Garcia et al. 2009GARCIA DC, STOPIGLIA AJ, MINGRONE LE AND FANTONI DT. 2009. Avaliação clínica de cães submetidos à parada circulatória total por diferentes períodos de tempo através da técnica “Inflow occlusion”. Pesq Vet Bras 29(2): 125-130.). The reversibility of this process directly depends on the duration of ischemia and the main way to prevent this is establishing reperfusion as soon as possible (Braunwald and Kloner 1985BRAUNWALD E AND KLONER RA. 1985. Myocardial reperfusion: a double-edged sword? J Clin Invest 76(5): 1713-1719.).
The cardiac glycogen is a potential source of myocardial energy, producing three molecules of ATP during glycolysis and the standard amount of ATP by citric acid cycle under aerobic conditions. To support the high-energy demand in myocardium, there is a stimulation of anaerobic metabolism, starting the glycogenolysis pathway to use glucose reserves of cardiac glycogen (Fraser et al. 1999FRASER H, LOPASCHUK GD AND CLANACHAN AS. 1999. Alteration of glycogen and glucose metabolism in ischaemic and post-ischaemic working rat hearts by adenosine A1 receptor stimulation. Br J Pharmacol 128(1): 197-205.). This substrate is classically described as an energy source during hypoxia or ischemia and contributes to glucose homeostasis during these processes. Previously studies have been shown that elevated glycogen levels in heart have cardioprotective effects against ischemic injury (Vale et al. 2016VALE DF, SILVA RM, AGUIAR RR, MONTEIRO GA, ANTUNES F, KALIL RAK, LOGULLO CJ AND OLIVEIRA ALA. 2016. The Correlation of Glycogen Metabolism in Rabbit Myocardial Ischemia. J Veterinar Sci Technol 1: 7., Omar et al. 2010OMAR MA, WANG L AND CLANACHAN AS. 2010. Cardioprotection by GSK-3 inhibition: role of enhanced glycogen synthesis and attenuation of calcium overload. Cardiovasc Res 86(3): 478-486.). Researches of Schaefer and Ramasamy (1997SCHAEFER S AND RAMASAMY R. 1997. Glycogen utilization and ischemic injury in the isolated rat heart. Cardiovasc Res 35(1): 90-98.) demonstrated a decrease in glycogen content after ischemia in isolated rat heart, but they did not explore key enzymes of glucose metabolism. Vale et al. (2016) showed that heart metabolic adaptations involving glycogen after ischemia and reperfusion injury are correlated to GSK-3β transcription, an enzyme associated with regulation of glycogen reserves.
Finally, heart ischemia is the major precursor of myocardial infarction, and has been the object of intense investigation by clinicians and basic medical sciences (Akinmoladun et al. 2016AKINMOLADUN AC, OLOWE JA, KOMOLAFE K, OGUNDELE J AND OLALEYE MT. 2016. Antioxidant activity and protective effects of cocoa and kola nut mistletoe (Globimetula cupulata) against ischemia/reperfusion injury in Langendorff-perfused rat hearts. J Food Drug Anal 24(2): 417-426.). Therefore, in this study, we demonstrate more details of metabolic changes associated to ischemia/reperfusion injury, associating the glycogen metabolism with gluconeogenesis, aiming at a better understanding in the homeostasis of glucose metabolism during these processes.
MATERIAL AND METHODS
ETHICAL APPROVAL
All this experiments were performed in accordance with the standards and ethics of animal experimentation, established by Brazilian Federal Law no. 11794/08 after approval of the Ethics Committee on Animal Use (CEUA) of Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF), under protocol number 171/2012. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
EXPERIMENTAL DESIGN
All procedures were made in the Unidade de Experimentação Animal (UEA) of the Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF). Fifteen New Zealand white rabbits (Oryctolagus cuniculus), male, weighing between 2.5 and 3.5 kilograms were used. The animals were provided by the rabbit sector of Universidade Federal de Viçosa (UFV). The rabbits passed for a quarantine, kept in the room at 22-27ºC, with controlled light and noise, housed in individual cages, where they received feed for rabbits (Presence® feed) and water ad libitum.
ANESTHETIC PROTOCOL
The anesthetic was induced in rabbits with ketamine chloridrate 10% (30mg.kg-1) (Ketamina Agener®, Agener União, São Paulo - Brazil) associated with midazolam (2mg.kg-1) (ampoule 5mg.ml-1, generic drug, São Paulo - Brazil) intramuscularly. Ten minutes after medication, animals were prepared for catheterization of the marginal vein and central auricular artery. Next step was the tracheal intubation by tracheostomy. During the anesthetic and surgical procedures, animals received an infusion of Ringer’s solution containing lactate (7ml/kg/h). Anesthetic maintenance was performed with isoflurane (1.5%) (Isoforine®, Cirstália, São Paulo - Brazil) in 100% oxygen in a semi-open circuit. Oxygen was provided to animals using a manual ventilator, except during Inflow occlusion.
CARDIAC CIRCULATORY ARREST OR INFLOW OCCLUSION
The rabbits were randomly divided into three groups of five animals: (1) control group, (2) ischemia group and (3) reperfusion group. All animals underwent right lateral thoracotomy in the 4th intercostal space. The cardiac circulatory arrest was performed in both (2) and (3) groups.
After thoracotomy, the pleural and pericardium spaces were opened (Figure 1a), and the cranial and caudal cava vein and the azygos vein were localized and dissected. Before the occlusion, a hyperventilation was performed for 30 seconds with the purpose of emptying the cardiac chambers. Subsequently, the cardiac circulatory arrest was established for 5 minutes (Figure 1b) using the inflow occlusion technique (Varco 1951VARCO RL. 1951. Temporary vena cavae occlusion for open heart surgery. Surgery 30: 42.). Then, the left ventricular biopsy was performed and the veins were released at the end of occlusion (Figure 1c). The same procedure was performed for reperfusion group, but the left ventricular biopsy was performed after 5 minutes of reperfusion. The control group was not submitted to occlusion of vessels and a biopsy was performed after 5 minutes of thoracotomy.
Application of Inflow occlusion technique. (a) Pericardium space opening to access the heart. (b) Occlusion of cranial and caudal vena with vascular clamps, at the moment of ischemia. (c) Clamping of the left ventricle and cardiac biopsy.
Previous studies demonstrated that a short period of time is sufficient to observe enzymes transcriptional changes (Vale et al. 2016VALE DF, SILVA RM, AGUIAR RR, MONTEIRO GA, ANTUNES F, KALIL RAK, LOGULLO CJ AND OLIVEIRA ALA. 2016. The Correlation of Glycogen Metabolism in Rabbit Myocardial Ischemia. J Veterinar Sci Technol 1: 7., Schomisch et al. 2005). Therefore, the 5 minutes of ischemia can be safely used to cause alterations on the transcription levels of enzymes.
RNA EXTRACTION
The left ventricular tissue was placed in RNAlater ® Stabilization Solution (Invitrogen Life Technologies, Inc. Carsbad, CA, USA) and stored at -20ºC for RNA extraction according to the Trizol® Reagent Protocol (Invitrogen Life Technologies, Inc., Carsbad, CA, USA).
Total RNA was extracted from 30mg of left ventricular tissue using Trizol reagent. One microgram of total RNA of each group (Control, ischemia and reperfusion) was reverse transcribed with the High-Capacity cDNA (Applied Biosystems, California, USA) to obtain the cDNA. Amplifications were Reverse Transcription Kit® performed on the Real Time PCR technique, as explained below.
RELATIVE QUANTIFICATION BY REAL-TIME PCR
A relative transcriptional analysis was conducted with cDNA as a template for quantitative PCR using the StepOne Plus TM-Applied. Serial dilutions of cDNA were used for calibration curve preparation. Reaction efficiencies between 85 and 100% were determined from the calibration curves for each set of primers in 10-μl reactions. Specific primers were designed to rabbit (Oryctolagys cuniculus) using sequences acquired in the GenBank. The primers used to amplify the targets are listed in Figure 2. The relative expression was determined for each group (control, ischemia and reperfusion) using the Relative Expression Software Tool (Pfaffl 2001PFAFFL MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9): e45.).
List of primers sequences. Cyclophylin A, Piruvate kinase, Lactate dehydrogenase and Phosphoenolpyruate carboxykinase.
The cyclophilin A gene was utilized as a reference to normalize the reactions (Blaauw et al. 2013BLAAUW E, LORENZEN-SCHMIDT I, BABIKER FA, MUNTS C, PRINZEN FW, SNOECKX LH, VAN BILSEN M, VAN DER VUSSE GJ AND VAN NIEUWENHOVEN FA. 2013. Stretch-Induced Upregulation of Connective Tissue Growth Factor in Rabbit Cardiomyocytes. J Cardiovasc Transl Res 6(5): 861-869.), and the cDNA from the control group was used as a calibrator for the assays. The relative expression of the calibrators was assigned a value of 1 unit. Statistical analyses (means and standard deviation) were performed on data from three independent experiments.
GLYCOGEN QUANTIFICATION
The left ventricular tissue (20 milligrams) was homogenized in 1 mL extraction buffer containing 200 mM sodium acetate with pH 4.8, and centrifuged at 10.000xg for 10 minutes. The supernatant was incubated with 1 unit α-amyloglucosidase (Sigma-Aldrich® Chemicals, São Paulo, SP, Brazil) in acetate buffer at 40ºC for 4 hours. The released glucose was detected with a commercial kit for glucose quantity (Glucox®, Doles Reagentes, Goiânia, GO, Brazil) at 510 nm of absorbance (A510). Endogenous glucose was subtracted from control conditions (no added α-amyloglucosidase). Glycogen content was determined using a standard curve (Bradford 1976BRADFORD MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.) and normalized by total protein content. The results are presented as a mean and standard deviation from three independent experiments.
STATISTICAL ANALYSIS
The analysis of variance (ANOVA) and Tukey tests were performed for all the results. The significance level (α) was 5% (P<0.05). Statistical tests were calculated using GraphPad PRISM® Software Version 6.0 (Graph Pad, USA).
RESULTS
GLUCONEOGENESIS IS PROBABLY CORRELATED WITH GLYCOGEN METABOLISM DURING ISCHEMIA AND REPERFUSION
The amount of glycogen was measured enzymatically in three studied groups (control, ischemia and reperfusion). We observed a reduction of glycogen levels after ischemia induction; however, during reperfusion, the glycogen amount remained unaltered compared to the control group (Figure 3a).
Glycogen as important reserve to maintain anaerobic glycolysis and energy homeostasis during ischemia. (a) Decreased glycogen content during ischemia. (b) Increased Pyruvate kinase transcription level during ischemia and reperfusion. The statistical analysis used was ANOVA with Tukey post-test (*P<0,05) (n=5/group).
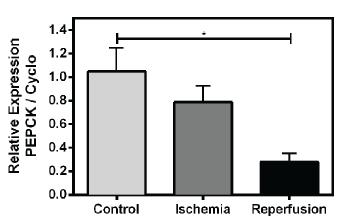
At the same moment of decreased glycogen, the PEPCK transcription, an important regulatory enzyme of gluconeogenesis, was higher (Figure 5). The opposite occurs during reperfusion, the PEPCK transcription decrease when the glycogen levels increases (Figures 5 and 3a).
Acidosis characterizes by the decrease of pH levels and lactemia and increased in Lactate dehydrogenase transcription. (a) Lactate levels were significantly higher in ischemia and reperfusion groups. (b) Lactate dehydrogenase transcription was significantly higher in ischemia group. (c) pH levels significantly reduced in ischemia and reperfusion groups. The statistical analysis used was ANOVA with Tukey post-test (*P<0,05) (n=5/group).
The phosphoenolpyruvate carboxykinase is probably correlated with glycogen metabolism during ischemia and reperfusion. PEPCK transcription was significantly higher in control and ischemia groups. The statistical analysis used was ANOVA with Tukey post-test (*P<0,05) (n=5/group).
LACTATE PRODUCTION MAY BE INVOLVED WITH GLUCONEOGENESIS
At the same moment PEPCK transcription increases, the lactate content is altered. The lactate levels were significantly higher when compared to the control group (Figure 4a). The transcriptional level of lactate dehydrogenase and pyruvate kinase also increased during ischemia, confirming the lactate production (Figure 4b).
HEMOGASOMETRY
In order to assess the hemogasometric variations during 5 minutes of ischemia and reperfusion, we analyzed the pH level in control rabbits or after inflow occlusion application. pH means were statistically significantly lower in the ischemia groups, compared to other treatments, and after reperfusion the pH values remained low (Figure 4c).
DISCUSSION
In the myocardial ischemia, the cardiac tissue undergoes glucose, nutrients and oxygen privation, although the heart energy demands remain as high as before the injury (Taegtmeyer et al. 1985TAEGTMEYER H, ROBERTS AFC AND RAINE AEG. 1985. Energy metabolism in reperfused heart muscle: metabolic correlates to return of function. J Am Coll Cardiol 6(4): 864-870.). In this context of hypoxia, cardiac myocytes may mobilize glycogen to maintain energy homeostasis, therefore sustaining anaerobic glycolysis. Such decrease in glycogen content was observed after ischemia induction at the same moment of increased pyruvate kinase transcription (Figure 3). This enzyme regulates the glycolysis and its increasing suggests that the glycogen mobilization is sustaining the glycolytic pathway during blood influx blocking. The lactate content and lactate dehydrogenase transcription also increased during ischemia, followed by a decrease in the blood pH, suggesting the activation of anaerobic glycolysis (Figure 4). Similar results were observed in rabbit hearts after inflow occlusion application (Vale et al. 2016VALE DF, SILVA RM, AGUIAR RR, MONTEIRO GA, ANTUNES F, KALIL RAK, LOGULLO CJ AND OLIVEIRA ALA. 2016. The Correlation of Glycogen Metabolism in Rabbit Myocardial Ischemia. J Veterinar Sci Technol 1: 7.). In that work, the glycogen synthase kinase 3 transcription, enzyme that regulates negatively the glycogen synthesis, also increased during ischemia, indicating that this enzyme is necessary to maintain the glycogen homeostasis and consequent heat integrity during ischemia and reperfusion process.
The acidosis in ischemia is well known; however, all the cellular process studied in this cardiac injury is referred to the investigation of glycolysis and, to a lesser extent, to the metabolism glycogen (Omar et al. 2010OMAR MA, WANG L AND CLANACHAN AS. 2010. Cardioprotection by GSK-3 inhibition: role of enhanced glycogen synthesis and attenuation of calcium overload. Cardiovasc Res 86(3): 478-486., Finegan et al. 2000, Wong et al. 1999WONG MS, LARA TM, KOBZIK L, ROUNDS JD, ROBINSON MK AND JACOBS DO. 1999. Hindlimb Ischemia-Reperfusion Increases Complement Deposition and Glycolysis. J Surg Res 85(1): 130-135., Carlsson 1988CARLSSON L. 1988. A crucial role of ongoing anaerobic glycolysis in attenuating acute ischemia-induced release of myocardial noradrenaline. J Mol Cell Cardiol 20(3): 247-253.). Another important pathway of carbohydrate metabolism is the gluconeogenesis, greatly neglected in heart.
Phosphoenolpyruvate carboxykinase (PEPCK) is a regulatory enzyme that catalyze the initial step of gluconeogenesis (Ballard and Oliver 1963BALLARD FJ AND OLIVER IT. 1963. Glycogen metabolism in embryonic chick and neonatal rat liver. Biochim Biophys Acta 71: 578-588., Ballard et al. 1967). Gluconeogenesis produces glucose from non-glycosidic compounds, and it is an important strategy for the maintenance of cell energy homeostasis. Under normal physiological conditions, when glucose levels become low, as in starvation, the gluconeogenic flux accelerates. In such cases, PEPCK increase its transcription and activity, since this enzyme is mainly regulated by transcription. Since the glucose metabolism in heart during ischemia is very important to sustain the hypoxia condition, the pathway responsible for recovering glucose should be studied. However, little is known about the PEPCK in heart.
Surprisingly, in control and ischemic groups the PEPCK transcription is higher compared to reperfusion (Figure 5). Most of the information about gluconeogenesis was obtained in mammal’s liver, and to a lesser extent, in kidneys and skeletal muscle (Hanson and Reshef 1997HANSON RW AND RESHEF L. 1997. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem 66: 581-611.). In these cases, the studies focus in starvation or glucose homeostasis. Nevertheless, some recent studies have been demonstrated the PEPCK involved in other mechanisms, as mitochondrial biogenesis and longevity (Hakimi et al. 2007HAKIMI P ET AL. 2007. Overexpression of the Cytosolic Form of Phosphoenolpyruvate Carboxykinase (GTP) in Skeletal Muscle Repatterns Energy Metabolism in the Mouse. J Biol Chem 282(45): 32844-32855.). PEPCK overexpression in skeletal muscle of mice greatly increased the number of mitochondria in this tissue, enhancing the muscle oxidative capacity. In this regard, the mutant mice did not accumulate lactate in their blood during maximal exercise (Hakimi et al. 2007). All these observations suggest that the skeletal muscle of mice overexpressing PEPCK presented particularities of the heart muscle. It is possible that, in rabbit heart during normoxia and hypoxia, the PEPCK is responsible to maintain the oxidative capacity and the recovery of lactate generated by aerobic metabolism, producing new glucose by gluconeogenesis. That would explain the high transcription level of this enzyme in both control and ischemic conditions (Figure 5). When the blood returns to irrigate the heart, the insulin signaling pathway activation possibly decreased the flux through gluconeogenesis pathway, explaining the decrease in PEPCK transcription, allowing the organ storage glycogen depletion during ischemia.
Taken together, these results show that the transcription of PEPCK correlates with heart metabolic adaptations after ischemia and reperfusion injuries. The glycogen reserves modulation and lactate production due to hypoxia condition may be involved in some level with gluconeogenesis in heart of rabbit. The enzyme GSK3 is being well studied in ischemic processes and has emerged over the years as a core component of energy metabolism, cell growth and of cardioprotection (Jope et al. 2007JOPE RS, YUSKAITIS CJ AND BEUREL E. 2007. Glycogen Synthase Kinase-3 (GSK3): Inflammation, Diseases, and Therapeutics. Neurochem Res 32(4-5): 577-595.); however, important regulators of glucose metabolism are neglected, as PEPCK. More studies of this enzyme and the relationship between GSK3 and PEPCK will contribute to further understanding its role in mammals during ischemia and reperfusion.
ACKNOWLEDGMENTS
The authors are grateful to the Brazilian agencies Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Instituto Nacional de Ciência e Tecnologia em Entomologia Molecular (INCT), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES) for their support to this project.
REFERENCES
- AKINMOLADUN AC, OLOWE JA, KOMOLAFE K, OGUNDELE J AND OLALEYE MT. 2016. Antioxidant activity and protective effects of cocoa and kola nut mistletoe (Globimetula cupulata) against ischemia/reperfusion injury in Langendorff-perfused rat hearts. J Food Drug Anal 24(2): 417-426.
- BALLARD FJ AND OLIVER IT. 1963. Glycogen metabolism in embryonic chick and neonatal rat liver. Biochim Biophys Acta 71: 578-588.
- BALLARD FJ, HANSON RW AND LEVEILLE GA. 1967. Phosphoenolpyruvate carboxykinase and the synthesis of glyceride-glycerol from pyruvate in adipose tissue. J Biol Chem 242(11): 2746-2750.
- BLAAUW E, LORENZEN-SCHMIDT I, BABIKER FA, MUNTS C, PRINZEN FW, SNOECKX LH, VAN BILSEN M, VAN DER VUSSE GJ AND VAN NIEUWENHOVEN FA. 2013. Stretch-Induced Upregulation of Connective Tissue Growth Factor in Rabbit Cardiomyocytes. J Cardiovasc Transl Res 6(5): 861-869.
- BRADFORD MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- BRAUNWALD E AND KLONER RA. 1985. Myocardial reperfusion: a double-edged sword? J Clin Invest 76(5): 1713-1719.
- CARLSSON L. 1988. A crucial role of ongoing anaerobic glycolysis in attenuating acute ischemia-induced release of myocardial noradrenaline. J Mol Cell Cardiol 20(3): 247-253.
- FERRARI R, PEPI P, FERRARI F, NESTA F, BENIGNO M AND VISIOLI O. 1998. Metabolic derangement in ischemic heart disease and its therapeutic control. Am J Cardiol 82(5A): 2K-13K.
- FOSSUM TW. 2005. Cirurgia de Pequenos Animais. 2a ed., São Paulo: Roca, 1335 p.
- FRASER H, LOPASCHUK GD AND CLANACHAN AS. 1999. Alteration of glycogen and glucose metabolism in ischaemic and post-ischaemic working rat hearts by adenosine A1 receptor stimulation. Br J Pharmacol 128(1): 197-205.
- GARCIA DC, STOPIGLIA AJ, MINGRONE LE AND FANTONI DT. 2009. Avaliação clínica de cães submetidos à parada circulatória total por diferentes períodos de tempo através da técnica “Inflow occlusion”. Pesq Vet Bras 29(2): 125-130.
- HAKIMI P ET AL. 2007. Overexpression of the Cytosolic Form of Phosphoenolpyruvate Carboxykinase (GTP) in Skeletal Muscle Repatterns Energy Metabolism in the Mouse. J Biol Chem 282(45): 32844-32855.
- HANSON RW AND RESHEF L. 1997. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem 66: 581-611.
- JOPE RS, YUSKAITIS CJ AND BEUREL E. 2007. Glycogen Synthase Kinase-3 (GSK3): Inflammation, Diseases, and Therapeutics. Neurochem Res 32(4-5): 577-595.
- OMAR MA, WANG L AND CLANACHAN AS. 2010. Cardioprotection by GSK-3 inhibition: role of enhanced glycogen synthesis and attenuation of calcium overload. Cardiovasc Res 86(3): 478-486.
- OPIE LH. 2004. Heart physiology: from cell to circulation. 4th ed., Lippincott: Williams and Wilkins, 640 p.
- PFAFFL MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9): e45.
- SCHAEFER S AND RAMASAMY R. 1997. Glycogen utilization and ischemic injury in the isolated rat heart. Cardiovasc Res 35(1): 90-98.
- SINGH J, DHALIWAL RS, BISWAL S AND SWAMI N. 2006. Inflow occlusion in the era of modern cardiac surgery. J Thorac Cardiovasc Surg 132(5): 1246.
- STOPIGLIA AJ, FREITAS RR, IRINO ET, POGLIANI FC, SIMÕES EA, KWASNICKA KL, FANTONI DT, JATENE FB. 2001. Avaliação clínica da parada circulatória total em cães (Canis familiaris). Acta Cir Bras 16(4): 211-217.
- TAEGTMEYER H, ROBERTS AFC AND RAINE AEG. 1985. Energy metabolism in reperfused heart muscle: metabolic correlates to return of function. J Am Coll Cardiol 6(4): 864-870.
- VALE DF, SILVA RM, AGUIAR RR, MONTEIRO GA, ANTUNES F, KALIL RAK, LOGULLO CJ AND OLIVEIRA ALA. 2016. The Correlation of Glycogen Metabolism in Rabbit Myocardial Ischemia. J Veterinar Sci Technol 1: 7.
- VANOVERSCHELDE JL, JANIER MF, BAKKE JE, MARSHALL DR AND BERGMANN SR. 1994. Rate of glycolysis during ischemia determines extent of ischemic injury and functional recovery after reperfusion. Am J Physiol 267(5 Pt 2): H1785-94.
- VARCO RL. 1951. Temporary vena cavae occlusion for open heart surgery. Surgery 30: 42.
- WONG MS, LARA TM, KOBZIK L, ROUNDS JD, ROBINSON MK AND JACOBS DO. 1999. Hindlimb Ischemia-Reperfusion Increases Complement Deposition and Glycolysis. J Surg Res 85(1): 130-135.
Publication Dates
-
Publication in this collection
31 Aug 2017 -
Date of issue
Jul-Sep 2017
History
-
Received
08 Nov 2016 -
Accepted
24 Mar 2017