Abstract
Abstract: The use of ulinastatin for pancreatitis and sepsis have been described. This study was designed to evaluate the effect of ulinastatin on vascular endothelial cell damage and coagulation in pregnant women with severe pre-eclampsia (PE).From October 2015 to November 2017 at Tianjin Central Hospital of gynecology and obstetrics in China. Eighty pregnant women with severe PE, who elected to deliver by cesarean section, were randomly assigned to a control group or an ulinastatin group. The plasma concentration of von Willebrand factor (vWF) and platelet granule membrane protein (GMP-140), platelet count, fibrinogen levels, prothrombin time (PT), and partial prothrombin activation time (APTT) were recorded before combined spinal-epidural anesthesia and 40 min after administration in both groups.Ulinastatin attenuates vascular endothelial cell damage in pregnant women with PE as indicated by decreased plasma concentrations of vWF and prolonged APTT.
Key words
Ulinastatin; Pre-eclampsia; Blood protection; vWF
INTRODUCTION
Pre-eclampsia (PE) is defined as new-onset hypertension (or, worsening hypertension in patients with pre-existing hypertension) occurring after 20 weeks of gestation, combined with either new-onset proteinuria (excess protein in the urine) or other signs or symptoms involving multiple organ systems.It is a pregnancy-specific systemic vascular disorder and is often accompanied by life-threatening events including eclampsia, renal failure, and HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets). Severe PE contributes to poor outcomes for both the mother and the baby if left untreated. Despite many theories, the etiology and pathogenesis of PE in pregnancy are not yet fully understood but are assumed to be complex and multifactorial.
One of the most important factors in the pathogenesis of hypertension in pregnancy is systemic endothelial activation and dysfunction (TakanoTAKANO H, INOUE K, SHIMADA A, SATO H, YANAGISAWA R and YOSHIKAWA T. 2009. Urinary trypsin inhibitor protects against liver injury and coagulation pathway dysregulation induced by lipopolysaccharide/D-galactosamine in mice. Lab Invest 89: 833-839. et al. 2009, TomimatsuTOMIMATSU T, MIMURA K, ENDO M, KUMASAWA K and KIMURA T. 2017. Pathophysiology of preeclampsia: an angiogenic imbalance and long-lasting systemic vascular dysfunction. Hypertens Res 40(4): 305-310. et al. 2017), and endothelial cell dysfunction is a hallmark of PE. Von Willebrand factor (vWF) is an important glycoprotein in the regulation of hemostasis and its level increases when vascular endothelial cells are injured. It binds platelet receptors and increases platelet adhesion to the sub-endothelial layer, thereby contributing to thrombus formation (RuggeriRUGGERI ZM. 2003. Von Willebrand factor, platelets and endothelial cell interactions. J Thromb Haemost 1: 1335-1342. 2003). The level of vWF in plasma can be used to evaluate the degree of vascular endothelial cell damage (HicksonHICKSON N, HAMPSHIRE D, CASTAMAN G, EIKENBOOM J, RODEGHIERO F, PEAKE I, GOODEVE A, MCMDM VWD and GROUPS Z-VS. 2011. Effect of the VWF promoter (GT)n repeat and single-nucleotide polymorphism c.-2527G>A on circulating von Willebrand factor levels under normal conditions. J Thromb Haemost 9: 603-605. et al. 2011). Elevated blood plasma concentrations of vWF are normal in pregnancy. However, some studies indicate that vWF blood plasma concentration is significantly higher in PE than in normal pregnancy (NadarNADAR SK, AL YEMENI E, BLANN AD and LIP GY. 2004. Thrombomodulin, von Willebrand factor and E-selectin as plasma markers of endothelial damage/dysfunction and activation in pregnancy induced hypertension. Thromb Res 113: 123-128. et al. 2004, ArefAREF S and GODA H. 2013. Increased VWF antigen levels and decreased ADAMTS13 activity in preeclampsia. Hematol 18: 237-241. and Goda 2013, MolvarecMOLVAREC A, RIGO J JR, BOZE T, DERZSY Z, CERVENAK L, MAKO V, GOMBOS T, UDVARDY ML, HARSFALVI J and PROHASZKA Z. 2009. Increased plasma von Willebrand factor antigen levels but normal von Willebrand factor cleaving protease (ADAMTS13) activity in preeclampsia. J Thromb Haemost 101: 305-311. et al. 2009, XiongXIONG Y et al. 2011. Alternations of maternal and cord plasma hemostasis in preeclampsia before and after delivery. Hypertens Pregn 30: 347-358. et al. 2011), and the plasma concentration of vWF may reflect both the severity and extent of damage to the endothelium in women with PE (Tomimatsu et al. 2017).
Ulinastatin, a high-molecular-weight (67,000 Dalton) human urinary trypsin inhibitor (UTI), is known to inhibit the activity of polymorphonuclear leukocyte elastase (PMNE). PMNE concentration correlates with the degree of coagulation and fibrinolysis. Ulinastatin has been widely used for the treatment of pancreatitis and sepsis, and may be considered a reasonable therapy for the treatment of vascular barrier dysfunction in inflammatory disorders (ChenCHEN J, WANG J, SU C, QIAN W, SUN L, SUN H, CHEN J, ZHANG H and ZHANG J. 2016. Urinary trypsin inhibitor attenuates LPS-induced endothelial barrier dysfunction by upregulation of vascular endothelial-cadherin expression. Inflammation Res 65(3): 213-224. et al. 2016). Ulinastatin has further been shown to decrease inflammatory reactions by decreasing inflammatory cytokine levels in experimental animal models . Whether it can decrease the plasma concentrations of vWF and alleviate vascular endothelial cell damage in pregnant women with PE has not been reported. In our study, we mainly observed the effect of ulinastatin on vWF, platelet granule membrane protein (GMP-140), platelet count, prothrombin time (PT), partial prothrombin activation time (APTT), and fibrinogen levels, as measures of vascular endothelial cell damage and blood coagulation in pregnant woman with severe PE after cesarean section.
MATERIALS AND METHODS
ABBREVIATIONS
vWF:von Willebrand factor; GMP -140: Platelet granule membrane protein; PT: Prothrombin time; APTT: Partial prothrombin activation time; CSEA:combined spinal-epidural anesthesia;UTI:urinary trypsin inhibitor;PMNE:polymorphonuclear leukocyte elastase;MAP:mean arterial pressures;DIC:disseminated intravascular coagulation.
PARTICIPANTS AND GROUP ASSIGNMENT
This was a single center, single-blind, placebo-controlled, parallel-group study conducted from October 2015 to November 2017 at Tianjin Central Hospital of gynecology and obstetrics in China. Our study was conducted in accordance with Helsinki Declaration principles. This study was approved by the committee of the Medical Ethics department from the Central Hospital of gynecology and obstetrics in Tianjin, and informed consent was obtained from each participant. On the basis of the diagnosis standards of severe PE (AmericanAMERICAN COLLEGE OF OBSTETRICIANS and GYNECOLOGISTS and TASK FORCE ON HYPERTENSION IN PREGNANCY. 2013. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 122: 1122-1131. College of Obstetricians and Gynecologists and Task Force on Hypertension in Pregnancy 2013, BrownBROWN MA et al. 2018. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertension 13: 291-310. et al. 2018) the inclusion criteria were as follows: 1. hypertension onset between 20 and 34 weeks, 2. SBP ≥ 160 or DBP ≥ 110, 3. proteinuria ≥ 2.0 g/24 h, 4. other maternal organ dysfunction, including acute kidney injury (AKI) (creatinine ≥ 90 µmol/L; 1 mg/dL), liver involvement (elevated transaminases, e.g., ALT or AST > 40 IU/L) with or without right upper quadrant or epigastric abdominal pain), and neurological complications (e.g., severe headaches, altered mental status, blindness, stroke, clonus, or persistent visual scotomata), and 5. uteroplacental dysfunction (e.g., fetal growth restriction, abnormal umbilical artery Doppler wave form analysis, or stillbirth). 80 pregnant women with severe PE who elected to undergo cesarean section were enrolled in our study. We applied the following criteria for exclusion from the study groups: placenta previa, placental abruption, twins or multiple gestations, fetal structural/genetic anomaly, maternal renal disease, inflammatory disease, cancer, metabolic disease, autoimmune disease, and other comorbidities associated with endothelial damage. The participants had the following characteristics: age, 24–39 years; body weight, 24–37 kg/m2; gestational age, 33–40 weeks; ASA classification, level II or III. Participants were randomly assigned following simple randomization procedures to 1 of 2 parallel groups, in a 1: 1 ratio, to receive either ulinastatin or normal saline as a control. An independent Data Monitoring Committee reviewed unblinded data for patient safety; no interim analyses for efficacy or futility were done during the trial. A computergenerated list of random numbers was used for allocation of the participants.
ANESTHESIA AND BLOOD SAMPLE COLLECTION
No patient was premedicated and 10·kg-1·h-1 of Ringer’s lactate solution was administered on arrival in the operating room. All parturients received oxygen (2L/min) via inhalation through a nasal catheter. Baseline values were measured including invasive mean arterial pressure (MAP), heart rates, and peripheral oxygen saturations.
All patients received combined spinal-epidural anesthesia (CSEA) in the left lateral position. Local anesthesia was provided at the site of epidural injection. All of the epidural puncturing points were at the L3/4 interspace. When the epidural needle punctured the endorhachis and negative pressure disappeared, the spinal anesthesia needle was inserted into the subarachnoid space and the needle core was taken out. After checking for clear cerebrospinal fluid, 0.5% hyperbaric ropivacaine 2 ml was injected into the subarachnoid space at an infusion speed of 2 ml/min. After withdrawal of the spinal anesthesia needle, the epidural catheter was inserted into the epidural space towards the head about 4 cm. In order to reduce aortocaval compression, the patient was placed in the supine position with 15° left lateral tilt using an obstetric wedge. Surgery started when a block level of T6 was achieved. After delivery of the fetus, the umbilical cord was cut and ligated. Ulinastatin (5000 IU/kg) (Grand number: 031310044, Guangdong Tian Pu Biochemical Pharmaceutical Co. LTD), dissolved in 20 ml of normal saline, was injected intravenously into parturients within 5 min. The dose of ulinastatin was chosen based on other studies and our own preliminary studies. The same volume of normal saline was injected intravenously into the control group. Phenylephrine (50 µg), in a volume of 1.5 ml, was administered in response to a 20% decrease from baseline MAP without bradycardia. Ephedrine (7.5 mg), in a volume of 1.5 ml, was administered in response to a 20% decrease from baseline MAP with bradycardia.
Before anesthesia (basic state) and 40 min after ulinastatin or saline administration, 5 ml of blood was collected from the elbow vein of patients. The 40-min time point for blood collection was chosen based on ulinastatin reaching peak concentrations 5 min after intravenous injection and its half-life of 40 min. We analyzed and treated the blood samples as follows: 2 ml of blood sample was placed into EDTA tubes for anti-coagulation and then centrifuged at 3000 rpm/min for 20 min. The supernatant was taken and preserved at -20℃. The plasma concentration of vWF and GMP-140 was later measured by ELISA (Shanghai Yuan-Mu Biological Technology Co. LTD); 1 ml of blood sample was mixed with EDTA and the platelet count and hemoglobin (Hb) levels were measured using an XE 2100 automatic blood analyzer (SYSMEX company, Japan). Blood sample (2 ml) was placed into sodium citrate tubes and centrifuged at 3000 rpm/min for 15 min. The supernatant was collected for the measurement of PT, APTT, and fibrinogen concentration using a CA7000 automatic blood analyzer (SYSMEX company).
STATISTICAL ANALYSIS
Statistical analysis of data was performed using SPSS 15.0. Data are expressed as the mean ± standard deviation(±s). Matched t-test was used to compare data within the same group. Group t-test and chi-square test were used to compare data between the groups. P-values less than 0.05 were considered significant.
RESULTS
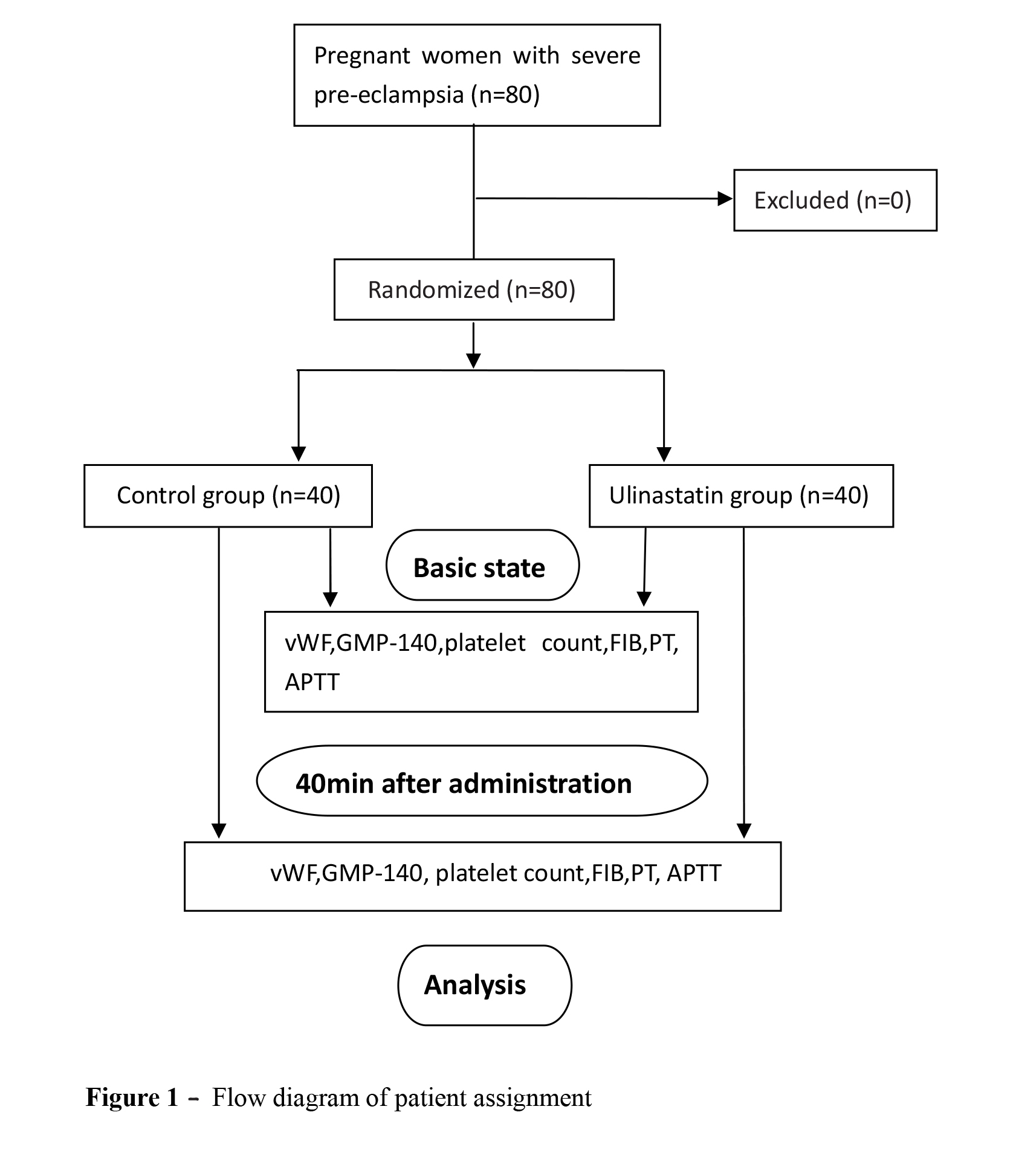
Patients were recruited from October 2015 to November 2017. The data of 80 patients were analyzed (Figure 1). Between the two groups, no significant differences were observed in patient characteristics including age, gestational age, body mass index, or fluid infusion volumes (Table I). Phenylephrine and ephedrine requirements were not significantly different between the two groups. Before the procedure and 40 min after the administration of ulinastatin, there were no significant differences in heart rate, MAP (mmHg), or Hb between the two groups.
Forty minutes after the administration of ulinastatin, the plasma concentration of vWF decreased significantly in the ulinastatin group compared to baseline levels (P<0.05) and also compared to the control group (P<0.05). There were no differences in GMP-140 and platelet count in the ulinastatin group or between the two groups (P<0.05) as shown in Table II. Forty minutes after the administration of ulinastatin, APTT was prolonged compared to baseline levels (P<0.05) as well as in comparison with the control group (P<0.05). There were no differences in fibrinogen levels or PT in the ulinastatin group or between the two groups (P<0.05) as shown in Table III.
DISCUSSION
Systemic endothelial dysfunction with the activation of endothelial cells is one of the most important factors in the pathogenesis of hypertensive disorders in pregnancy (SzarkaSZARKA A, RIGO J JR, LAZAR L, BEKO G and MOLVAREC A. 2010. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol 11: 59. et al. 2010), and is a marker of PE (BoeldtBOELDT DS and BIRD IM. 2017. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol 232: R27-R44. and Bird 2017). Endothelial dysfunction contributes to major symptoms of PE, such as hypertension, edema, proteinuria, and improper platelet aggregation. vWF is a glycoprotein synthesized and secreted by vascular endothelial cells. When vascular endothelial cells are injured, vWF is released into blood. Therefore, increased levels of vWF in plasma can be used to indicate the degree of damage to vascular endothelial cells. Several studies show that vWF antigen levels markedly increase in patients with PE or HELLP syndrome (HulsteinHULSTEIN JJ, VAN RUNNARD HEIMEL PJ, FRANX A, LENTING PJ, BRUINSE HW, SILENCE K, DE GROOT PG and FIJNHEER R. 2006. Acute activation of the endothelium results in increased levels of active von Willebrand factor in hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome. J Thromb Haemost 4: 2569-2575. et al. 2006). The normal reference range of vWF in plasma is 1075 ±296 U/L (Hulstein et al. 2005HULSTEIN JJ, DE GROOT PG, SILENCE K, VEYRADIER A, FIJNHEER R and LENTING PJ. 2005. A novel nanobody that detects the gain-of-function phenotype of von Willebrand factor in ADAMTS13 deficiency and von Willebrand disease type 2B. Blood 106: 3035-3042.). The plasma concentrations of vWF in pregnant women are higher than in non-pregnant women (GermainGERMAIN AM, ROMANIK MC, GUERRA I, SOLARI S, REYES MS, JOHNSON RJ, PRICE K, KARUMANCHI SA and VALDES G. 2007. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension 49: 90-95. et al. 2007, MyattMYATT L and WEBSTER RP. 2009. Vascular biology of preeclampsia. J Thromb Haemost 7: 375-384. and Webster 2009), and hypertensive pregnant women had higher vWF than normal pregnant women (KarthikeyanKARTHIKEYAN VJ, LIP GY, BAGHDADI S, LANE DA, BEEVERS DG and BLANN AD. 2012. Angiogenin and hemoxygenase in pregnancy: influence of hypertension. Angiology 63: 194-198. et al. 2012). PE is associated with an overall pro-inflammatory systemic environment. In maternal circulation, elevated levels of pro-inflammatory cytokines might play a central role in the excessive systemic inflammatory response and induce endothelial dysfunction (LauLAU SY, GUILD SJ, BARRETT CJ, CHEN Q, MCCOWAN L, JORDAN V and CHAMLEY LW. 2013. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis. Am J Reproduct Immunol 70: 412-427. et al. 2013, Szarka et al. 2010). The levels of interleukin (IL)-6 and IL-8 are higher in pregnant women with PE than in those without PE (SalazarSALAZAR GARCIA MD, MOBLEY Y, HENSON J, DAVIES M, SKARIAH A, DAMBAEVA S, GILMAN-SACHS A, BEAMAN K, LAMPLEY C and KWAK-KIM J. 2018. Early pregnancy immune biomarkers in peripheral blood may predict preeclampsia. J Reproduct Immunol 125: 25-31. Garcia et al. 2018). Higher concentrations of IL-8 were reported in PE women than in normal pregnancies, and the concentration of IL-8 was associated with the severity of PE .
As serine protease inhibitors, UTIs have been used in patients with sepsis, disseminated intravascular coagulation (DIC), shock, and pancreatitis. UTIs decrease inflammatory cytokine levels in experimental animal models of inflammation. Data further show that prophylactic UTIs maintain the endothelial barrier function and increase cadherin expression on vascular endothelium under inflammatory conditions (Chen et al. 2016). A previous study indicated that the administration of ulinastatin via peritoneal lavage effectively lowered the levels of inflammatory mediators in the serum, alleviating the severe systemic inflammatory response (FengFENG C, LI B, WANG LL, CHEN LI, ZHOU X, LV FQ and LI TS. 2015. Effect of peritoneal lavage with ulinastatin on the expression of NF-kappaB and TNF-alpha in multiple organs of rats with severe acute pancreatitis. Exp Ther Med 10(6): 2029-2034. et al. 2015). Ulinastatin suppresses the production of tumor necrosis factor-α (TNF-α) (AosasaAOSASA S, ONO S, MOCHIZUKI H, TSUJIMOTO H, UENO C and MATSUMOTO A. 2001. Mechanism of the inhibitory effect of protease inhibitor on tumor necrosis factor alpha production of monocytes. Shock 15: 101-105. et al. 2001) and IL-6 and IL 8 (SatoSATO Y, ISHIKAWA S, OTAKI A, TAKAHASHI T, HASEGAWA Y, SUZUKI M, YAMAGISHI T and MORISHITA Y. 2000. Induction of acute-phase reactive substances during open-heart surgery and efficacy of ulinastatin. Inhibiting cytokines and postoperative organ injury. Jpn J Thorac Cardiovasc Surg 48(7): 428-434. et al. 2000). Therefore, it was hypothesized that ulinastatin would decrease inflammatory reactions in PE in this study. In this study, the plasma concentration of vWF was significantly lower 40 min after administration than at baseline or in the control group. This indicates that ulinastatin alleviated endothelial cell damage likely by inhibiting the release of inflammatory mediators.
Some studies have described the effects ulinastatin on coagulation, but the mechanism of action remains poorly understood, and the results are inconsistent. In a study using ulinastatin before aortic cross clamping in atrioventricular valve surgery with cardiopulmonary bypass (ParkPARK JB, KIM SH, LEE SA, CHUNG JW, KIM JS and CHEE HK. 2013. Effects of ulinastatin on postoperative blood loss and hemostasis in atrioventricular valve surgery with cardiopulmonary bypass. Korean J Thorac Cardiovasc Surg 46: 185-191. et al. 2013), the postoperative platelet count was statistically lower than in the control group, and there were no significant differences in PT, APTT, or fibrinogen levels. Another study reported that ulinastatin alleviates the prolongation of PT and APTT, and prevents decreases in fibrinogen and platelet counts in mice with liver injury (Takano et al. 2009); however, the effect of ulinastatin on platelet count and PT was not remarkable. Additionally, increased GMP-140 (also known as P-selectin or CD62P) in plasma is a specific marker of platelet activation; however, ulinastatin did not have any effects on plasma GMP-140 in this study. The level of fibrinogen in all patients was higher than normal, and ulinastatin had no effect on fibrinogen levels. Despite these data, after administration of ulinastatin, APTT was prolonged in the ulinastatin group, although all APTT values were in the normal range. APTT reflects the intrinsic coagulation system, of which most of the intrinsic coagulation factors are serine proteases (NishiyamaNISHIYAMA T, YOKOYAMA T and YAMASHITA K. 2006. Effects of a protease inhibitor, ulinastatin, on coagulation and fibrinolysis in abdominal surgery. J Anesthes 20: 179-182. et al. 2006). Ulinastatin is a broad-spectrum, nonspecific serine inhibitor; thus, it may play an important role in anticoagulation by inhibiting these intrinsic coagulation factors (KimKIM NY, SHIM JK, BANG SO, SIM JS, SONG JW and KWAK YL. 2013. Effects of ulinastatin on coagulation in high-risk patients undergoing off-pump coronary artery bypass graft surgery. Korean J Anesthesiol 64: 105-111. et al. 2013).
There were some limitations in this study: this was a single-center study, ulinastatin was injected only once, and the experimental period was short. The level of plasma inflammatory mediators was also not detected. A long term, multicenter clinical trial is needed to further define the effect of ulinastatin on vascular endothelial cells in pregnant women with severe PE.
CONCLUSIONS
In summary, ulinastatin can alleviate vascular endothelial cell injury in pregnant women with severe PE after cesarean section, suggesting its clinical utility in the treatment of PE.
ACKNOWLEGMENTS
The study was supported by Tianjin Central Hospital of gynecology and obstetrics, Health and Family planning commission of Tianjin .ChiCTR1800014898. On behalf of all authors, the corresponding author states that there is no conflict of interest.
REFERENCES
- AMERICAN COLLEGE OF OBSTETRICIANS and GYNECOLOGISTS and TASK FORCE ON HYPERTENSION IN PREGNANCY. 2013. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 122: 1122-1131.
- AOSASA S, ONO S, MOCHIZUKI H, TSUJIMOTO H, UENO C and MATSUMOTO A. 2001. Mechanism of the inhibitory effect of protease inhibitor on tumor necrosis factor alpha production of monocytes. Shock 15: 101-105.
- AREF S and GODA H. 2013. Increased VWF antigen levels and decreased ADAMTS13 activity in preeclampsia. Hematol 18: 237-241.
- BOELDT DS and BIRD IM. 2017. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol 232: R27-R44.
- BROWN MA et al. 2018. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertension 13: 291-310.
- CHEN J, WANG J, SU C, QIAN W, SUN L, SUN H, CHEN J, ZHANG H and ZHANG J. 2016. Urinary trypsin inhibitor attenuates LPS-induced endothelial barrier dysfunction by upregulation of vascular endothelial-cadherin expression. Inflammation Res 65(3): 213-224.
- FENG C, LI B, WANG LL, CHEN LI, ZHOU X, LV FQ and LI TS. 2015. Effect of peritoneal lavage with ulinastatin on the expression of NF-kappaB and TNF-alpha in multiple organs of rats with severe acute pancreatitis. Exp Ther Med 10(6): 2029-2034.
- GERMAIN AM, ROMANIK MC, GUERRA I, SOLARI S, REYES MS, JOHNSON RJ, PRICE K, KARUMANCHI SA and VALDES G. 2007. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension 49: 90-95.
- HICKSON N, HAMPSHIRE D, CASTAMAN G, EIKENBOOM J, RODEGHIERO F, PEAKE I, GOODEVE A, MCMDM VWD and GROUPS Z-VS. 2011. Effect of the VWF promoter (GT)n repeat and single-nucleotide polymorphism c.-2527G>A on circulating von Willebrand factor levels under normal conditions. J Thromb Haemost 9: 603-605.
- HULSTEIN JJ, DE GROOT PG, SILENCE K, VEYRADIER A, FIJNHEER R and LENTING PJ. 2005. A novel nanobody that detects the gain-of-function phenotype of von Willebrand factor in ADAMTS13 deficiency and von Willebrand disease type 2B. Blood 106: 3035-3042.
- HULSTEIN JJ, VAN RUNNARD HEIMEL PJ, FRANX A, LENTING PJ, BRUINSE HW, SILENCE K, DE GROOT PG and FIJNHEER R. 2006. Acute activation of the endothelium results in increased levels of active von Willebrand factor in hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome. J Thromb Haemost 4: 2569-2575.
- KARTHIKEYAN VJ, LIP GY, BAGHDADI S, LANE DA, BEEVERS DG and BLANN AD. 2012. Angiogenin and hemoxygenase in pregnancy: influence of hypertension. Angiology 63: 194-198.
- KIM NY, SHIM JK, BANG SO, SIM JS, SONG JW and KWAK YL. 2013. Effects of ulinastatin on coagulation in high-risk patients undergoing off-pump coronary artery bypass graft surgery. Korean J Anesthesiol 64: 105-111.
- LAU SY, GUILD SJ, BARRETT CJ, CHEN Q, MCCOWAN L, JORDAN V and CHAMLEY LW. 2013. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis. Am J Reproduct Immunol 70: 412-427.
- MOLVAREC A, RIGO J JR, BOZE T, DERZSY Z, CERVENAK L, MAKO V, GOMBOS T, UDVARDY ML, HARSFALVI J and PROHASZKA Z. 2009. Increased plasma von Willebrand factor antigen levels but normal von Willebrand factor cleaving protease (ADAMTS13) activity in preeclampsia. J Thromb Haemost 101: 305-311.
- MYATT L and WEBSTER RP. 2009. Vascular biology of preeclampsia. J Thromb Haemost 7: 375-384.
- NADAR SK, AL YEMENI E, BLANN AD and LIP GY. 2004. Thrombomodulin, von Willebrand factor and E-selectin as plasma markers of endothelial damage/dysfunction and activation in pregnancy induced hypertension. Thromb Res 113: 123-128.
- NISHIYAMA T, YOKOYAMA T and YAMASHITA K. 2006. Effects of a protease inhibitor, ulinastatin, on coagulation and fibrinolysis in abdominal surgery. J Anesthes 20: 179-182.
- PARK JB, KIM SH, LEE SA, CHUNG JW, KIM JS and CHEE HK. 2013. Effects of ulinastatin on postoperative blood loss and hemostasis in atrioventricular valve surgery with cardiopulmonary bypass. Korean J Thorac Cardiovasc Surg 46: 185-191.
- RUGGERI ZM. 2003. Von Willebrand factor, platelets and endothelial cell interactions. J Thromb Haemost 1: 1335-1342.
- SALAZAR GARCIA MD, MOBLEY Y, HENSON J, DAVIES M, SKARIAH A, DAMBAEVA S, GILMAN-SACHS A, BEAMAN K, LAMPLEY C and KWAK-KIM J. 2018. Early pregnancy immune biomarkers in peripheral blood may predict preeclampsia. J Reproduct Immunol 125: 25-31.
- SATO Y, ISHIKAWA S, OTAKI A, TAKAHASHI T, HASEGAWA Y, SUZUKI M, YAMAGISHI T and MORISHITA Y. 2000. Induction of acute-phase reactive substances during open-heart surgery and efficacy of ulinastatin. Inhibiting cytokines and postoperative organ injury. Jpn J Thorac Cardiovasc Surg 48(7): 428-434.
- SZARKA A, RIGO J JR, LAZAR L, BEKO G and MOLVAREC A. 2010. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol 11: 59.
- SZPERA-GOZDZIEWICZ A, MAJCHEREK M, BORUCZKOWSKI M, GOZDZIEWICZ T, DWORACKI G, WICHEREK L and BREBOROWICZ GH. 2017. Circulating endothelial cells, circulating endothelial progenitor cells, and von Willebrand factor in pregnancies complicated by hypertensive disorders. Am J Reprod Immunol 77(3): 10.1111/aji.12625.
- TAKANO H, INOUE K, SHIMADA A, SATO H, YANAGISAWA R and YOSHIKAWA T. 2009. Urinary trypsin inhibitor protects against liver injury and coagulation pathway dysregulation induced by lipopolysaccharide/D-galactosamine in mice. Lab Invest 89: 833-839.
- TOMIMATSU T, MIMURA K, ENDO M, KUMASAWA K and KIMURA T. 2017. Pathophysiology of preeclampsia: an angiogenic imbalance and long-lasting systemic vascular dysfunction. Hypertens Res 40(4): 305-310.
- XIONG Y et al. 2011. Alternations of maternal and cord plasma hemostasis in preeclampsia before and after delivery. Hypertens Pregn 30: 347-358.
Publication Dates
-
Publication in this collection
30 Sept 2019 -
Date of issue
2019
History
-
Received
4 Aug 2018 -
Accepted
23 Oct 2018