ABSTRACT
It is not unusual to find epiphytic bromeliads in mangroves, but most studies on mangrove vegetation do not record their presence. This study aimed to evaluate the diversity and distribution of epiphytic bromeliads in a subtropical mangrove. The richness, abundance and life form (atmospheric and tank) of bromeliads were recorded and compared among host tree species and waterline proximity. The effects of diameter and height of host trees on the abundance of bromeliads were also assessed. The mangrove was composed of Avicennia schaueriana, Laguncularia racemosa and Rhizophora mangle. We recorded seven bromeliad species of the genera Tillandsia and Vriesea. The waterline proximity did not affect the abundance or diversity of bromeliads, but atmospheric forms were predominant near the waterline, whereas tank bromeliads were more frequent in the interior of the mangrove. The three mangrove species hosted bromeliads, but L. racemosa was the preferred host. The species composition showed that the distribution of bromeliads is more related to the host species than to the distance from the waterline. Bromeliad abundance increased with tree size. Bromeliads can be biological indicators of ecosystem health; therefore, inventories and host tree preferences are necessary knowledge for an adequate management of sensitive ecosystems as mangroves.
Key words:
Bromeliaceae; bromeliad-host relationship; species richness; waterline distance
INTRODUCTION
Vascular epiphytes can be found in diverse habitats, on host plants with architectural and phenological features that are favorable for their establishment (Graham and Andrade 2004GRAHAM EA and ANDRADE JL. 2004. Drought tolerance associated with vertical stratification of two co-occurring epiphytic bromeliads in a tropical dry forest. Am J Bot 91: 699-706., Zotz and Schultz 2008ZOTZ G and SCHULTZ S. 2008. The vascular epiphytes of a lowland forest in Panama-species composition and spatial structure. Plant Ecol 195: 131-141., Cach-Pérez et al. 2013). Since epiphytes are sensitive to human impact and climate change, the structure of epiphytic communities has been used as a conservation index (Nadkarni and Solano 2002, Wolf 2005WOLF JHD. 2005. The response of epiphytes to anthropogenic disturbance of pine-oak forests in the highlands of Chiapas, Mexico. For Ecol Manage 212: 376-393., Hayasaka et al. 2012HAYASAKA D, KIMURA N, FUJIWARA K, THAWATCHAI W and NAKAMURA T. 2012. Relationship between microenvironment of mangrove forests and epiphytic fern species richness along the Pan Yi river, Thailand. J Trop For Sci 24: 265-274.).
The Bromeliaceae is a rich family of vascular plants, with about 3160 species, of which more than 60% are epiphytes (Zotz 2013ZOTZ G. 2013. The systematic distribution of vascular epiphytes - a critical update. Bot J Linn Soc 171: 453-481.). The composition and distribution of epiphytic bromeliads are influenced by the characteristics of hosts. The substrate offered by each host tree promotes specificity to the epiphyte-host relationship (Zotz and Vollrath 2003, Zotz and Schultz 2008, Benavides et al. 2011BENAVIDES AM, VASCO A, DUQUE AJ and DUIVENVOORDEN JF. 2011. Association of vascular epiphytes with landscape units and phorophytes in humid lowland forests of Colombian Amazonia. J Trop Ecol 27: 223-237.). Epiphytic bromeliads can show two life forms: tank bromeliads, whose leaves are arranged in a rosette where they accumulate water and organic debris; and atmospheric bromeliads, whose leaves are narrow and do not serve as a reservoir (Benzing 1990BENZING DH. 1990. Vascular epiphytes: general biology and related biota. Cambridge: Cambridge University Press, 354 p., Givnish et al. 2007GIVNISH TJ, MILLAM KC, BERRY PE and SYTSMA KJ. 2007. Phylogeny, adaptive radiation, and historical biogeography of Bromeliaceae inferred from ndhF sequence data. Aliso 23: 3-26.). Each life form shows specific requirements that also influence their distribution pattern (Benzing 1990). Tank bromeliads also play an important ecological role, increasing the local diversity, since many invertebrates and vertebrates use their reservoir for shelter, breeding and feeding (Mestre et al. 2001MESTRE LAM, ARANHA JMR and ESPER MLP. 2001. Macroinvertebrate fauna associated to the bromeliad Vriesea inflata of the Atlantic Forest (Paraná State, Southern Brazil). Braz Arch Biol Technol 44: 89-94., Lopez et al. 2005LOPEZ LCS, FILIZOLA B, DEISS I and RIOS RI. 2005. Phoretic behaviour of bromeliad annelids (Dero) and ostracods (Elpidium) using frogs and lizards as dispersal vectors. Hydrobiologia 549: 15-22., Ngai and Srivastava 2006NGAI JT and SRIVASTAVA DS. 2006. Predators accelerate nutrient cycling in a bromeliad ecosystem. Science 314: 963.).
The spatial distribution of epiphytic bromeliads depends on the relationship between their particular requirements (for reproduction, fixing, germination, growth and survival) and biotic (availability of host trees, dispersers and pollinators) and abiotic factors (temperature, luminous intensity and atmospheric humidity) (Nieder et al. 2000NIEDER J, ENGWALD S, KLAWUN M and BARTHLOTT W. 2000. Spatial distribution of vascular epiphytes (including hemiepiphytes) in a lowland Amazonian rain forest (Surumoni crane plot) of southern Venezuela. Biotropica 32: 385-396., Zotz and Vollrath 2003ZOTZ G and VOLLRATH B. 2003. The epiphyte vegetation of the palm Socratea exorrhiza - correlations with tree size, tree age and bryophyte cover. J Trop Ecol 19: 81-90., Benavides et al. 2011BENAVIDES AM, VASCO A, DUQUE AJ and DUIVENVOORDEN JF. 2011. Association of vascular epiphytes with landscape units and phorophytes in humid lowland forests of Colombian Amazonia. J Trop Ecol 27: 223-237.). Vertically, the composition and abundance of species can vary between the different heights of the host trees, depending on the strategy of nutrient acquisition, time of substrate availability and microclimatic conditions (Bonnet and Queiroz 2006BONNET A and QUEIROZ MH. 2006. Estratificação vertical de bromélias epifíticas em diferentes estádios sucessionais da floresta ombrófila densa, Ilha de Santa Catarina, Brasil. Braz J Bot 29: 217-228.). Horizontally, the distribution of epiphytic vegetation along ecosystems varies according to the relative humidity, the forest structure and the host species arrangement (Nieder et al. 2000, Zotz and Schultz 2008, Cach-Pérez et al. 2013).
It is not unusual to find epiphytic bromeliads in mangroves, which are important ecosystems in tropical and subtropical coastlines (Vannucci 2001VANNUCCI M. 2001. What is so special about mangroves? Braz J Biol 61: 599-603.). Mangroves have great functional diversity and high productivity (Ashton and Macintosh 2002ASHTON EC and MACINTOSH DJ. 2002. Preliminary assessment of the plant diversity and community ecology of the Sematan mangrove forest, Sarawak, Malaysia. For Ecol Manage 166: 111-129., Bhomia et al. 2016BHOMIA RK, KAUFFAN JB and MCFADDEN TN. 2016. Ecosystem carbon stocks of mangrove forests along the Pacific and Caribbean coasts of Honduras. Wetlands Ecol Manage 24: 187.). They are recognized by being great organic matter transformers and sources of income and services, such as minimizing the effects of coastal disturbance and climate change (Alongi 2008ALONGI DM. 2008. Mangrove forests: resilience protection from tsunamis, and responses to global climate change. Estuar Coast Shelf Sci 76: 1-13., Lima and Colpo 2014LIMA RG and COLPO DK. 2014. Leaf-litter decomposition of the mangrove species Avicennia schaueriana, Laguncularia racemosa and Rhizophora mangle. J Mar Biol Assoc UK 94: 233-239.). However, mangroves are very sensitive to human activities, and are in decline around the world due to the exploitation of their areas and natural resources (Penha-Lopes et al. 2011, Santos et al. 2011SANTOS HF, CARMO FL, PAES JES, ROSADO AS and PEIXOTO RS. 2011. Bioremediation of mangroves impacted by petroleum. Water Air Soil Pollut Focus 216: 329-350. ). Mangrove degradation causes the decrease and fragmentation of biodiversity (Ebrahimi-Sirizi and Riyahi-Bakhtiyari 2012, Bayen 2012BAYEN S. 2012. Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: A review. Environ Manage 48: 84-101.). Therefore, describing and monitoring the biodiversity in mangroves could be a valuable tool for the conservation and management of this ecosystem (Hayasaka et al. 2012HAYASAKA D, KIMURA N, FUJIWARA K, THAWATCHAI W and NAKAMURA T. 2012. Relationship between microenvironment of mangrove forests and epiphytic fern species richness along the Pan Yi river, Thailand. J Trop For Sci 24: 265-274.).
Most studies describing the flora of mangroves assess the diversity and community structure of trees but do not record the presence of epiphytes (Ashton and Macintosh 2002ASHTON EC and MACINTOSH DJ. 2002. Preliminary assessment of the plant diversity and community ecology of the Sematan mangrove forest, Sarawak, Malaysia. For Ecol Manage 166: 111-129., Colpo et al. 2011COLPO KD, CHACUR MM, GUIMARÃES FJ and NEGREIROS-FRANSOZO ML. 2011. Subtropical Brazilian mangroves as a refuge of crab (Decapoda: Brachyura) diversity. Biodivers Conserv 20: 3239-3250., Bhomia et al. 2016BHOMIA RK, KAUFFAN JB and MCFADDEN TN. 2016. Ecosystem carbon stocks of mangrove forests along the Pacific and Caribbean coasts of Honduras. Wetlands Ecol Manage 24: 187., MacKenzie et al. 2016, Chen et al. 2016CHEN Q, ZHAO Q, Li J, JIAN S and REN H. 2016. Mangrove succession enriches the sediment microbial community in South China. Sci Rep 6: 27468. ). Although epiphytes are very abundant in dense mangroves, few studies have assessed the richness of epiphytic species or specifically of bromeliads (Robertson and Platt 2001ROBERTSON KM and PLATT WJ. 2001. Effects of multiple disturbances (fire and hurricane) on epiphyte community dynamics in a subtropical forest, Florida, USA. Biotropica 33: 573-582. , Zotz and Reuter 2009ZOTZ G and REUTER N. 2009. The effect of exposure to sea water on germination and vegetative growth of an epiphytic bromeliad. J Trop Ecol 25: 311-319. , Cach-Pérez et al. 2013). To understand the distribution patterns of epiphytes and to preserve them, it is important to know not only the diversity, but also the bromeliad-host relationships (Magalhães and Lopes 2015MAGALHÃES JLL and LOPES MA. 2015. Species richness and abundance of low-trunk herb epiphytes in relation to host tree size and bark type, eastern Amazonia. Rev Árvore 39: 457-466.).
Due to the lack of studies and information about epiphytic bromeliads in mangrove forests, and considering that the diversity of these sensitive species can be a tool for mangrove conservation, this study aimed to assess the diversity of epiphytic bromeliads in a subtropical mangrove, evaluating their distribution and relationship with their host trees.
MATERIALS AND METHODS
The study area was a mangrove fragment of the Itapanhaú river (23° 49' 07'' S, 46° 09' 07'' W), located in Bertioga, central coast of the State of São Paulo, Brazil. This area is located in the Serra do Mar State Park. The average annual temperature is approximately 27ºC, the air relative humidity is greater than 80%, and the annual rainfall is 3,200 mm (Maia et al. 2008MAIA VC, MAGENTA MAG and MARTINS SE. 2008. Occurrence and characterization of insect galls at resting areas of Bertioga (São Paulo, Brazil). Biota Neotrop 8: 167-197.).
Two transects were established in the study area: one at the mangrove-river interface (edge transect) and the other at 100 m from the mangrove-river interface (interior transect) (experimental design modified from Ashton and Macintosh 2002ASHTON EC and MACINTOSH DJ. 2002. Preliminary assessment of the plant diversity and community ecology of the Sematan mangrove forest, Sarawak, Malaysia. For Ecol Manage 166: 111-129., and MacKenzie et al. 2016). This distance was established because the transition-mangrove zone starts at about 150 m from the waterline, with the presence of non-mangrove species. In each transect, we randomly demarcated 10 quadrats of 25 m². In each quadrat, all trees with circumference larger than 5 cm were registered, identified, measured (DBH = diameter at breast height, in cm) and inspected regarding the presence of bromeliads. The height of host trees was also measured with a clinometer.
The bromeliads in each host tree were identified, counted, photographed and characterized as tank or atmospheric bromeliads. With exception of Tillandsia usneoides, the actual abundance of each species of bromeliads could be recorded, since it was possible to identify and record each ramet. Abundance of T. usneoides was estimated as the degree of cover in the host tree on a scale from 0 to 4, according to Bonnet et al. (2007BONNET A, CURCIO GR, BARDDAL ML, RODERJAN CV and LAVORANTI OJ. 2007. Distribuição horizontal de bromélias epifíticas na planície do Rio Iguaçu, Paraná, Brasil. R Bras Bioci 5: 513-515.). The proportion of individuals of each bromeliad species in the three host species was also calculated.
The structure of the mangrove forest in the study area was evaluated by a two-way ANOVA, which compared the density of each tree species (fixed and orthogonal factor with three levels: Avicennia schaueriana, Laguncularia racemosa and Rhizophora mangle) between transects (fixed and orthogonal factor with two levels: edge and interior). The proportion of trees with and without bromeliads in each transect was compared by chi-square test.
The effects of host species (fixed and orthogonal factor with three levels: A. schaueriana, L. racemosa and R. mangle) and transects (fixed and orthogonal factor with two levels: edge and interior) on the abundance of bromeliads were evaluated by a two-way ANOVA. The Cochran test assessed the homogeneity of variances. Chi-square tests were used to compare the proportion of tank and atmospheric bromeliads in each transect. The proportion of each bromeliad on each host species was also determined.
Variations in the composition of bromeliad species between transects and host tree species were evaluated by cluster analysis, which was carried out based on a Bray Curtis similarity index. We also performed a multiple regression analysis (GLM) to test the effects of DBH and height of host trees (predictor variables) on the abundance of bromeliads (dependent variable).
RESULTS
The mangrove forest in the study area was composed of three tree species: Avicennia schaueriana Stapf and Leechman, Laguncularia racemosa C.F. Gaertn and Rhizophora mangle L. The density of trees showed no differences between the edge (5,400±2,185 trees.ha-1) and the interior (4,040±1,091 trees.ha-1) transects, but the density of A. schaueriana was lower than that of the other species in the forest (Table I and Figure 1). Most of the trees were free of bromeliads (Chi-square: p<0.001). Only 23% of trees in the edge and 32% in the interior were used by epiphytes (Figure 2).
Summary of the two-way ANOVA comparing A: the density of each tree species between transects and B: the abundance of bromeliads in each species of host tree between transects. Transects is a fixed and orthogonal factor, with two levels: edge and interior. Tree species is a fixed and orthogonal factor, with three levels: Avicennia schaueriana, Laguncularia racemosa and Rhizophora mangle .
Proportion of trees without and with bromeliads in the edge and interior transects of Itapanhaú mangrove. Chi-square results.
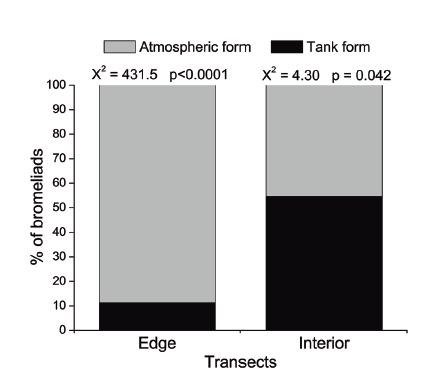
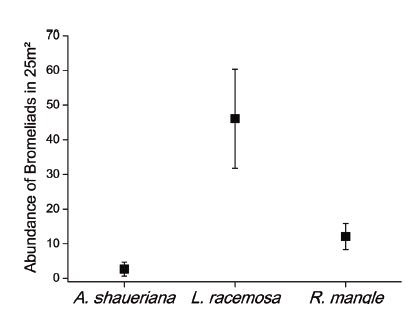
We recorded seven bromeliads species of the genera Tillandsia and Vriesea. In each transect, we found six bromeliad species. A total of 1,165 individuals, of which 47.9% were seedlings were registered in the studied mangrove. Furthermore, the sum of degree of cover of T. usneoides was at 60 (Table II). Tillandsia gardneri was the most abundant species, which was found in all host species, while V. gigantea was the rarest species, only one individual were recorded in L. racemosa (Table II). The abundance of bromeliads in the edge (73±79.2 bromeliads.25m-²) and the interior (48.8±46.1 bromeliads.25m-²) transects was similar (Table I). In the edge transect, 88.7% of bromeliads were atmospheric (c² = 431.5; p<0.0001), whereas in the interior transect, tank bromeliads predominated (54.7%) (c² = 4.301; p = 0.0425) (Figure 3). The three mangrove species hosted bromeliads, but the abundance and richness of bromeliads in each host species were different. Laguncularia racemosa seemed to be the preferred host because greater abundance (Figure 4) and richness (Table II) of bromeliads were recorded in this tree. Four bromeliads species were recorded in R. mangle and only T. gardneri was found in A. schaueriana (Table II).
Richness, life form, and abundance of bromeliads recorded in the mangrove of the Itapanhaú river. Proportion of each bromeliad species in the host trees. * degree of cover as abundance estimation.
Proportion of atmospheric and tank bromeliads in the edge and interior transects of Itapanhaú mangrove. Chi-square results.
Abundance of bromeliads (mean and standard error in 25m²) in each host tree species recorded in Itapanhaú mangrove.
According to the composition of bromeliad species, the cluster analysis grouped the host tree species (especially L. racemosa and R. mangle) at 58% similarity (Bray Curtis coefficient) but not the transects (edge and interior) (Figure 5). This result suggests that the distribution of epiphytic bromeliads in the mangroves is more associated with the host tree than with the distance from the waterline. The DBH of the host tree showed a positive effect on bromeliad abundance, while the height was not related (Table III). Therefore, larger DBH of host trees is associated with greater abundance of bromeliads.
Summary of multiple regression analysis (GLM), testing the relationship between predictor variables (diameter at breast height - DBH - and height of host trees) and dependent variable (abundance of bromeliads).
Cluster analysis based on bromeliad abundances recorded in each host tree species and in each transect (E = edge, I - interior). The Bray Curtis coefficient grouped the host species (L. racemosa and R. mangle) at 58% of similarity, independently of the transect.
DISCUSSION
The conditions of light, air humidity, temperature, and physical variants provided by host trees create microenvironments that affect the spatial distribution patterns and settlement of epiphytic bromeliads in a forest (Zotz and Vollrath 2003ZOTZ G and VOLLRATH B. 2003. The epiphyte vegetation of the palm Socratea exorrhiza - correlations with tree size, tree age and bryophyte cover. J Trop Ecol 19: 81-90., Padmawathe et al. 2004PADMAWATHE R, QURESHI Q and RAWAT GS. 2004. Effects of selective logging on vascular epiphyte diversity in a moist lowland forest of Eastern Himalaya, India. Biol Conserv 119: 81-92., Zotz and Schultz 2008, Hayasaka et al. 2012HAYASAKA D, KIMURA N, FUJIWARA K, THAWATCHAI W and NAKAMURA T. 2012. Relationship between microenvironment of mangrove forests and epiphytic fern species richness along the Pan Yi river, Thailand. J Trop For Sci 24: 265-274., Cach-Pérez et al. 2013). In the mangrove of Itapanhaú river, the distribution of bromeliads was more affected by the characteristics of the host tree than by the distance to the waterline. In the study area, L. racemosa and trees with greatest diameter provided better conditions for the colonization of bromeliads.
The topography, type of substrate, frequency of tides, availability of freshwater and nutrients influence the diversity and structure of mangrove forests (Schaeffer-Novelli et al. 1990). Avicennia schaueriana, L. racemosa and R. mangle, the tree species recorded in this study, are common in subtropical Brazilian mangroves (Colpo et al. 2011COLPO KD, CHACUR MM, GUIMARÃES FJ and NEGREIROS-FRANSOZO ML. 2011. Subtropical Brazilian mangroves as a refuge of crab (Decapoda: Brachyura) diversity. Biodivers Conserv 20: 3239-3250.). The arrangement of trees in the forest is an important factor that affects the distribution of epiphytes, since each host species provides different substrates, conditions and microclimates for epiphytic development (Benzing 1990BENZING DH. 1990. Vascular epiphytes: general biology and related biota. Cambridge: Cambridge University Press, 354 p., Bonnet and Queiroz 2006BONNET A and QUEIROZ MH. 2006. Estratificação vertical de bromélias epifíticas em diferentes estádios sucessionais da floresta ombrófila densa, Ilha de Santa Catarina, Brasil. Braz J Bot 29: 217-228.). However, in the present study, we found no differences in the density of each tree species or in the proportion of trees with bromeliads between the edge and interior transects. This homogeneous structure of forest possibly promoted the similar abundance and richness of bromeliads in both transects. In this sector of the Brazilian coast, the coastal plains are small, and consequently the mangrove areas are less extensive (Colpo et al. 2011). Therefore, it is probable that the distance between the river and the interior of the forest was insufficient to reveal differences in mangrove structure or in bromeliad diversity and abundance.
In this study, the most important difference found between transects was the proportion of atmospheric and tank bromeliads. Atmospheric bromeliads, with more intermittent water supply but better photoprotection (Benzing 2000BENZING DH. 2000. Bromeliaceae: profile of an adaptive radiation. Cambridge: Cambridge University Press , 690 p., Pierce 2007PIERCE S. 2007. The jeweled armor of Tillandsia - multifaceted or elongated trichomes provide photoprotection. Aliso 23: 44-52., Cach-Pérez et al. 2016), predominated in the edge transect, whereas tank bromeliads, which have their own water reservoir and show lower capacity of dissipation of excess radiation (Arruda and Costa 2003ARRUDA RCO and COSTA AF. 2003. Foliar anatomy of five Vriesea Sect. Xiphion (Bromeliaceae) species. Selbyana 24: 180-189., Woods et al. 2015WOODS CL, CARDELÚS CL and DEWALT SJ. 2015. Microhabitat associations of vascular epiphytes in a wet tropical forest canopy. J Ecol 103: 421-430., Cach-Pérez et al. 2016), were more abundant in the interior of the mangrove. In mangroves, air humidity and incident radiation near the river are greater than in the forest interior (Bonnet et al. 2007BONNET A, CURCIO GR, BARDDAL ML, RODERJAN CV and LAVORANTI OJ. 2007. Distribuição horizontal de bromélias epifíticas na planície do Rio Iguaçu, Paraná, Brasil. R Bras Bioci 5: 513-515.). In the studied mangrove, probably these environmental factors differed between transects and apparently provided the distribution pattern of atmospheric and tank bromeliads.
Based on the composition of bromeliad assemblages, our results suggest that the distribution of these epiphytes is more related to the host tree species than to the distance from the waterline. Many epiphytes show preferences for specific characteristics of the host trees (Benzing 2000BENZING DH. 2000. Bromeliaceae: profile of an adaptive radiation. Cambridge: Cambridge University Press , 690 p., Benavides et al. 2011BENAVIDES AM, VASCO A, DUQUE AJ and DUIVENVOORDEN JF. 2011. Association of vascular epiphytes with landscape units and phorophytes in humid lowland forests of Colombian Amazonia. J Trop Ecol 27: 223-237., Wagner et al. 2015WAGNER K, MENDIETA-LEIVA G and ZOTZ G. 2015. Host specificity in vascular epiphytes: a review of methodology, empirical evidence and potential mechanisms. AoB Plants 7: plu092.). Therefore, phylogenetically analogous trees or those that share morphology or functional traits may host similar species of epiphytes (Chaves et al. 2016CHAVES CJN, DYONISIO JC and ROSSATO DR. 2016. Host trait combinations drive abundance and canopy distribution of atmospheric bromeliad assemblages. AoB Plants 8: plw010. ). The bark structure of the tree is an important feature that affects the preference of bromeliads, since the bark influences the microclimate near the trunk (Callaway et al. 2002CALLAWAY RM, REINHART KO, MOORE GW, MOORE DJ and PENNINGS SC. 2002. Epiphyte host preferences and host traits: mechanisms for species-specific interactions. Oecologia 132: 221-230., Vergara-Torres et al. 2010, Wagner et al. 2015). Increased roughness of the bark provides greater capacity of retention of organic matter and humidity, promoting the germination of seeds and the development of epiphytes (Benzing 1990, Laube and Zotz 2006LAUBE S and ZOTZ G. 2006. Neither host-specific nor random: vascular epiphytes on three tree species in a Panamanian lowland forest. Ann Bot 97: 1103-1114., Tewari et al. 2009TEWARI LM, TEWARI G, NAILWAL T and PANGTEY YPS. 2009. Bark factors affecting the distribution of epiphytic ferns communities. Nat Sci 7: 76-81., Chomba et al. 2011CHOMBA C, SENZOTA R, CHABWELA H and NYIRENDA V. 2011. The influence of host tree morphology and stem size on epiphyte biomass distribution in Lusenga Plains National Park, Zambia. J Ecol Nat Environ 3: 370-380., Wagner et al. 2015). In the present study, all bromeliad species were found on L. racemosa, which is the mangrove species with greatest bark roughness (Marcelli 1992MARCELLI MP. 1992. Ecologia liquência nos manguezais do Sul-Sudeste Brasileiro. Bibliotheca Lichenologica, 47. Berlin: J Cramer, 288 p., Schaeffer-Novelli 1995). Therefore, we can assume that this tree species is the most favorable host for bromeliad establishment in this Brazilian subtropical mangrove. Besides the bark traits, the size of attachment area and the time available for colonization positively influence the settlement and development of bromeliads (Flores-Palacios and García-Franco 2006). We found that mature trees with larger trunks (DBH) show greater abundance of bromeliads.
Epiphyte colonization depends on the conditions and support provided by the host tree; therefore, epiphytes can be considered useful biological indicators of ecosystem health (Hayasaka et al. 2012HAYASAKA D, KIMURA N, FUJIWARA K, THAWATCHAI W and NAKAMURA T. 2012. Relationship between microenvironment of mangrove forests and epiphytic fern species richness along the Pan Yi river, Thailand. J Trop For Sci 24: 265-274., Sáyago et al. 2013SÁYAGO R, LOPEZARAIZA-MIKEL M, QUESADA M, ÁLVAREZ-AÑORVE MY, CASCANTE-MARÍN A and BASTIDA JM. 2013. Evaluating factors that predict the structure of a commensalistic epiphyte - phorophyte network. Proc R Soc B 280: 20122821). We can predict a rich bromeliad flora in mangroves with good development and conservation status, especially in mangrove forests with great abundance of mature specimens of L. racemosa. In many regions, the deterioration of mangroves is faster than the production of information. Therefore, bromeliad species inventories and assessment on host tree preferences are necessary knowledge for an adequate management of mangroves.
ACKNOWLEDGMENTS
We thank Marina Baldan, Maurício Abib and Gabriela Dolcinotti for help in fieldwork, and Paulo Sampaio for assistance in bromeliad identification. We also thank the Universidade Estadual Paulista - Campus São Vicente for providing structural support.
REFERENCES
- ALONGI DM. 2008. Mangrove forests: resilience protection from tsunamis, and responses to global climate change. Estuar Coast Shelf Sci 76: 1-13.
- ARRUDA RCO and COSTA AF. 2003. Foliar anatomy of five Vriesea Sect. Xiphion (Bromeliaceae) species. Selbyana 24: 180-189.
- ASHTON EC and MACINTOSH DJ. 2002. Preliminary assessment of the plant diversity and community ecology of the Sematan mangrove forest, Sarawak, Malaysia. For Ecol Manage 166: 111-129.
- BAYEN S. 2012. Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: A review. Environ Manage 48: 84-101.
- BENAVIDES AM, VASCO A, DUQUE AJ and DUIVENVOORDEN JF. 2011. Association of vascular epiphytes with landscape units and phorophytes in humid lowland forests of Colombian Amazonia. J Trop Ecol 27: 223-237.
- BENZING DH. 1990. Vascular epiphytes: general biology and related biota. Cambridge: Cambridge University Press, 354 p.
- BENZING DH. 2000. Bromeliaceae: profile of an adaptive radiation. Cambridge: Cambridge University Press , 690 p.
- BHOMIA RK, KAUFFAN JB and MCFADDEN TN. 2016. Ecosystem carbon stocks of mangrove forests along the Pacific and Caribbean coasts of Honduras. Wetlands Ecol Manage 24: 187.
- BONNET A, CURCIO GR, BARDDAL ML, RODERJAN CV and LAVORANTI OJ. 2007. Distribuição horizontal de bromélias epifíticas na planície do Rio Iguaçu, Paraná, Brasil. R Bras Bioci 5: 513-515.
- BONNET A and QUEIROZ MH. 2006. Estratificação vertical de bromélias epifíticas em diferentes estádios sucessionais da floresta ombrófila densa, Ilha de Santa Catarina, Brasil. Braz J Bot 29: 217-228.
- CACH-PÉREZ MJ, ANDRADE JL, CETZAL-IX W and REYES-GARCÍA C. 2016. Environmental influence on the inter- and intraspecific variation in the density and morphology of stomata and trichomes of epiphytic bromeliads of the Yucatan Peninsula. Bot J Linn Soc 181: 441-458.
- CACH-PÉREZ MJ, ANDRADE JL, CHILPA-GALVÁN N, TAMAYO-CHIM M, ORELLANA R and REYES-GARCÍA C. 2013. Climatic and structural factors influencing epiphytic bromeliad community assemblage along a gradient of water-limited environments in the Yucatan Peninsula, Mexico. Trop Conserv Sci 6: 283-302.
- CALLAWAY RM, REINHART KO, MOORE GW, MOORE DJ and PENNINGS SC. 2002. Epiphyte host preferences and host traits: mechanisms for species-specific interactions. Oecologia 132: 221-230.
- CHAVES CJN, DYONISIO JC and ROSSATO DR. 2016. Host trait combinations drive abundance and canopy distribution of atmospheric bromeliad assemblages. AoB Plants 8: plw010.
- CHEN Q, ZHAO Q, Li J, JIAN S and REN H. 2016. Mangrove succession enriches the sediment microbial community in South China. Sci Rep 6: 27468.
- CHOMBA C, SENZOTA R, CHABWELA H and NYIRENDA V. 2011. The influence of host tree morphology and stem size on epiphyte biomass distribution in Lusenga Plains National Park, Zambia. J Ecol Nat Environ 3: 370-380.
- COLPO KD, CHACUR MM, GUIMARÃES FJ and NEGREIROS-FRANSOZO ML. 2011. Subtropical Brazilian mangroves as a refuge of crab (Decapoda: Brachyura) diversity. Biodivers Conserv 20: 3239-3250.
- EBRAHIMI-SIRIZI Z and RIYAHI-BAKHTIYARI A. 2012. Petroleum pollution in mangrove forests sediments from Qeshm Island and Khamir Port-Persian Gulf, Iran. Environ Monit Assess 185: 4019-4032.
- FLORES-PALACIOS A and GARCÍA-FRANCO G. 2006. The relationship between tree size and epiphyte species richness: testing four different hypotheses. J Biogeogr 33: 323-330.
- GIVNISH TJ, MILLAM KC, BERRY PE and SYTSMA KJ. 2007. Phylogeny, adaptive radiation, and historical biogeography of Bromeliaceae inferred from ndhF sequence data. Aliso 23: 3-26.
- GRAHAM EA and ANDRADE JL. 2004. Drought tolerance associated with vertical stratification of two co-occurring epiphytic bromeliads in a tropical dry forest. Am J Bot 91: 699-706.
- HAYASAKA D, KIMURA N, FUJIWARA K, THAWATCHAI W and NAKAMURA T. 2012. Relationship between microenvironment of mangrove forests and epiphytic fern species richness along the Pan Yi river, Thailand. J Trop For Sci 24: 265-274.
- LAUBE S and ZOTZ G. 2006. Neither host-specific nor random: vascular epiphytes on three tree species in a Panamanian lowland forest. Ann Bot 97: 1103-1114.
- LIMA RG and COLPO DK. 2014. Leaf-litter decomposition of the mangrove species Avicennia schaueriana, Laguncularia racemosa and Rhizophora mangle. J Mar Biol Assoc UK 94: 233-239.
- LOPEZ LCS, FILIZOLA B, DEISS I and RIOS RI. 2005. Phoretic behaviour of bromeliad annelids (Dero) and ostracods (Elpidium) using frogs and lizards as dispersal vectors. Hydrobiologia 549: 15-22.
- MACKENZIE RA, FOULK PB, Val KLUMP J, WECKERLY K, PURBOSPITO J, MURDIYARSO D, DONATO DC and NGOC NAM V. 2016. Sedimentation and belowground carbon accumulation rates in mangrove forests that differ in diversity and land use: a tale of two mangroves. Wetlands Ecol Manage 24: 245-261.
- MAGALHÃES JLL and LOPES MA. 2015. Species richness and abundance of low-trunk herb epiphytes in relation to host tree size and bark type, eastern Amazonia. Rev Árvore 39: 457-466.
- MAIA VC, MAGENTA MAG and MARTINS SE. 2008. Occurrence and characterization of insect galls at resting areas of Bertioga (São Paulo, Brazil). Biota Neotrop 8: 167-197.
- MARCELLI MP. 1992. Ecologia liquência nos manguezais do Sul-Sudeste Brasileiro. Bibliotheca Lichenologica, 47. Berlin: J Cramer, 288 p.
- MESTRE LAM, ARANHA JMR and ESPER MLP. 2001. Macroinvertebrate fauna associated to the bromeliad Vriesea inflata of the Atlantic Forest (Paraná State, Southern Brazil). Braz Arch Biol Technol 44: 89-94.
- NADKAENI NM and SOLANO R. 2002. Potential effects of climate change on canopy communities in a tropical cloud forest: an experimental approach. Oecologia 131: 580-586.
- NGAI JT and SRIVASTAVA DS. 2006. Predators accelerate nutrient cycling in a bromeliad ecosystem. Science 314: 963.
- NIEDER J, ENGWALD S, KLAWUN M and BARTHLOTT W. 2000. Spatial distribution of vascular epiphytes (including hemiepiphytes) in a lowland Amazonian rain forest (Surumoni crane plot) of southern Venezuela. Biotropica 32: 385-396.
- PADMAWATHE R, QURESHI Q and RAWAT GS. 2004. Effects of selective logging on vascular epiphyte diversity in a moist lowland forest of Eastern Himalaya, India. Biol Conserv 119: 81-92.
- PENHA-LOPES G, TORRES P, CANNICCI S, NARCISO L and PAULA J. 2011. Monitoring antropogenic sewage pollution on mangrove creeks in southern Mozambique: a test of Palaemon concinnus Dana, 1852 (Palaemonidae) as a biological indicator. Environ Pollut 159: 636-645.
- PIERCE S. 2007. The jeweled armor of Tillandsia - multifaceted or elongated trichomes provide photoprotection. Aliso 23: 44-52.
- ROBERTSON KM and PLATT WJ. 2001. Effects of multiple disturbances (fire and hurricane) on epiphyte community dynamics in a subtropical forest, Florida, USA. Biotropica 33: 573-582.
- SANTOS HF, CARMO FL, PAES JES, ROSADO AS and PEIXOTO RS. 2011. Bioremediation of mangroves impacted by petroleum. Water Air Soil Pollut Focus 216: 329-350.
- SÁYAGO R, LOPEZARAIZA-MIKEL M, QUESADA M, ÁLVAREZ-AÑORVE MY, CASCANTE-MARÍN A and BASTIDA JM. 2013. Evaluating factors that predict the structure of a commensalistic epiphyte - phorophyte network. Proc R Soc B 280: 20122821
- SCHAEFFER-NOVELLI Y. 1995. Manguezal: ecossistema entre a terra e o mar. São Paulo: Caribbean Ecological Research, 64 p.
- SCHAEFFER-NOVELLI Y, CINTRÓN-MOLERO G and ADAIME RR. 1990. Variability of mangrove ecosystems along the Brazilian coast. Estuaries 13: 201-218.
- TEWARI LM, TEWARI G, NAILWAL T and PANGTEY YPS. 2009. Bark factors affecting the distribution of epiphytic ferns communities. Nat Sci 7: 76-81.
- VANNUCCI M. 2001. What is so special about mangroves? Braz J Biol 61: 599-603.
- VERGARA-TORRES CA, PACHECO-ÁLVAREZ MC and FLORES-PALACIOS A. 2010. Host preference and host limitation of vascular epiphytes in a tropical dry forest of central Mexico. J Trop Ecol 26: 563-570.
- WAGNER K, MENDIETA-LEIVA G and ZOTZ G. 2015. Host specificity in vascular epiphytes: a review of methodology, empirical evidence and potential mechanisms. AoB Plants 7: plu092.
- WOLF JHD. 2005. The response of epiphytes to anthropogenic disturbance of pine-oak forests in the highlands of Chiapas, Mexico. For Ecol Manage 212: 376-393.
- WOODS CL, CARDELÚS CL and DEWALT SJ. 2015. Microhabitat associations of vascular epiphytes in a wet tropical forest canopy. J Ecol 103: 421-430.
- ZOTZ G. 2013. The systematic distribution of vascular epiphytes - a critical update. Bot J Linn Soc 171: 453-481.
- ZOTZ G and REUTER N. 2009. The effect of exposure to sea water on germination and vegetative growth of an epiphytic bromeliad. J Trop Ecol 25: 311-319.
- ZOTZ G and SCHULTZ S. 2008. The vascular epiphytes of a lowland forest in Panama-species composition and spatial structure. Plant Ecol 195: 131-141.
- ZOTZ G and VOLLRATH B. 2003. The epiphyte vegetation of the palm Socratea exorrhiza - correlations with tree size, tree age and bryophyte cover. J Trop Ecol 19: 81-90.
Publication Dates
-
Publication in this collection
02 May 2017 -
Date of issue
Apr-Jun 2017
History
-
Received
17 Oct 2016 -
Accepted
19 Dec 2016