Abstract
Abstract: We used skeletochronology to compare age, size, reproductive parameters and growth patterns of two related, anuran amphibians from Northern Argentina: Leptodactylus bufonius(n=69) andL. latinasus(n=56), in order to better understand their coexistence in syntopy. Previous studies showed that the two species overlap in their dietary requirements and utilize the same habitats for feeding and breeding. We found that their life-history patterns are significantly different,L. bufoniusbeing larger, and having a higher reproductive output and lifespan, compared to the smaller and shorter-livingL. latinasus. Since none of the species exhibited sexual size dimorphism, and both acquired sexual maturity after the first year of life, we suggest that the differences in the observed life-history parameters must appear during early stages of development, during larval and/or juvenile stages.
Key words
age; Anura; body size; growth; Leptodactylus bufonius; Leptodactylus latinansus
INTRODUCTION
Body size, growth and lifespan are central life history traits related to fitness, and ultimately to reproductive success and survival (CalderCALDER WA. 1984. Size, function and life history. Boston: Harvard University Press, 431 p. 1984, Schmidt-Nielsen 1984SCHMIDT-NIELSEN K. 1984. Scaling. Why is animal size so important? New York: Cambridge University Press, 241 p., BrownBROWN JH, MARQUET PA and TAPER ML. 1993. Evolution of body size: consequences of an energetic definition of fitness. Am Nat 142: 573-584. et al. 1993, StearnsSTEARNS SC. 2000. Life history evolution: successes, limitations, and prospects. Naturwissenschaften 87: 476-486. 2000, MetcalfeMETCALFE NB and MONAGHAN P. 2003. Growth versus lifespan: perspectives from evolutionary ecology. Exp Gerontol 38: 935-940. and Monaghan 2003), which makes the study of intra- and interspecific variation in these traits an essential goal for understanding life-history evolution. In amphibians, several factors may contribute to the variation in adult body size, either within species or among them. Traditionally, it has been considered that variation in body size can simply reflect an underlying pattern of variation in the age structure of populations (Díaz-PaniaguaDÍAZ-PANIAGUA C and MATEO JA. 1999. Geographic variation in body size and life-history traits in Bosca’s newt (Triturusboscai). Herpetol J 9: 21-27. and Mateo 1999, MiaudMIAUD C, GUYETANT R and ELMBERG J. 1999. Variations in life-history traits in the common frog Rana temporaria (Amphibia, Anura): a literature review and new data from the French Alps. J Zool 249: 61-73. et al. 1999, LaugenLAUGEN AT, LAURILA A, JÖNSSON KI, SÖDERMAN F and MERILÄ J. 2005. Do common frogs (Rana temporaria) follow Bergmann’s rule? Evol Ecol Res 7: 717-731. et al. 2005). Variations in juvenile growth rates to sexual maturity and age at maturity may promote divergences in adult body size between species and populations or sexual size dimorphism within a population (HemelaarHEMELAAR A. 1985. An improved method to estimate the number of year rings resorbed in phalanges of Bufo bufo (L.) and its implications to populations from different latitudes. Amphibia-Reptilia 6: 323-341. 1985, Monnet and Cherry 2002MONNET JM and CHERRY MI. 2002. Sexual size dimorphism in anurans. P Roy Soc Lond B Bio 269: 2301-2307.). Thus, an important factor in the analysis of variation of body size in amphibians is the indeterminate growth pattern that they exhibit, which becomes asymptotic when sexual maturity is reached (HallidayHALLIDAY TR and VERRELL P. 1988. Body size and age in amphibians and reptiles. J Herpetol 22: 253-265. and Verrel 1988). Fast growth and early sexual maturity is one of the life strategies that determines increased efficacy at the expense of reaching a smaller adult size (RoffROFF D. 1993.The evolution of life histories. New York: Chapman and Hall, 535 p. 1993, Stearns 1992). In contrast, the benefits of delaying reproduction are generally related to the benefits associated with large body size, which is positively related to fecundity and breeding success (HowardHOWARD RD. 1980. Mating behaviour and mating success in wood frogs Rana sylvatica. Anim Behav 28: 705-716. 1980, BervenBERVEN KA. 1981. Mate choice in the wood frog, Rana sylvatica. Evolution 35: 707-722. 1981), jumping performance (TejedoTEJEDO M, SEMLITSCH RD and HOTZ H. 2000. Covariation of morphology and jumping performance in newly metamorphosed water frogs: effects of larval growth history. Copeia 2000: 448-458. et al. 2000) and competition (Tejedo 1988TEJEDO M, REQUES R and ESTEBAN M. 1997. Actual and osteochronological estimated age of natterjack toads (Bufo calamita). Herpetol J 7: 81-82.). Large body size is also associated with higher survivorship and clutch size (Berven 1982aBERVEN KA. 1982a. The genetic basis of altitudinal variation in the wood frog Rana sylvatica. I. An experimental analysis of life history traits. Evolution 36: 962-983., GibbonsGIBBONS MM and MCCARTHY TK. 1986. The reproductive output of frogs Rana temporaria (L.) with particular reference to body size and age. J Herpetol 209: 579-593. and McCarthy 1986, BegonBEGON M, HARPER JL and TOWNSEND CR. 1990. Ecology, individuals, populations and communities. London: Blackwell Scientific Publications, 945 p. et al. 1990, Stearns 1992STEARNS SC. 1992. The evolution of life histories. Oxford: Oxford University Press, 249 p.). In addition, the environmental conditions experienced by the mother (non-genetic factors), are an important determinant of offspring adult body size, due to the maternally induced variation in egg size (KaplanKAPLAN RH. 1998. Maternal effects, developmental plasticity, and life history evolution. In: Mousseau TE and Fox CW (Eds), Maternal effects as adaptations. New York: Oxford University Press, New York, USA, p. 244-260. 1998). For example, a smaller egg size may determine a smaller size at metamorphosis (BernardoBERNARDO J. 1996. Maternal effects in animal ecology. Am Zool 36: 83-105. 1996) and, when compensatory growth does not take place (Metcalfe and Monaghan 2003, HectorHECTOR KL and NAKAGAWA S. 2012. Quantitative analysis of compensatory and catch-up growth in diverse taxa. J Anim Ecol 81: 583-593. and Nakagawa 2012), it can determine small adult body size (Bernardo 1996, RäsänenRÄSÄNEN K, LAURILA A and MERILÄ J. 2003. Geographic variation in acid stress tolerance of the moor frog, Rana arvalis. II. Adaptive maternal effects. Evolution 57: 363-371.etal et al. 2003, 2005RÄSÄNEN K, LAURILA A and MERILÄ J. 2005. Maternal investment in egg size: environment- and population-specific effects on offspring performance. Oecologia 142: 546-553., Laugen et al. 2005, MarangoniMARANGONI F. 2006. Variación clinal en el tamaño del cuerpo a escala microgeográfica en dos especies de anuros (Pelobates cultripes y Bufo calamita). PhD thesis, Sevilla, Spain, Universidad de Sevilla, 298 p. 2006).
Skeletochronology is a useful technique to estimate individual age in amphibians, and discriminate variations in growth rates and age-related parameters such as age and size at sexual maturity, longevity, and potential reproductive lifespan (SinschSINSCH U. 2015. Skeletochronological assessment of demographic life-history traits in amphibians. Herpetol J 25: 5-13. 2015). These life-history parameters also allow explaining the actual pattern of sexual size dimorphism in amphibians (MarangoniMARANGONI F, BARRASSO DA, CAJADE R and AGOSTINI G. 2012. Body size, age and growth pattern of Physalaemus fernandezae (Anura: Leiuperidae) of Argentina. NW J Zool 8: 63-71. et al. 2012, CajadeCAJADE R, MARANGONI F and GANGENOVA E. 2013. Age, body size and growth pattern of Argenteohyla siemersi pederseni (Anura: Hylidae) in northeastern Argentina. J Nat Hist 47: 237-251. et al. 2013, Quiroga et al. 2015). Skeletochronology is based on the presence of cyclic and annular bone growth, which can be visualized in cross-sections of bones (CastanetCASTANET J. 1982. Recherches sur la croissance du tissu osseux des reptiles. Application: la méthode squelettochronologique. Thèse de Doctorat d’État, Paris. 1982, Castanet and Smirina 1990CASTANET J and SIMIRINA E. 1990. Introduction to the skeletochronological method in amphibians and reptiles. Ann Sci Nat Zool Paris 11: 191-196.). This method is commonly and successfully used for evaluating the age of many species of amphibians and reptiles, providing an age estimate through non-lethal means (Castanet and Smirina 1990, Marangoni et al. 2009MARANGONI F, SCHAEFER EF, CAJADE R and TEJEDO M. 2009. Growth marks formation and chronology of two neotropical anuran species. J Herpetol 43: 446-450., 2012, Sinsch 2015SINSCH U, PELSTER B and LUDWIG G. 2015. Large‐scale variation of size‐and age‐related life‐history traits in the common frog: a sensitive test case for macroecological rules. J Zool 297: 32-43.).
The comparative study of life-history traits in related amphibian species which undergo similar environmental conditions is a good way to understand interspecific interactions and explain how differences in life-history strategies allow the coexistence of these species (MacArthurMACARTHUR R and LEVIN SR. 1967. The limiting similarity, convergence and divergence of coexisting species. Am Nat 101: 377-385. and Levin 1967, MacArthur 1970MACARTHUR RH. 1970. Species packing and competitive equilibrium for many species. Theor Popul Biol 1: 1-11., Pianka 1975PIANKA ER. 1975. Niche relations of desert lizards. In: Cody ML and Diamond JM (Eds), Ecology and Evolution of Communities. Boston: Harvard University Press, Massachusetts, USA, p. 292-314., ToftTOFT CA. 1980. Feeding ecology of thirteen syntopic species of anurans in a seasonal tropical environment. Oecologia 45: 131-141. 1980, 1981TOFT CA. 1981. Feeding ecology of Panamanian litter anurans: patterns in diet and foraging mode. J Herpetol 15: 139-144.). We used skeletochronology to compare the life-history patterns of two closely-related species of the Leptodactylus fuscus group (Heyer 1978HEYER WR. 1978. Systematics of the fuscus group of the frog genus Leptodactylus (Amphibia, Leptodactylidae). Nat Hist Mus LA 29: 1-85.), which occupy the same habitats in the wet Chaco region of northern Argentina. Specifically, we estimated and compared body size, age, growth and reproductive parameters for the two species, aiming to explain the life-history strategies that allow their coexistence.
MATERIALS AND METHODS
STUDY SPECIES
The monophyletic genus Leptodactylus (FitzingerFITZINGER LJ. 1826. Neue Classification der Reptilien nach ihren Natürlichen Verwandtschaften nebst einer Verwandtschafts - Tafel und einem Verzeichnisse der Reptilien - Sammlung des K.K. Zoologischen Museums zu Wien. J.G. Heubner, Wien. 1826) has a predominantly Neotropical distribution and is composed of 75 currently recognized species includeDE LA ESPADA JM. 1875. Vertebrados del viaje al Pacifico verificado de 1862 a 1865 por una comision de naturalistas enviada por el gobierno Español. Batracios. Madrid, 208 p.d in four groups: Leptodactylus fuscus (30 species), L. melanonotus (17 species), L. latrans (8 species), L. pentadactylus (17 species) and three species unassigned to any species group (De Sá et al. 2014DE SÁ RO, GRANT T, CAMARGO A, HEYER RW, PONSSA ML and STANLEY E. 2014. Systematics of the Neotropical genus Leptodactylus Fitzinger, 1826 (Anura: Leptodactylidae): phylogeny, the relevance of non-molecular evidence, and species accounts. S Am J Herpetol 9: S1-S128.). The study species, Leptodactylus latinasus (Jiménez de la Espada 1875) and L. bufonius (BoulengerBOULENGER GA. 1894. XXXVIII.-List of reptiles and batrachians collected by Dr. J. Bohls near Asuncion, Paraguay. J Nat Hist 13: 342-348. 1894), belong to the monophyletic L. fuscus clade (De Sá et al. 2014). Leptodactylus latinasus is distributed in Argentina (Vaira et al. 2012VAIRA M et al. 2012. Categorización del estado de conservación de los anfibios de la República Argentina. Cuad Herpetol (AHA) 26: 131-159. ), Bolivia, Paraguay, south and east throughout Uruguay and southern Brazil (LavillaLAVILLA E, HEYER R, KWET A and LANGONE J. 2004. Leptodactylus latinasus. The IUCN Red List of Threatened Species 2004: e.T57139A11590252. http://dx.doi.org/10.2305/IUCN.UK.2004.RLTS.T57139A11590252.en. et al. 2004). Leptodactylus bufonius is distributed in Argentina (CabreraCABRERA A and WILLINK A. 1980. Biogeografía de América Latina (Latin America Biogeography).Washington: Secretaría General de la Organización de los Estados Americanos, 122 p. and Willink 1980, CarnevaliCARNEVALI R. 1994. Fitogeografía de la provincia de Corrientes. Corrientes: Gobierno de la Provincia de Corrientes, 324 p. 1994, Vaira et al. 2012), southern Bolivia, Paraguay, and central Brazil (Heyer 1978). The two species are common, and sharing habitats scattered throughout the Chaco and Espinal ecoregions (Cabrera and Willink 1980, Carnevali 1994, Vaira et al. 2012). Males of both species construct mud nests at the edge of ponds and other low-lying depressions, and call near semi-permanent or ephemeral water bodies, from inside or close to the chambers (Heyer 1978, CeiCEI JM. 1980. Amphibians of Argentina. Monitore Zoologico Italiano n.s. Monografia 2: 609. 1980, FaggioniFAGGIONI G, SOUZA F, UETANABARO M, LANDGREF-FILHO P, FURMAN J and PRADO C. 2017. Reproductive biology of the nest building vizcacheras frog Leptodactylus bufonius (Amphibia, Anura, Leptodactylidae), including a description of unusual courtship behaviour. Herpetol J 27: 73-80. et al. 2017). The two species share the same reproductive mode (DuellmanDUELLMAN WE and TRUEB L. 1986. Biology of Amphibians. New York: MacGraw-Hill, 670 p. and Trueb1986) and their trophic niche overlaps to a great extent (DuréDURÉ M and KEHR A. 2004. Influence of microhabitat on the trophic ecology of two leptodactylids from northeastern Argentina. Herpetologica 60: 295-303. and Kehr 2004).
STUDY SITE
Fieldwork was carried out in the area called “El Perichón” (27°25′53.1′′ S, 58°44′44.8′′ W), 10 km northeast from Corrientes city, Argentina, where L. latinasus and L. bufonius live in syntopy. This area is characterized by the presence of numerous temporary and semi-permanent ponds. Mean annual temperature is 21.5°C and the mean annual precipitation is 1500 mm, without a pronounced dry season, although periods of rain shortages occur every 4-6 years (Carnevali 1994). The original vegetation was Schinopsis balansae “quebracho” forest, which is currently extremely degraded and largely replaced by sclerophyllous forest, with prevalence of Acacia caven, Celtis spp., Prosopis affinis, Prosopis nigra, and numerous colonies of Aechmea distichantha and Bromelia spp. (Carnevali 1994). Eight out of thirteen species of Leptodactylus genus reported for Argentina are present in the study area: five belong to L. fuscus group (L. bufonius, L. elenae, L. gracilis, L. latinasus, and L. mystacinus), two to the L. latrans group (L. latrans and L. chaquensis) and one to the L. melanonotus group (L. podicipinus).
SAMPLING
We sampled 56 L. latinasus (34 males, 17 females and five juveniles), and 69 L. bufonius (56 males, 9 females and four juveniles), from autumn 2007 to late spring 2008. The frogs were captured between 20:00 and 23:00 h. The sampling followed the ethical standards imposed by the Dirección de Recursos Naturales of the Corrientes province, Argentina. Most males (26) were captured by hand when they were calling on the ground away from ponds, hidden in crevices (L. latinasus) or near the cone-shaped nests (L. bufonius). Remaining males and females were collected during migration or at the edge of the breeding ponds. In these cases, sex and sexual maturity was determined by the presence of dark vocal sac (males), or ova that could be visualized through the skin (females). Frogs were separated by sex, placed in independent plastic containers (12 cm diameter x 6.5 cm height), and brought to the laboratory.
We measured snout-vent length (SVL) and right hind-limb length (HL) by placing each frog on laminated graph paper (accuracy ± 1 mm).We measured the head width (HW), arm length (AL) and tibia length (TL) to the nearest 0.1 mm with digital calipers. We measured body mass (BM) to the nearest 0.01 g, using an Acculab electronic balance (Acculab Scales, Titusville, NJ). In addition, we measured 67 specimens of related Leptodactylus species from the Collection of Laboratorio de Genética Evolutiva (Instituto de Biología Subtropical (CONICET-UNaM), Posadas, Misiones, Argentina), which we considered useful for further comparisons: 13 Leptodactylus furnarius, 18 L. laticeps and 36 L. plaumanni (Appendix A, B). All measurements were taken according to DuellmanDUELLMAN WE. 1970. The hylid frogs of Middle America. Monographs, Museum of Natural History, University of Kansas 1: 1-753. (1970).
Most individuals (109 out of 125; 87%) were released back into their original ponds within 24-48 h after their capture. Ten L. latinasus and six L. bufonius females were preserved for the analysis of reproductive traits, and further genetics and morphological studies, and deposited in the Collection of Laboratorio de Genética Evolutiva, Instituto de Biología Subtropical (CONICET-UNaM), Posadas, Misiones, Argentina (see Appendix A for specimen codes).
SEXUAL SIZE DIMORPHISM
We checked for significant differences in size parameters (i.e. SVL, BM, HW, AM and TL) between sexes, using multi- and univariate analyses of variance (with type III Sum of Squares). We used Pearson correlation coefficient adjusted for small sample sizes (radj) to analyze the associations between these parameters.
We assessed the sexual size dimorphism (SSD) for each body measurement using the sexual dimorphism index (SDI), following LovichLOVICH JE and GIBBONS JW. 1992. A review of techniques for quantifying sexual size dimorphism. Growth Develop Aging 56: 269-281. and Gibbons (1992): SDI = mean sizelarger sex/mean sizesmaller sex, with the result arbitrarily defined as positive when females are larger than males, and negative when males are larger.
SKELETOCHRONOLOGY
We clipped the third toe of the right leg of 28 L. latinasus (12 males, 12 females, four juveniles) and 35 L. bufonius (24 males, 7 females, four juveniles) (Table II), and stored them in 70% ethanol at room temperature for age estimation through skeletochronology. We followed the standard protocols used in skeletochronology (e.g. Smirina 1972SMIRINA EM. 1972. Annual layers in bones of Rana temporaria. Zool Zh 51: 1529-1534.). We selected the third phalanx of the toe, which was washed in water for 30 min, and decalcified in 5% nitric acid for 30-45 min. Afterwards, the samples were washed in running tap water for 5 min and kept overnight in distilled water. Then, the phalanges were frozen (Tissue-Tek O.C.T. Compound, Sakura Finetek) and cross-sectioned at 16 μm using a cryo-microtome. Sections were stained for 3-6 h at room temperature with Ehrlich´s hematoxylin (TejedoTEJEDO M. 1988. Fighting for females in the toad Bufo calamita is affected by the operational sex ratio. Anim Behav 36: 1765-1769. et al. 1997). 15 to 20 of these sections were permanently mounted in aqueous synthetic resin (Aquatex®, Merck KgaA, Germany) on glass microscope slides. Cross sections were examined light microscopically at magnifications of 20x using a Nikon Optiphot microscope. A Canon PowerShot A570 was used to take digital images from those diaphysis sections in which the size of the medullar cavity was at its minimum and that of periosteal bone at its maximum. Cross sections were viewed and measured using the computer package Image-Pro Plus Version 1.1 (Media Cybernetics 1993-1994MEDIACYBERNETICS. 1993-1994. Image-Pro Plus. Version 4.5.0.29. Media Cybernetics, Silver Spring, Maryland, USA.). In a first step of the analysis, we recorded the presence/absence of the line of metamorphosis (LM) and of lines of arrested growth (LAGs). The number of LAGs visible in each cross section was assessed by FM and independently by AC. In those frogs with no remnant of the line of metamorphosis we estimated the degree of resorption by osteometrical analysis, following the method of SagorSAGOR ES, OULLET M, BARTEN E and GREEN DM. 1998. Skeletochronology and geographic variation in age structure in the wood Frog, Rana sylvatica. J Herpetol 34: 469-474. et al. (1998). In a second step, we distinguished annual growth marks (LAGs sensu stricto) from non-annual ones (irregular interruptions of the aestivation periods), following SinschSINSCH U, OROMI N and SANUY D. 2007. Growth marks in Natterjack Toad (Bufocalamita) bones: histological correlates of hibernation and aestivation periods. Herpetol J 17: 129-137. et al. (2007). The age of maturity was defined as the lowest age recorded in a reproductive frog of a given population.
AGE-RELATED PARAMETERS
We computed the following age-related parameters (sensuLeskovarLESKOVAR C, OROMI N, SANUY D and SINSCH U. 2006. Demographic life history traits of reproductive Natterjack Toads (Bufocalamita) vary between northern and southern latitudes. Amphibia-Reptilia 27: 365-375. et al. 2006): (1) age at maturity: the minimum number of LAGs counted in breeding individuals; (2) longevity: the maximum number of LAGs counted in breeding individuals; (3) potential reproductive lifespan: the difference between longevity and age at maturity; (4) mean lifespan; (5) size at maturity: the average snout-vent length of all individuals with the minimum number of LAGs. We used a two-sample Kolmogorov-Smirnov and Mann-Whitney test to check for differences in the shape of age distribution and median age between males and females. We used linear regressions to analyze the associations between age and body size parameters.
GROWTH PATTERNS
We used the packages FSA (Ogle 2018OGLE DH. 2018. FSA: Fisheries Stock Analysis. R package version 0.8.18.) and nlstools (BatyBATY F, RITZ C, CHARLES S, BRUTSCHE M, FLANDROIS JP and DELIGNETTE-MULLER ML. 2015. A Toolbox for Nonlinear Regression in R: The Package nlstools. J StatSoftw 66: 1-21. et al. 2015) in R Studio version 1.1.423 (© 2009-2018 RStudio, Inc.) to compute von BertalanffyBERTALANFFY L VON. 1938. A quantitative theory of organic growth. Hum Biol 10: 181-213.’s growth model (Bertalanffy 1938) following BevertonBEVERTON RJH and HOLT SJ. 1957. On the dynamics of exploited fish populations. London: Fishery Invest Ser II, Vol. XIX, Ministry of Agriculture, Fisheries, and Food, 533 p. and Holt (1957): SVLt = SVLmax x (1-e-k x (t- t0)) , where SVLt is the expected or average SVL at time (or age) t, SVLmax is the asymptotic average SVL, k is the growth rate coefficient and t0 is the time or age when the average SVL was zero. We fitted von Bertalanffy growth model and estimated growth parameters (VBGPs) by nonlinear least squares regression. Two estimated VBGPs were considered significantly different at the 0.95 level when their confidence intervals (CI 95%) did not overlap. We used the value of 10.9 mm as the mean size at metamorphosis (0 LAGs) found in L. bufonius by Vera and Ponssa (2014)VERA MC and PONSSA ML. 2014. Skeletogenesis in anurans: cranial and postcranial development in metamorphic and postmetamorphic stages of Leptodactylus bufonius (Anura: Leptodactylidae). Acta Zool-Stockholm 95: 44-62., to adjust the growth model, since no freshly metamorphosed individuals could be captured from the studied area. Based on the known life-history patterns of the species (i.e. breeding period and larval development) and the moment of sampling, we assigned the age of 0.25 LAGs to L. bufonius and L. latinasus juveniles, assuming that only 3 months elapsed since their metamorphosis.
REPRODUCTIVE TRAITS
Reproductive traits were measured in 16 females: ten L. latinasus and six L. bufonius. We determined the ovarian mass (OM) as the difference between the body mass before and after ovary removal. The ovarian complement (OC) represents the total number of mature ova from each gravid female and is considered a measure of their fertility or reproductive potential (CrumpCRUMP ML. 1974. Reproductive strategies in a tropical anuran community. Miscellaneous publication, University of Kansas, Museum of Natural History 61: 1-68. 1974, BassoBASSO NG. 1990. Estrategias adaptativas en una comunidad subtropical de anuros. Cuad Herpetol (AHA) 1: 1-71. 1990). We removed and weighed approximately 10% of each ovary and counted the mature ova under a Nikon C-DS magnifying glass. Mature ova had well-defined black and yellow poles and pronounced larger size, consistent with the post-vitellogenesis class (Crump 1974). We photographed a random sample of about 200 ova from each ovary with a digital Nikon Coolpix S10 camera, mounted on a Nikon C-DS magnifying glass. We measured the longest and shortest perpendicular axes of 100 ova per sample to the nearest 0.01 mm using Image-Pro Plus 1.1 (Media Cybernetics 1993-94). We determined mature ovum size (OS) by square rooting the product of the two axis measurements. We estimated the ovarian size factor (OSF) which correlates the number and size of mature ova to body length, following DuellmanDUELLMAN WE and CRUMP ML. 1974. Speciation in frogs of the Hyla parviceps group in the upper Amazon Basin. Occas. Pap Mus Nat Hist Univ Kansas 23: 1-40. and Crump (1974): OSF = (OC x OS)/SVL. Finally, we estimated the reproductive effort (RE) following Prado et al. (2000): RE = (OM/BM) x 100, where the body mass is the final weight of the female after oviposition. We used Pearson product-moment correlation coefficient adjusted for small sample sizes (radj) to analyze the associations between size and reproductive parameters.
STATISTICAL ANALYSIS
Body size variables were log-transformed in order to achieve normality. We tested all data for normality and homoscedasticity using Shapiro-Wilk and Levene tests and chose the statistic tests accordingly. We used multi- and univariate analyses of variance to test for differences in body size between the sexes. We used linear regressions to test the association between body size, age and clutch characteristics. All statistical analyses were performed using STATISTICA 6.0 package (StatSoft Inc. 2001STATSOFT. 2001. Statistica (Data analysis software system).Version 6. StatSoft, Tulsa.).
RESULTS
BODY SIZE AND SEXUAL SIZE DIMORPHISM
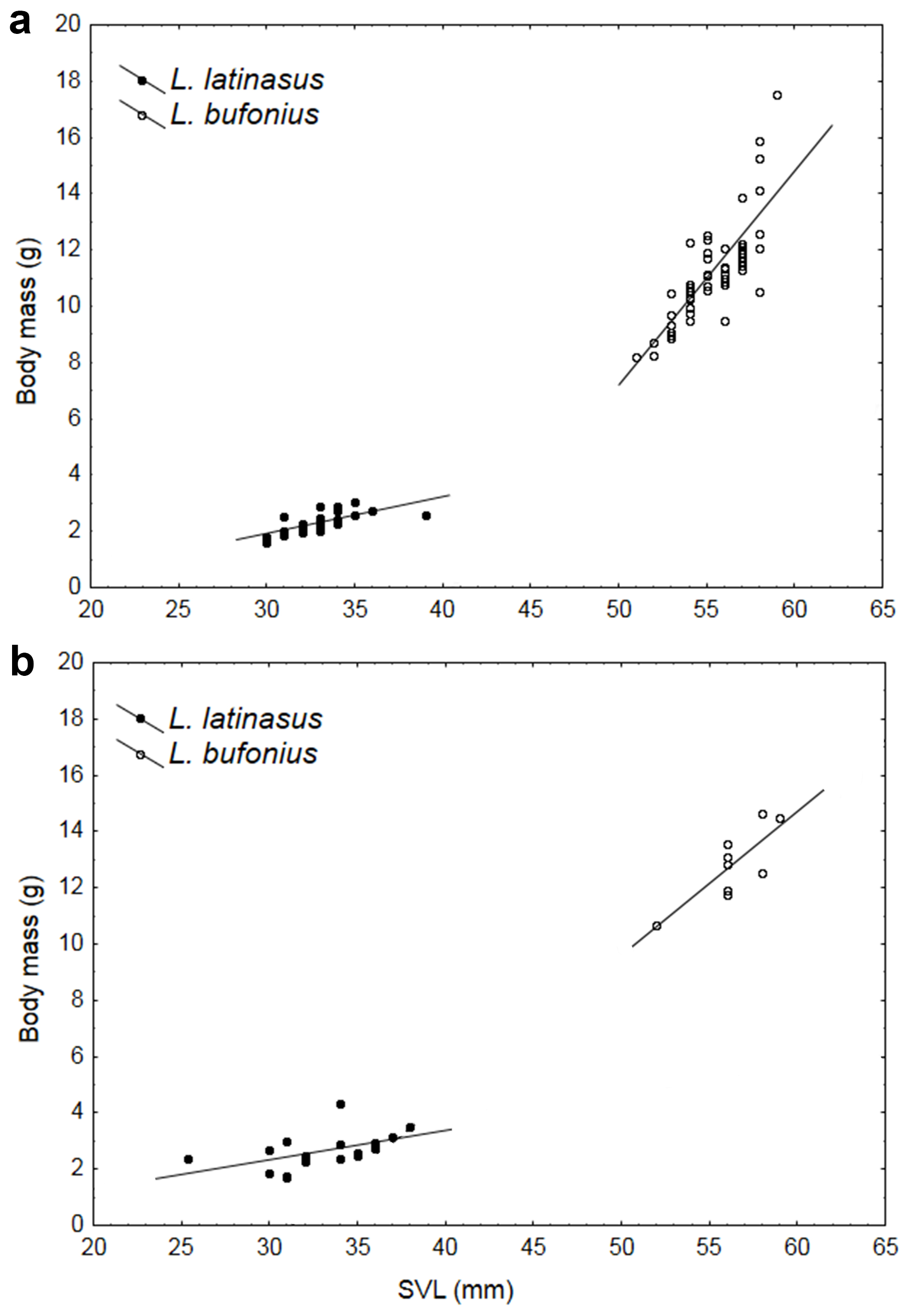
Both species (Table I) showed no significant effect on all measured morphometric variables (L. latinasus: Wilk´s λ=0.771, F6,39=1.920, P=0.101; L. bufonius: Wilk´s λ=0.846, F6,58=1.750, P=0.125). The values of sexual dimorphism index (SDI) were negative for head width (-1.00) in L. latinasus and arm length (-1.01) in L. bufonius, showing that the males were larger than females in these variables, whereas the females were larger than males in the remaining variables studied (Table I). We found a positive and significant correlation between body mass and SVL in both species. This correlation showed differences in the slope between the two species, with body mass increasing faster with SVL in L. bufonius compared to L. latinasus (Fig. 1).
Snout-vent length (SVL) and body mass (BM) relationships in Leptodactylus latinasus and L. bufonius males (a) and females (b).
Mean ± SD values of body mass (BM), snout-vent length (SVL), right hind leg length (HLR), head width (HW), tibia length (TL) and arm length (AL) of male and female Leptodactylus latinasus and L. bufonius from northeastern Argentina. SDI = sexual dimorphism index. Sample size is provided in parentheses.
AGE-RELATED PARAMETERS
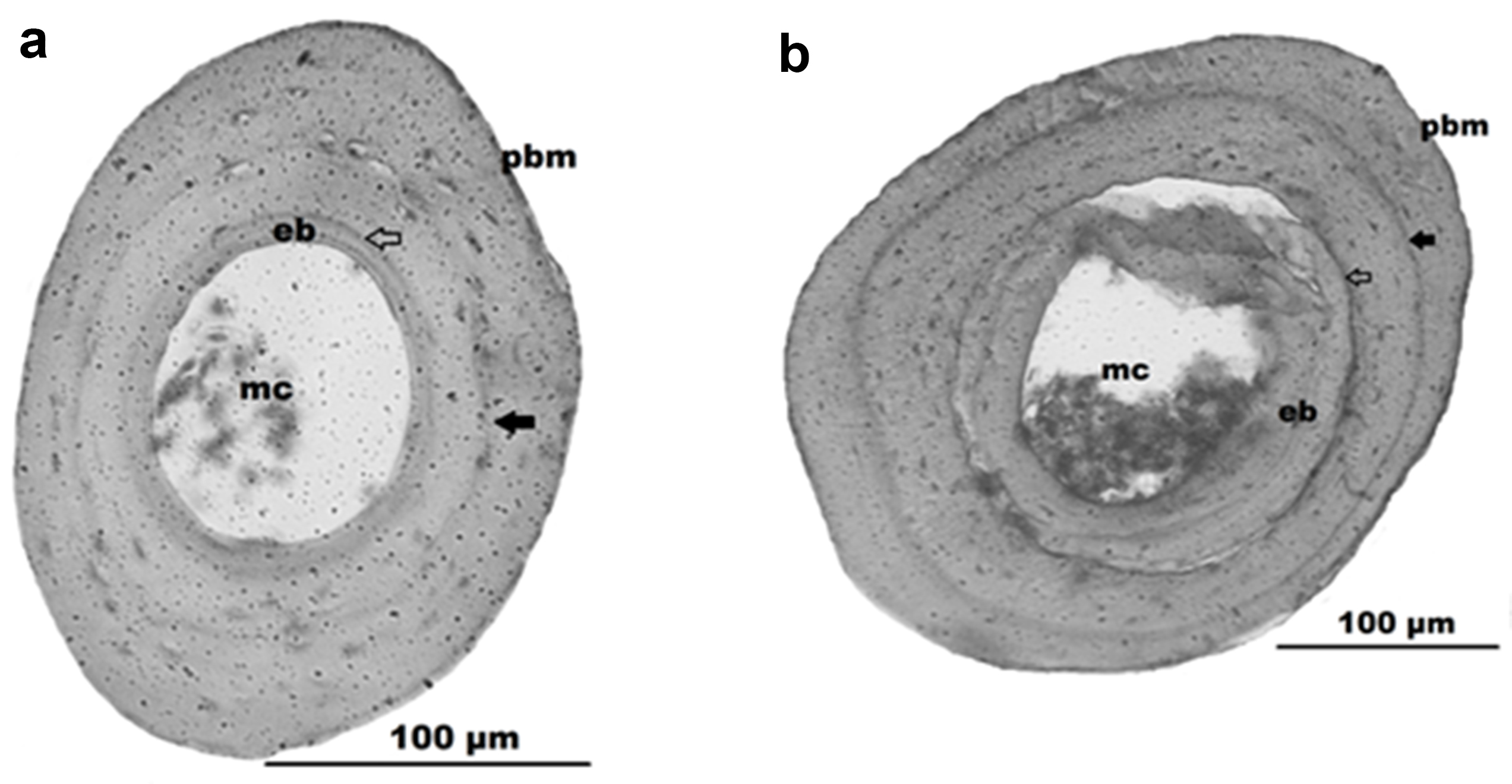
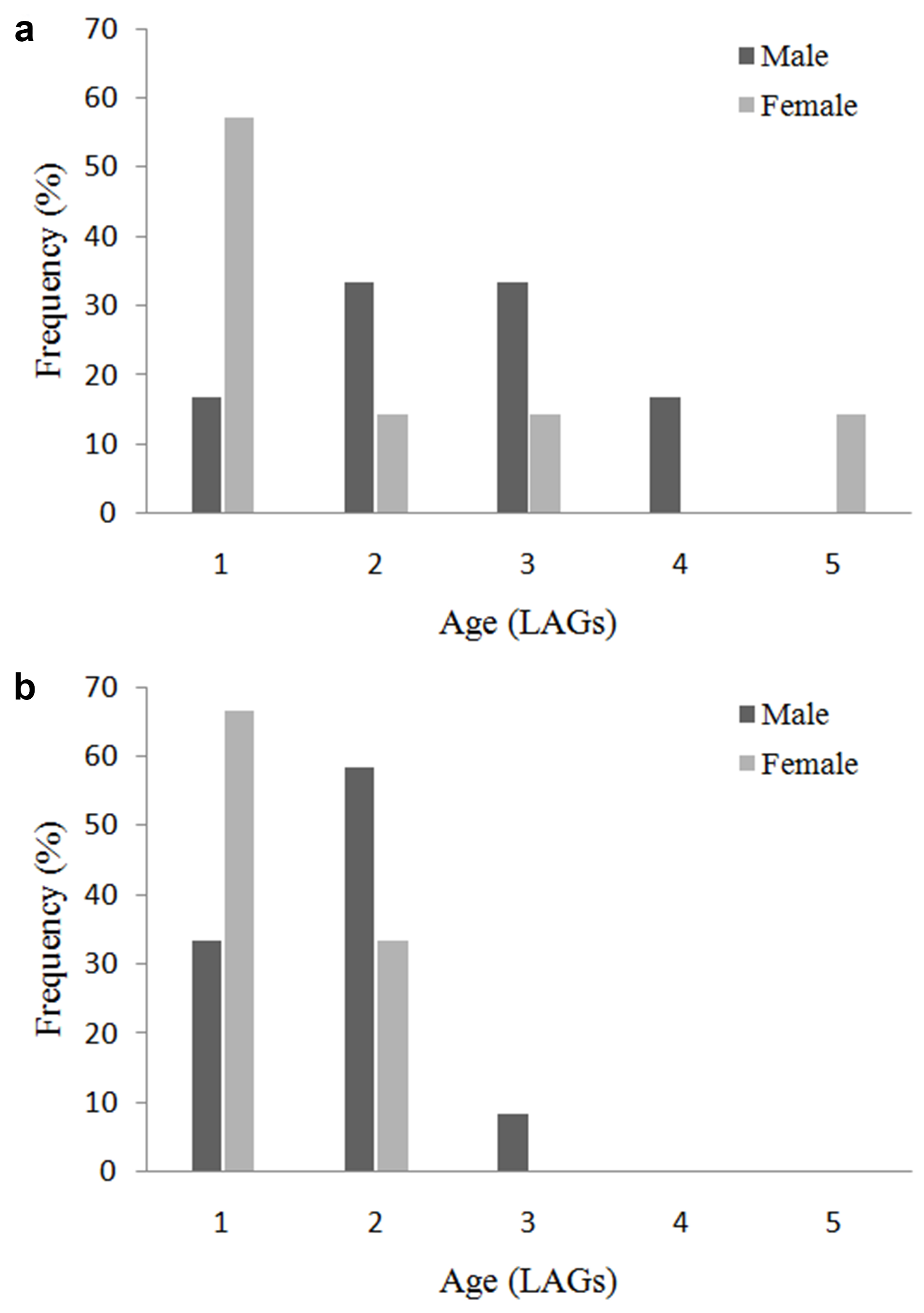
All sections showed recognizable bone structures that allowed age determination. We found well-defined LAGs in the periosteal bone of these sections, and they were relatively easy to count in order to assess individual age (Fig. 2). The descriptive statistics of the studied life history-traits are summarized in Table II, and the age structure is presented in Fig. 3. The minimum number of LAGs found in reproductive individuals was one in both species. One-year old L. latinasus males were on average smaller than one-year old females, whereas in L. bufonius males were bigger than females within the one-year old age class. On average, males were older than females in both species; however, the differences in the median lifespan between sexes were significant only in L. latinasus (Mann-Whitney U test, Z = 2.849, P = 0.004). Longevity in L. latinasus was three years in males and two years in females, while females were older in L. bufonius (five years in females and four years in males). Thus, the difference in the potential reproductive lifespan (PRLS) between sexes was one year in both species. We found no LAGs in the cross sections of juveniles.
Cross sections through a phalanx of Leptodactylus latinasus (a) and L. bufonius (b). An arrowhead indicates the lines of arrested growth (LAGs), medullar cavity (mc), endosteal bone (eb), periosteal bone margin (pbm).
Age-related traits of Leptodactylus latinasus and L. bufonius. AM = age at maturity (i.e. the minimum age in the sample, in LAGs); Longevity = maximum age in the sample (LAGs); PRLS = potential reproductive lifespan (years); Mean and maximum size at AM = mean and maximum snout-vent length of first-year breeders (mm). Sample size is provided in parentheses.± SEageAM ± SD size at AM
GROWTH PATTERNS
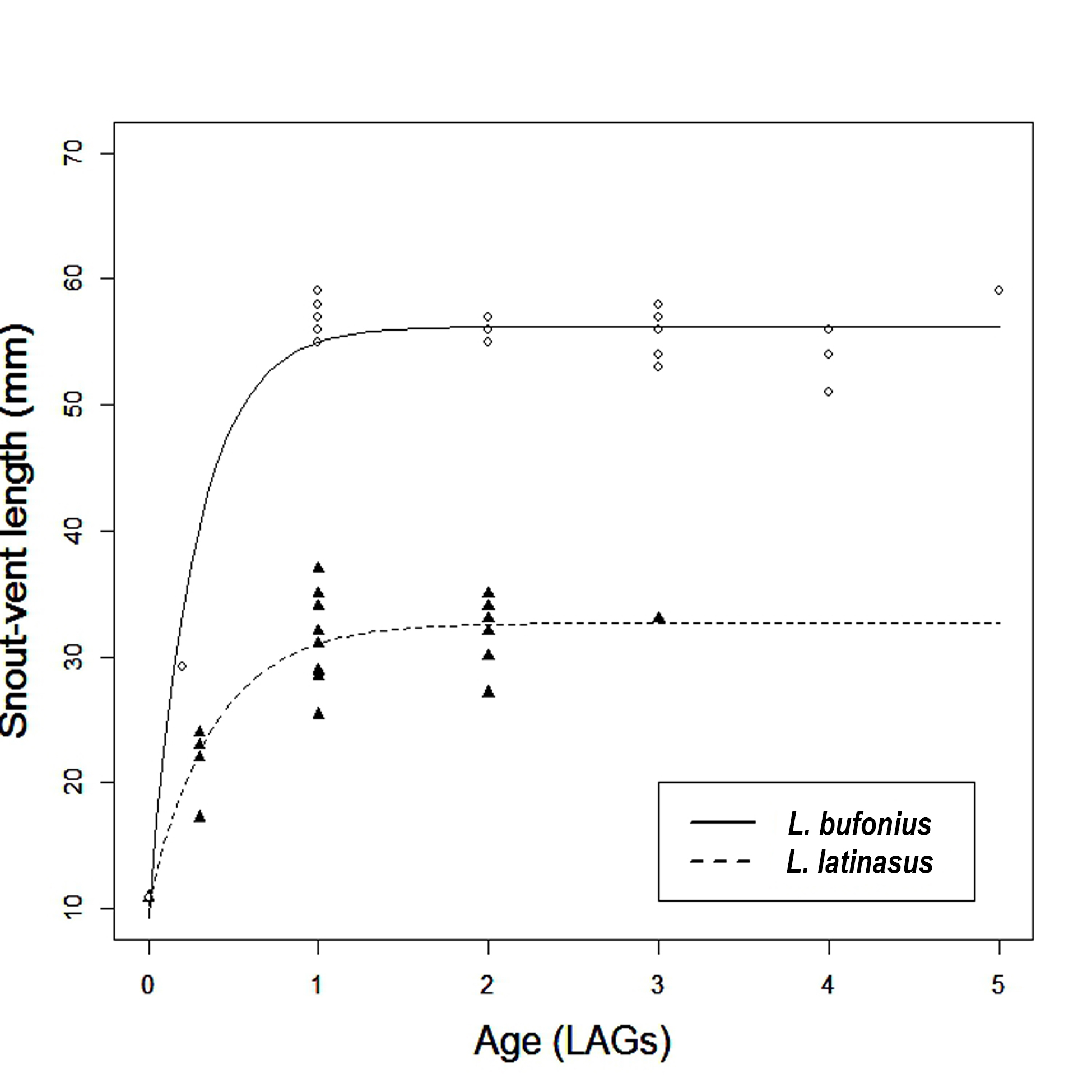
Since MANOVA on all morphometric variables measured showed no significant effects of sex in both species, we computed Von Bertalanffy’s growth model using pooled data of males and females (Fig. 4). The asymptotic average snout-vent length was significantly higher in L. bufonius (SVLmax ± SE = 56.22 ± 0.43, CI 95%= 55.35 – 57.09, K ± SE = 3.62 ± 0.44, CI 95% = 2.71 – 4.52) than in L. latinasus (SVLmax ± SE = 32.68 ± 0.816, CI 95% = 31.00 – 34.36, K ± SE = 2.60 ± 0.55, CI 95% = 1.46 - 3.75). Although we found no significant differences in the growth coefficient, the growth rates during the first year of life, from metamorphosis to sexual maturity, appear to be distinct in the two species: L. latinasus had a mean SVL of only 32.6 mm (n=12 one-year old individuals: four males, eight females), compared to L. bufonius which attained a mean SVL of 56.8 mm in the same age class (n=8 one-year old individuals: four males, four females). In addition, there were significant differences both in the size and age distribution of the two species, L. bufonius being larger (Mann-Whitney U-test, U=50, P<0.001) and having a higher average lifespan compared to L. latinasus (M-W, U= 257, P= 0.006).
Growth patterns in Leptodactylus latinasus (black triangles) and L. bufonius (open circles).
REPRODUCTIVE TRAITS VS FEMALES’ SIZE
The descriptive statistics of the reproductive variables in both species are presented in Table III. Following the differences in body size between species, all reproductive traits were higher in L. bufonius than L. latinasus. However, the relationships among reproductive variables (i.e. ovarian complement, ovarian mass and ovum size), female size (i.e. snout-vent length, body mass) and age in L .latinasus and L. bufonius were statistically non-significant (Table IV). In L. latinasus, the mean OC increased with SVL, BM and age. Similarly, OM increased with BM and age, but the relationship with SVL was negative. OS increased with age, but decreased with SVL and BM. On the other hand, in L. bufonius, OC increased with age, but showed a negative relationship with SVL and BM. Moreover, OM showed a positive relationship with SVL and age, but had a negative relationship with BM. Finally, OS increased significantly with SVL, BM and age.
Reproductive traits of Leptodactylus latinasus and L. bufonius females. SVL = snout-vent length; OM = ovarian mass; OC = ovarian complement; OS = ovum size; RE = reproductive effort; OSF = ovarian size factor. Values are presented as Mean ± SD. Sample size is provided in parentheses.
Relationship between reproductive variables (i.e. ovarian complement, ovarian mass, ovum size), body size (snout-vent length, body mass) and age in Leptodactylus latinasus and L. bufonius females. All variables were log-transformed. All relationships were statistically not significant.
DISCUSSION
INTRASPECIFIC DIFFERENCES IN BODY SIZE AND AGE-RELATED PARAMETERS
Several non-mutually exclusive factors may contribute to SSD in amphibians, such as environmental conditions, phylogeny, genetic drift, or natural and sexual selection (Berven 1982a, bBERVEN KA. 1982b. The genetic basis of altitudinal variation in the frog Rana sylvatica II. An experimental analysis of larval development. Oecologia 52: 360-369., MarangoniMARANGONI F and TEJEDO M. 2008. Variation in body size and metamorphic traits of Iberian spade foot toads over a short geographic distance. J Zool Lond 275: 97-105. and Tejedo 2008, CogălniceanuCOGĂLNICEANU D, ROŞIORU D, SZEKELY P, SZEKELY D, BUHACIUC E, STĂNESCU F and MIAUD C. 2014. Age and body size in populations of two syntopic spadefoot toads (genus Pelobates) at the limit of their ranges. J Herp 48: 537-545. et al. 2014). In most anurans, females are larger than males and in overall this is explained by the positive correlation between female body size and reproductive output (Shine 1979SHINE R. 1979. Sexual selection and sexual dimorphism in the Amphibia. Copeia 1979: 297-306., Duellman and Trueb 1986). However, in some cases males are larger than females or there is no SSD (Shine 1979, Silva et al. 2005, Zina and Haddad 2005). We did not find a significant SSD in the studied L. latinasus and L. bufonius populations, in any of the analyzed morphological variables. However, a female-biased SSD was reported in other populations of L. bufonius (Heyer 1978, ReadingREADING CJ and JOFRÉ GM. 2003. Reproduction in the nest building vizcacheras frog Leptodactylus bufonius in central Argentina. Amphibia-Reptilia 24: 415-427. and Jofré 2003, Schaefer 2007, Faggioni et al. 2017, but see Duré and Kehr 2004) and L. latinasus (Heyer 1978, Duré and Kehr 2004, Schaefer 2007, Ponssa and Barrionuevo 2012, AttademoATTADEMO MA, BIONDA C. PELTZER PM, LAJMANOVICH RC, SEIB SN, BASSO A AND JUNGES CM. 2014. Age, size at sexual maturity, longevity, and reproductive potential of Leptodactylus latinasus and Leptodactylus mystacinus in a soybean crop and a native forest from mideastern Argentina. Rev Mex Biodivers 85: 315-317. et al. 2014), and likewise, in the other 11 species of the genus distributed in Argentina (Appendix B). Regarding species of the Leptodactylus fuscus group, where males construct subterranean chambers, Heyer (1978) hypothesized a relationship between burrowing habits and sexual dimorphism, males having larger heads compared to females (Faggioni et al. 2017). We found no SSD in the head width of either species studied, but our results are similar to those obtained by Ponssa and Barrionuevo (2012).
Several species of Leptodactylus exhibit male combat, a main source that have been widely proposed to explain the existence of sexual size dimorphism in anurans (Shine 1979, BlanckenhornBLANCKENHORN WU. 2000. The evolution of body size: what keeps organisms small? Q Rev Biol 75: 385-407. 2000, Monnet and Cherry 2002), but although we observed male-male interaction in L. bufonius, with the consequent emission of territorial calls (F. Marangoni, personal observation), we never observed male combat in either of the two species studied (F. Marangoni, personal observation). Thus, we suggest that the absence of male combat could be another possible explanation for the absence of sexual size dimorphism in these species.
Variation in age structure promoting considerable variation in adult body size has been widely demonstrated in amphibians (Díaz-Paniagua and Mateo 1999, Miaud et al. 1999, Laugen et al . 2005, Marangoni et al. 2006, 2012, Cajade et al. 2013, QuirogaQUIROGA LN, SANABRIA EA and MARANGONI F. 2015. Sexual size dimorphism and age in Odontophrynus cf. barrioi (Anura: Odontophrynidae) from the Monte Desert, Argentina. J Herpetol 49: 627-632. et al. 2015, Sinsch et al. 2015). In addition, contrasting life-strategies related to growth rates, age and body size at sexual maturity of males versus females can also shape sexual size dimorphism in amphibians (Hemelaar 1988, HallidayHALLIDAY TR and TEJEDO M. 1995. Intrasexual selection and alternative mating behaviour. In: Heatwole H and Sullivan BK (Eds), Amphibian Biology, Social Behaviour, p. 419-468. and Tejedo 1995). We found that sexual maturity was reached after the first year of life in males and females of both species studied, which could also explain the absence of a significant sexual size dimorphism. BassoBASSO NG and KEHR AI. 1991. Postmetamorphic growth and population structure of the frog Leptodactylus latinasus (Anura: Leptodactylidae). Stud Neotrop Fauna E 26: 39-44. and Kehr (1991) also found that L. latinasus attains sexual maturity after the first year of life. Similar age at maturity and longevity (one and five years, respectively) and no SDD was also found in a related species - L. latrans, by LópezLÓPEZ JA, ANTONIAZZI CE, LLANES RE and GHIRARDI R. 2017. Age structure, growth pattern, sexual maturity, and longevity of Leptodactylus latrans (Anura: Leptodactylidae) in temperate wetlands. Amphibia-Reptilia 38: 371-379. et al. (2017) and the authors proposed that the lack of SSD is probably due to the lack of differences in the age structure of males and females, females having only a slightly delayed sexual maturity. In contrast, other studies found that males and females attained sexual maturity after the second year of life in L. bufonius (Reading and Jofré 2003) and L. latinasus (Attademo et al. 2014). Attademo et al. (2014) found that age at maturity and longevity where 3 and 7 years respectively, in L. mystacinus.
INTERSPECIFIC DIFFERENCES IN LIFE-HISTORY TRAITS
The observed differences in adult body size paralleled the differences in age-related parameters (longevity and PRLS), all reproductive traits, and growth pattern in both species. Observed SVL of first-breeders suggest that distinct growth patterns occur before sexual maturity in the two species, L. bufonius achieving a larger body size compared to L. latinasus, during the same amount of time. This pattern is also evident from the SVL-BM relationship, body mass increasing faster with SVL in L. bufonius compared to L. latinasus (Fig. 1). Overall, L. bufonius is larger than L. latinasus, and females have a higher reproductive investment. In addition, the potential reproductive lifespan (PRLS) is also higher in L. bufonius, which increases the potential reproductive success of the species (Halliday and Verrel 1988, Halliday and TejedoHEMELAAR A. 1988. Age, growth and other population characteristics of Bufo bufo from different latitudes and altitudes. J Herpetol 22: 369-388. 1995, Blanckenhorn 2000). Overall, our study indicates that L. bufonius exhibits a more successful life-history strategy and therefore has better chances to displace L. latinasus in competition for resources. However, Duré and Kehr (2004) showed that L. latinasus and L. bufonius exhibit niche complementarity, which means that under satisfactory levels of food and space availability, competition should not be an issue, and thus explaining the coexistence of the two species in syntopy. Furthermore, competition is avoided through spatial segregation between L. latinasus and L. bufonius: for example, although males of both species construct mud nests at the edge of ponds and other low-lying depressions (Heyer 1978, Cei 1980), there are subtle differences in their microhabitat preferences and reproductive behavior (see CrumpCRUMP ML. 1995. Leptodactylus bufonius (NCN). Reproduction. Herpetol Rev 26: 97-98. 1995), L. latinasus being usually associated to crevices in the ground, while L. bufonius constructs cone-shaped nests at the edge of the ponds (Shoemaker and McClanahan 1973SHOEMAKER VH and MCCLANAHAN LL. 1973.Nitrogen excretion in the larvae of a land nesting frog (Leptodactylus bufonius).Comp Biochem Physiol 44A: 1149-1156., Crump 1995, F. Marangoni, personal observation during present study). Since both species use the same ponds for breeding, at the same time, interspecific interaction is most likely to occur during larval stages. However, little is known regarding the length of larval development, dietary requirements, foraging behavior of the tadpoles, or size at metamorphosis. Hence, studies regarding growth, diet and foraging behavior during early-stages of life in both species are required to fully understand the mechanisms that shape their life-histories and allow their coexistence.
ACKNOWLEGMENTS
We are grateful to V. I. Gomez for invaluable help during fieldwork. We acknowledge L. Rossi and A. Ibañez for their assistance in the sample processing. We acknowledge M. Sanchez-Negrette and M. Montenegro for providing the corresponding permits to use the cryostat microtome at the Cátedra de Patología General y Sistemática and Cátedra de Histología y Embriología, Facultad de Ciencias Veterinarias, Universidad Nacional del Nordeste. We thank D. Baldo for acces to the database of amphibian collections of the Laboratorio de Genética Evolutiva del Instituto de Biología Subtropical (CONICET-UNaM). We thank J. J. Neiff for providing the Microscopy Laboratory at the Centro de Ecología Aplicada del Litoral (CECOAL, CONICET // UNNE). We thank also H. Duarte, a native speaker, for correcting the English draft of this manuscript. D. Cogălniceanu reviewed earlier versions of the manuscript and provided useful comments that helped improve it. The authors have complied with all applicable Institutional Animal Careguide lines. The collecting permit was granted by Dirección de Recursos Naturales of the Corrientes province, Argentina. This project was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina, through fellowships to F. Marangoni. We are very grateful for the continuous support of the CONICET and the Facultad de Ciencias Exactas y Naturales y Agrimensura, Universidad Nacional del Nordeste (FACENA, UNNE).
REFERENCES
- ATTADEMO MA, BIONDA C. PELTZER PM, LAJMANOVICH RC, SEIB SN, BASSO A AND JUNGES CM. 2014. Age, size at sexual maturity, longevity, and reproductive potential of Leptodactylus latinasus and Leptodactylus mystacinus in a soybean crop and a native forest from mideastern Argentina. Rev Mex Biodivers 85: 315-317.
- BASSO NG. 1990. Estrategias adaptativas en una comunidad subtropical de anuros. Cuad Herpetol (AHA) 1: 1-71.
- BASSO NG and KEHR AI. 1991. Postmetamorphic growth and population structure of the frog Leptodactylus latinasus (Anura: Leptodactylidae). Stud Neotrop Fauna E 26: 39-44.
- BATY F, RITZ C, CHARLES S, BRUTSCHE M, FLANDROIS JP and DELIGNETTE-MULLER ML. 2015. A Toolbox for Nonlinear Regression in R: The Package nlstools. J StatSoftw 66: 1-21.
- BEGON M, HARPER JL and TOWNSEND CR. 1990. Ecology, individuals, populations and communities. London: Blackwell Scientific Publications, 945 p.
- BERNARDO J. 1996. Maternal effects in animal ecology. Am Zool 36: 83-105.
- BERTALANFFY L VON. 1938. A quantitative theory of organic growth. Hum Biol 10: 181-213.
- BERVEN KA. 1981. Mate choice in the wood frog, Rana sylvatica. Evolution 35: 707-722.
- BERVEN KA. 1982a. The genetic basis of altitudinal variation in the wood frog Rana sylvatica. I. An experimental analysis of life history traits. Evolution 36: 962-983.
- BERVEN KA. 1982b. The genetic basis of altitudinal variation in the frog Rana sylvatica II. An experimental analysis of larval development. Oecologia 52: 360-369.
- BEVERTON RJH and HOLT SJ. 1957. On the dynamics of exploited fish populations. London: Fishery Invest Ser II, Vol. XIX, Ministry of Agriculture, Fisheries, and Food, 533 p.
- BLANCKENHORN WU. 2000. The evolution of body size: what keeps organisms small? Q Rev Biol 75: 385-407.
- BOULENGER GA. 1894. XXXVIII.-List of reptiles and batrachians collected by Dr. J. Bohls near Asuncion, Paraguay. J Nat Hist 13: 342-348.
- BROWN JH, MARQUET PA and TAPER ML. 1993. Evolution of body size: consequences of an energetic definition of fitness. Am Nat 142: 573-584.
- CABRERA A and WILLINK A. 1980. Biogeografía de América Latina (Latin America Biogeography).Washington: Secretaría General de la Organización de los Estados Americanos, 122 p.
- CAJADE R, MARANGONI F and GANGENOVA E. 2013. Age, body size and growth pattern of Argenteohyla siemersi pederseni (Anura: Hylidae) in northeastern Argentina. J Nat Hist 47: 237-251.
- CALDER WA. 1984. Size, function and life history. Boston: Harvard University Press, 431 p.
- CARNEVALI R. 1994. Fitogeografía de la provincia de Corrientes. Corrientes: Gobierno de la Provincia de Corrientes, 324 p.
- CASTANET J. 1982. Recherches sur la croissance du tissu osseux des reptiles. Application: la méthode squelettochronologique. Thèse de Doctorat d’État, Paris.
- CASTANET J and SIMIRINA E. 1990. Introduction to the skeletochronological method in amphibians and reptiles. Ann Sci Nat Zool Paris 11: 191-196.
- CEI JM. 1980. Amphibians of Argentina. Monitore Zoologico Italiano n.s. Monografia 2: 609.
- COGĂLNICEANU D, ROŞIORU D, SZEKELY P, SZEKELY D, BUHACIUC E, STĂNESCU F and MIAUD C. 2014. Age and body size in populations of two syntopic spadefoot toads (genus Pelobates) at the limit of their ranges. J Herp 48: 537-545.
- CRUMP ML. 1974. Reproductive strategies in a tropical anuran community. Miscellaneous publication, University of Kansas, Museum of Natural History 61: 1-68.
- CRUMP ML. 1995. Leptodactylus bufonius (NCN). Reproduction. Herpetol Rev 26: 97-98.
- DE-CARVALHO CB, FREITAS EB, FARIA RG, BATISTA RC, BATISTA CC, COELHO WA and BOCCHIGLIERI A. 2008. História natural de Leptodactylus mystacinus e Leptodactylus fuscus (Anura: Leptodactylidae) no Cerrado do Brasil Central. Biota Neotrop 8: 105-115.
- DE LA ESPADA JM. 1875. Vertebrados del viaje al Pacifico verificado de 1862 a 1865 por una comision de naturalistas enviada por el gobierno Español. Batracios. Madrid, 208 p.
- DE SÁ RO, GRANT T, CAMARGO A, HEYER RW, PONSSA ML and STANLEY E. 2014. Systematics of the Neotropical genus Leptodactylus Fitzinger, 1826 (Anura: Leptodactylidae): phylogeny, the relevance of non-molecular evidence, and species accounts. S Am J Herpetol 9: S1-S128.
- DÍAZ-PANIAGUA C and MATEO JA. 1999. Geographic variation in body size and life-history traits in Bosca’s newt (Triturusboscai). Herpetol J 9: 21-27.
- DUELLMAN WE. 1970. The hylid frogs of Middle America. Monographs, Museum of Natural History, University of Kansas 1: 1-753.
- DUELLMAN WE and CRUMP ML. 1974. Speciation in frogs of the Hyla parviceps group in the upper Amazon Basin. Occas. Pap Mus Nat Hist Univ Kansas 23: 1-40.
- DUELLMAN WE and TRUEB L. 1986. Biology of Amphibians. New York: MacGraw-Hill, 670 p.
- DURÉ M and KEHR A. 2004. Influence of microhabitat on the trophic ecology of two leptodactylids from northeastern Argentina. Herpetologica 60: 295-303.
- FAGGIONI G, SOUZA F, UETANABARO M, LANDGREF-FILHO P, FURMAN J and PRADO C. 2017. Reproductive biology of the nest building vizcacheras frog Leptodactylus bufonius (Amphibia, Anura, Leptodactylidae), including a description of unusual courtship behaviour. Herpetol J 27: 73-80.
- FITZINGER LJ. 1826. Neue Classification der Reptilien nach ihren Natürlichen Verwandtschaften nebst einer Verwandtschafts - Tafel und einem Verzeichnisse der Reptilien - Sammlung des K.K. Zoologischen Museums zu Wien. J.G. Heubner, Wien.
- GIARETTA AA and KOKUBUM MN DE C. 2003. Reproductive ecology of Leptodactylus furnarius Sazima & Bokermann, 1978, a frog that lays eggs in underground chambers (Anura: Leptodactylidae). Herpetozoa 16: 115-126.
- GIBBONS MM and MCCARTHY TK. 1986. The reproductive output of frogs Rana temporaria (L.) with particular reference to body size and age. J Herpetol 209: 579-593.
- HALLIDAY TR and TEJEDO M. 1995. Intrasexual selection and alternative mating behaviour. In: Heatwole H and Sullivan BK (Eds), Amphibian Biology, Social Behaviour, p. 419-468.
- HALLIDAY TR and VERRELL P. 1988. Body size and age in amphibians and reptiles. J Herpetol 22: 253-265.
- HECTOR KL and NAKAGAWA S. 2012. Quantitative analysis of compensatory and catch-up growth in diverse taxa. J Anim Ecol 81: 583-593.
- HEMELAAR A. 1985. An improved method to estimate the number of year rings resorbed in phalanges of Bufo bufo (L.) and its implications to populations from different latitudes. Amphibia-Reptilia 6: 323-341.
- HEMELAAR A. 1988. Age, growth and other population characteristics of Bufo bufo from different latitudes and altitudes. J Herpetol 22: 369-388.
- HEYER WR. 1978. Systematics of the fuscus group of the frog genus Leptodactylus (Amphibia, Leptodactylidae). Nat Hist Mus LA 29: 1-85.
- HOWARD RD. 1980. Mating behaviour and mating success in wood frogs Rana sylvatica. Anim Behav 28: 705-716.
- KAPLAN RH. 1998. Maternal effects, developmental plasticity, and life history evolution. In: Mousseau TE and Fox CW (Eds), Maternal effects as adaptations. New York: Oxford University Press, New York, USA, p. 244-260.
- LAUGEN AT, LAURILA A, JÖNSSON KI, SÖDERMAN F and MERILÄ J. 2005. Do common frogs (Rana temporaria) follow Bergmann’s rule? Evol Ecol Res 7: 717-731.
- LAVILLA E, HEYER R, KWET A and LANGONE J. 2004. Leptodactylus latinasus. The IUCN Red List of Threatened Species 2004: e.T57139A11590252. http://dx.doi.org/10.2305/IUCN.UK.2004.RLTS.T57139A11590252.en.
- LESKOVAR C, OROMI N, SANUY D and SINSCH U. 2006. Demographic life history traits of reproductive Natterjack Toads (Bufocalamita) vary between northern and southern latitudes. Amphibia-Reptilia 27: 365-375.
- LÓPEZ JA, ANTONIAZZI CE, LLANES RE and GHIRARDI R. 2017. Age structure, growth pattern, sexual maturity, and longevity of Leptodactylus latrans (Anura: Leptodactylidae) in temperate wetlands. Amphibia-Reptilia 38: 371-379.
- LOVICH JE and GIBBONS JW. 1992. A review of techniques for quantifying sexual size dimorphism. Growth Develop Aging 56: 269-281.
- LUCAS EM, BRASILEIRO CA, OYAMAGUCHI HM and MARTINS M. 2008. The reproductive ecology of Leptodactylus fuscus (Anura, Leptodactylidae): new data from natural temporary ponds in the Brazilian Cerrado and a review throughout its distribution. J Nat Hist 42: 2305-2320.
- MACARTHUR R and LEVIN SR. 1967. The limiting similarity, convergence and divergence of coexisting species. Am Nat 101: 377-385.
- MACARTHUR RH. 1970. Species packing and competitive equilibrium for many species. Theor Popul Biol 1: 1-11.
- MARAGNO FP and CECHIN SZ. 2009. Reproductive biology of Leptodactylus fuscus (Anura, Leptodactylidae) in the subtropical climate, Rio Grande do Sul, Brazil. Iheringia Sér Zool 99: 237-241.
- MARANGONI F. 2006. Variación clinal en el tamaño del cuerpo a escala microgeográfica en dos especies de anuros (Pelobates cultripes y Bufo calamita). PhD thesis, Sevilla, Spain, Universidad de Sevilla, 298 p.
- MARANGONI F, BARRASSO DA, CAJADE R and AGOSTINI G. 2012. Body size, age and growth pattern of Physalaemus fernandezae (Anura: Leiuperidae) of Argentina. NW J Zool 8: 63-71.
- MARANGONI F, SCHAEFER EF, CAJADE R and TEJEDO M. 2009. Growth marks formation and chronology of two neotropical anuran species. J Herpetol 43: 446-450.
- MARANGONI F and TEJEDO M. 2008. Variation in body size and metamorphic traits of Iberian spade foot toads over a short geographic distance. J Zool Lond 275: 97-105.
- MARTINS M. 1988. Biología reprodutiva de Leptodactylus fuscus em BoaVista, Roraima (Amphinia: Anura). Rev Bras Biol 48: 969-977.
- MEDIACYBERNETICS. 1993-1994. Image-Pro Plus. Version 4.5.0.29. Media Cybernetics, Silver Spring, Maryland, USA.
- METCALFE NB and MONAGHAN P. 2003. Growth versus lifespan: perspectives from evolutionary ecology. Exp Gerontol 38: 935-940.
- MIAUD C, GUYETANT R and ELMBERG J. 1999. Variations in life-history traits in the common frog Rana temporaria (Amphibia, Anura): a literature review and new data from the French Alps. J Zool 249: 61-73.
- MONNET JM and CHERRY MI. 2002. Sexual size dimorphism in anurans. P Roy Soc Lond B Bio 269: 2301-2307.
- OGLE DH. 2018. FSA: Fisheries Stock Analysis. R package version 0.8.18.
- OLIVEIRA FILHO JC and GIARETTA AA. 2008. Reproductive behavior of Leptodactylus mystacinus (Anura, Leptodactylidae) with notes on courtship call of other Leptodactylus species. Iheringia Sér Zool 98: 508-515.
- PIANKA ER. 1975. Niche relations of desert lizards. In: Cody ML and Diamond JM (Eds), Ecology and Evolution of Communities. Boston: Harvard University Press, Massachusetts, USA, p. 292-314.
- PONSSA ML and BARRIONUEVO JS. 2012. Sexual dimorphism in Leptodactylus latinasus (Anura, Leptodactylidae): nasal capsule anatomy, morphometric characters and performance associated with burrowing behavior. Acta Zool-Stockholm 93: 57-67.
- PRADO CPA, UETANABARO M and LOPES FS. 2000. Reproductive strategies of Leptodactylus chaquensis and L. podicipinus in the Pantanal, Brazil. J Herpetol 34: 135-139.
- QUIROGA LN, SANABRIA EA and MARANGONI F. 2015. Sexual size dimorphism and age in Odontophrynus cf. barrioi (Anura: Odontophrynidae) from the Monte Desert, Argentina. J Herpetol 49: 627-632.
- RÄSÄNEN K, LAURILA A and MERILÄ J. 2003. Geographic variation in acid stress tolerance of the moor frog, Rana arvalis. II. Adaptive maternal effects. Evolution 57: 363-371.
- RÄSÄNEN K, LAURILA A and MERILÄ J. 2005. Maternal investment in egg size: environment- and population-specific effects on offspring performance. Oecologia 142: 546-553.
- READING CJ and JOFRÉ GM. 2003. Reproduction in the nest building vizcacheras frog Leptodactylus bufonius in central Argentina. Amphibia-Reptilia 24: 415-427.
- RODRIGUES DJ, UETANABARO M and PRADO CPA. 2004. Seasonaland ontogenetic variation in diet composition of Leptodactylus podicipinus (Anura, Leptodactylidae) in the southern Pantanal, Brazil. Rev Esp Herpetol 2004: 19-28.
- ROFF D. 1993.The evolution of life histories. New York: Chapman and Hall, 535 p.
- SAGOR ES, OULLET M, BARTEN E and GREEN DM. 1998. Skeletochronology and geographic variation in age structure in the wood Frog, Rana sylvatica. J Herpetol 34: 469-474.
- SCHAEFER EF. 2007. Restricciones cuantitativas asociadas con los modos reproductivos de los anfibios en áreas de impacto por la actividad arrocera en la provincia de Corrientes. PhD thesis, La Plata, Buenos Aires, Argentina, Universidad Nacional de La Plata.
- SCHAEFER EF, HAMANN MI, KEHR AI, GONZÁLEZ CE and DURÉ MI. 2006. Trophic, reproductive and parasitological aspects of the ecology of Leptodactylus chaquensis (Anura: Leptodactylidae) in Argentina. Herpetol J 16: 387-394.
- SCHMIDT-NIELSEN K. 1984. Scaling. Why is animal size so important? New York: Cambridge University Press, 241 p.
- SHINE R. 1979. Sexual selection and sexual dimorphism in the Amphibia. Copeia 1979: 297-306.
- SHOEMAKER VH and MCCLANAHAN LL. 1973.Nitrogen excretion in the larvae of a land nesting frog (Leptodactylus bufonius).Comp Biochem Physiol 44A: 1149-1156.
- SILVA WR, GIARETTA AA and FACURE KG. 2005. On the natural history of the South American pepper frog, Leptodactylus labyrinthicus (Spix, 1824) (Anura: Leptodactylidae). J Nat Hist 39: 555-566.
- SINSCH U. 2015. Skeletochronological assessment of demographic life-history traits in amphibians. Herpetol J 25: 5-13.
- SINSCH U, OROMI N and SANUY D. 2007. Growth marks in Natterjack Toad (Bufocalamita) bones: histological correlates of hibernation and aestivation periods. Herpetol J 17: 129-137.
- SINSCH U, PELSTER B and LUDWIG G. 2015. Large‐scale variation of size‐and age‐related life‐history traits in the common frog: a sensitive test case for macroecological rules. J Zool 297: 32-43.
- SMIRINA EM. 1972. Annual layers in bones of Rana temporaria. Zool Zh 51: 1529-1534.
- STATSOFT. 2001. Statistica (Data analysis software system).Version 6. StatSoft, Tulsa.
- STEARNS SC. 1992. The evolution of life histories. Oxford: Oxford University Press, 249 p.
- STEARNS SC. 2000. Life history evolution: successes, limitations, and prospects. Naturwissenschaften 87: 476-486.
- TEJEDO M. 1988. Fighting for females in the toad Bufo calamita is affected by the operational sex ratio. Anim Behav 36: 1765-1769.
- TEJEDO M, REQUES R and ESTEBAN M. 1997. Actual and osteochronological estimated age of natterjack toads (Bufo calamita). Herpetol J 7: 81-82.
- TEJEDO M, SEMLITSCH RD and HOTZ H. 2000. Covariation of morphology and jumping performance in newly metamorphosed water frogs: effects of larval growth history. Copeia 2000: 448-458.
- TOFT CA. 1980. Feeding ecology of thirteen syntopic species of anurans in a seasonal tropical environment. Oecologia 45: 131-141.
- TOFT CA. 1981. Feeding ecology of Panamanian litter anurans: patterns in diet and foraging mode. J Herpetol 15: 139-144.
- VAIRA M et al. 2012. Categorización del estado de conservación de los anfibios de la República Argentina. Cuad Herpetol (AHA) 26: 131-159.
- VERA MC and PONSSA ML. 2014. Skeletogenesis in anurans: cranial and postcranial development in metamorphic and postmetamorphic stages of Leptodactylus bufonius (Anura: Leptodactylidae). Acta Zool-Stockholm 95: 44-62.
- ZINA J and HADDAD CFB. 2005. Reproductive activity and vocalizations of Leptodactylus labyrinthicus (Anura: Leptodactylidae) in southeastern Brazil. Biota Neotrop 5: 1-11.
APPENDIX A
Species, location and registration numbers of all individuals deposited and measured at the Collection of the Laboratorio de Genética Evolutiva, Instituto de Biología Subtropical (CONICET-UNaM), Posadas, Misiones, Argentina (LGE):
L. bufonius: Charata, Chaco: 05226, 05235, 05236, 05247, 05248, 05249, 05250, 05251, 05252, 05253, 05254. Perichón, Corrientes: 20058, 20059, 20060, 20061, 20062, 20063, 20064, 20065, 20066, 20067, 20068, 20069, 20070, 20071, 12163, 12947, 12948, 12949, 12950, 12951, 13330, 13419, 13437, 13438, 13439, 13440. Fuerte Esperanza, Chaco: 13006, 05863, 05864, 05865, 05866, 05867, 05868, 05869, 05870, 05871, 05872, 05873, 05874, 05875, 05876, 05877, 05878, 05879, 05880, 05881, 05882, 05883, 05886, 05887, 05888, 05889, 05890, 05891, 05892, 05893, 05894, 05895, 05896, 05897, 05898, 05899, 13022, 13078, 13079, 13317, 13370, 13371, 13373, 13405, 50898, 5899.
L. latinasus: Perichón, Corrientes: 20072, 20073, 20074, 20075, 20076, 20077, 20078, 20079, 20080, 20081, 20082, 20083, 20084, 20085, 20086, 20087.
L. laticeps: Chaco, Formosa: 12083, 12084, 12100, 12101, 12150, 12164, 15282, 15283, 15284, 15285, 15286, 15287, 15289, 15290, 15291, 15292, 15293, 15294.
L. furnarius: Corrientes and Misiones: 03438, 03439, 03493, 03666, 03867, 04119, 04163, 04694, 07889,12854, 12855, 12856, 12857.
L. plaumanni: Misiones: 02067, 03373, 03374, 03375, 03386, 03427, 03430, 03431, 03537, 03543, 03545, 03546, 03556, 03557, 03929, 03930, 04243, 04244, 04823, 05086, 05104, 07034, 07077, 09662, 09663, 09664, 09665, 09666, 09667, 09668, 09669, 09672, 09673, 09674, 09675, 19927.
APPENDIX B
Average SVL of males and females of the 13 species of the genus Leptodactylus and the SDI computed for each population. # = species belong to the L. fuscus group. LGE = Collection of the Laboratorio de Genética Evolutiva, Instituto de Biología Subtropical (CONICET-UNaM), Posadas, Misiones, Argentina. Mean ± SD. n=sample size. Significant differences in SVL between males and females (P< 0.05) are marked with *;▲= not analyzed.| Species | Country | Coordinates | Males SVL | Females SVL | SDI | Source |
|---|---|---|---|---|---|---|
| L. bufonius # | Argentina | 29°48’S, 64°43’W | 56.40 ± 2.58 (62) | 60.00 ± 2.87 (53) | 1.06* | Reading and Jofré 2003 |
| L. bufonius # | Several | Several | 51.60 ± 2.0 | 53.60 ± 2.3 | 1.04* | Heyer 1978 |
| L. bufonius # | Argentina | 27°17’34.8” S, 61°09’01.4” W | 46.68 ± 1.02 (9) | 45.69 ± 1.76 (3) | -1.02 | LGE |
| L. bufonius # | Argentina | 25°04’39.24” S, 61°37’52.33” W | 53.26 ± 3.05 (36) | 59.87 ± 3.64 (10) | 1.12* | LGE |
| L. bufonius # | Argentina | 27°30’ S, 58°45’W | 44.20 ± 4.2(12) | 43.80 ± 6.9 (8) | -1.01 | Duré and Kehr 2004 |
| L. bufonius # | Argentina | 27°26’ S, 58°44’ W | 46.10 ± 1.94 (11) | 47.90 ± 2.32 (11) | 1.04 | Schaefer 2007SCHAEFER EF. 2007. Restricciones cuantitativas asociadas con los modos reproductivos de los anfibios en áreas de impacto por la actividad arrocera en la provincia de Corrientes. PhD thesis, La Plata, Buenos Aires, Argentina, Universidad Nacional de La Plata. |
| L. bufonius # | Argentina | 27°25’53.2” S, 58°44’44.8” W | 55.30 ± 1.8 (56) | 56.33 ± 2 (9) | 1.02 | Present study |
| L. bufonius # | Brazil | 21º42’39’’ S, 57º43’16’’ W | 46.03 ± 2.91 (25) | 47.66 ± 3.11 (31) | 1.04* | Faggioni et al. 2017 |
| L. chaquensis | Brazil | 57°00’ W, 19°34’ S | 71.34 ± 5.11 (34) | 71.31 ± 4.51 (50) | -1.00 | Prado et al. 2000PRADO CPA, UETANABARO M and LOPES FS. 2000. Reproductive strategies of Leptodactylus chaquensis and L. podicipinus in the Pantanal, Brazil. J Herpetol 34: 135-139. |
| L. chaquensis | Argentina | 27°30’ S, 58°45’ W | 62.90 ± 5.43 (21) | 65.30 ± 7.82 (14) | 1.04 | Schaefer et al. 2006SCHAEFER EF, HAMANN MI, KEHR AI, GONZÁLEZ CE and DURÉ MI. 2006. Trophic, reproductive and parasitological aspects of the ecology of Leptodactylus chaquensis (Anura: Leptodactylidae) in Argentina. Herpetol J 16: 387-394. |
| L. chaquensis | Argentina | 27° 26’ S, 58°44’ W | 63.00 ± 5.45 (27) | 61.38 ± 7.67 (35) | -1.03 | Schaefer 2007 |
| L. elenae # | Several | Several | 42.70 ± 2.5 | 42.80 ± 3.1 | 1.00* | Heyer 1978 |
| L. furnarius | Brazil | 18°55’ S, 48°17’ W | 38.00 ± 1.13 (19) | 42.40 ± 1.58 (52) | 1.12* | Giaretta and Kokubum 2003GIARETTA AA and KOKUBUM MN DE C. 2003. Reproductive ecology of Leptodactylus furnarius Sazima & Bokermann, 1978, a frog that lays eggs in underground chambers (Anura: Leptodactylidae). Herpetozoa 16: 115-126. |
| L. furnarius | Argentina | Several | 36.62 ± 1.70 (11) | 41.79 ± (2) | 1.14▲ | LGE |
| L. fuscus | Brazil | - | 43.60 ± 2.4 (135) | 45.60 ± 2.2 (13) | 1.05* | Lucas et al. 2008LUCAS EM, BRASILEIRO CA, OYAMAGUCHI HM and MARTINS M. 2008. The reproductive ecology of Leptodactylus fuscus (Anura, Leptodactylidae): new data from natural temporary ponds in the Brazilian Cerrado and a review throughout its distribution. J Nat Hist 42: 2305-2320. |
| L. fuscus | Brazil | - | 43.00 (39.9-46.8) (28) | 43.70 (41.9-46.3) (28) | 1.02 | Maragno and Cechin 2009MARAGNO FP and CECHIN SZ. 2009. Reproductive biology of Leptodactylus fuscus (Anura, Leptodactylidae) in the subtropical climate, Rio Grande do Sul, Brazil. Iheringia Sér Zool 99: 237-241. |
| L. fuscus | Brazil | 2°48’ N, 60°12’ W | 36.20 ± 1.3 (25) | 39.50 ± 1.3 (25) | 1.09* | Martins 1988MARTINS M. 1988. Biología reprodutiva de Leptodactylus fuscus em BoaVista, Roraima (Amphinia: Anura). Rev Bras Biol 48: 969-977. |
| L. fuscus | Brazil | 16°13’50” S, 48°04’49” W | 46.90 ± 2.7 (13) | 49.30 ± 2.6 (6) | 1.05▲ | De-Carvalho et al. 2008DE-CARVALHO CB, FREITAS EB, FARIA RG, BATISTA RC, BATISTA CC, COELHO WA and BOCCHIGLIERI A. 2008. História natural de Leptodactylus mystacinus e Leptodactylus fuscus (Anura: Leptodactylidae) no Cerrado do Brasil Central. Biota Neotrop 8: 105-115. |
| L. gracilis # | Several | Several | 43.00 ± 4.8 | 43.00 ± 3.7 | 1.00 | Heyer 1978 |
| L. labyrinthicus | Brazil | 18°55’ S, 48°17’ W | 136.50 ± 17.2 (16) | 127.30 ± 12.7 (12) | -1.07 | Silva et al. 2005SILVA WR, GIARETTA AA and FACURE KG. 2005. On the natural history of the South American pepper frog, Leptodactylus labyrinthicus (Spix, 1824) (Anura: Leptodactylidae). J Nat Hist 39: 555-566. |
| L. labyrinthicus | Brazil | 22°15’ S, 47°49’ W | 170.00 ± 18.9 (5) | 157.00 ± 10.4 (5) | -1.08 | Zina and Haddad 2005ZINA J and HADDAD CFB. 2005. Reproductive activity and vocalizations of Leptodactylus labyrinthicus (Anura: Leptodactylidae) in southeastern Brazil. Biota Neotrop 5: 1-11. |
| L. labyrinthicus | Brazil | 22°16’ S, 47°42’ W | 152.30 ± 10.6 (10) | 155.00 ± 12.3 (8) | 1.02 | Zina and Haddad 2005 |
| L. laticeps | Argentina | Several | 94.61± 3.00 (8) | 99.32 ± 10.2 (10) | 1.05 | LGE |
| L. latinasus | Several | Several | 31.20 ± 1.7 | 33.00 ± 1.9 | 1.06* | Heyer 1978 |
| L. latinasus | Argentina | - | 30.30 ± 0.9 (7) | 32.20 ± 2.2 (6) | 1.06▲ | Ponssa and Barrionuevo 2012PONSSA ML and BARRIONUEVO JS. 2012. Sexual dimorphism in Leptodactylus latinasus (Anura, Leptodactylidae): nasal capsule anatomy, morphometric characters and performance associated with burrowing behavior. Acta Zool-Stockholm 93: 57-67. |
| L. latinasus | Argentina | 30°00’10.83’’ S 57°22’31.61’’ W | 30.43 ± 1.67 (21) | 32.59 ± 1.65 (19) | 1.07 | R. Cajade and J.M. Piñeiro, unpublished data |
| L. latinasus | Argentina | 27°26’S, 58°44’W | 28.35 ± 1.62 (60) | 29.30 ± 1.92 (50) | 1.03* | Schaefer 2007 |
| L. latinasus | Argentina | 27°3’S, 58°45’W | 27.76 ± 2.2 (43) | 28.50 ± 2.4 (27) | 1.02 | Duré and Kehr 2004 |
| L. latinasus | Argentina | 27°25’53.2’’S, 58°44’44.8’’ W | 32.38 ± 2.94 (34) | 33.02 ± 3.15 (17) | 1.02 | Present study |
| L. latrans | Argentina | Several | 65.41 ± 28.84 (94) | 63.59 ± 26.72 (89) | -1.03 | López et al. 2017 |
| L. mystacinus # | Several | Several | 53.00 ± 4.6 | 56.50 ± 2.7 | 1.07* | Heyer 1978 |
| L. mystacinus # | Brazil | 16°13’50” S, 48°04’49” W | 55.80 ± 2.2 (17) | 60.80 ± 5.5 (18) | 1.09▲ | De-Carvalho et al. 2008 |
| L. mystacinus # | Brazil | - | 52.90 ± 2.8 (7) | 57.90 ± 3.1 (6) | 1.09* | Oliveira Filho and Giaretta 2008OLIVEIRA FILHO JC and GIARETTA AA. 2008. Reproductive behavior of Leptodactylus mystacinus (Anura, Leptodactylidae) with notes on courtship call of other Leptodactylus species. Iheringia Sér Zool 98: 508-515. |
| L. plaumanni | Argentina | 26°13’15.6” S, 53°49’16.2” W | 38.64 ± 2.57 (27) | 41.78 ± 2.42 (9) | 1.08* | LGE |
| L. podicipinus | Brazil | 19°34’ S, 57°00’ W | 35.19 ± 1.34 (21) | 39.47 ± 2.13 (36) | 1.12* | Prado et al. 2000 |
| L. podicipinus | Brazil | 19°34’ S, 57°00’ W | 32.20 ± 3.4 (55) | 38.00 ± 3.7 (53) | 1.18▲ | Rodrigues et al. 2004RODRIGUES DJ, UETANABARO M and PRADO CPA. 2004. Seasonaland ontogenetic variation in diet composition of Leptodactylus podicipinus (Anura, Leptodactylidae) in the southern Pantanal, Brazil. Rev Esp Herpetol 2004: 19-28. |
Publication Dates
-
Publication in this collection
29 July 2019 -
Date of issue
2019
History
-
Received
23 May 2018 -
Accepted
25 Aug 2018