Abstract
Coronavirus is associated with several infectious diseases that cause outbreaks in humans, such as SARS in 2002-2003 and MERS in 2012. In December 2019, COVID-19, promoted by the SARS-CoV-2 virus, was first reported in Wuhan (China) as a new coronavirus disease. This outbreak quickly reached a pandemic status, affecting at least 185 countries and territories to date on all continents. The first case of COVID-19 reported in São Paulo city (Brazil) occurred in February 26th. Days later, 182 suspected cases in 16 states were being monitored. In May 30th, 514,849 cases and 29,314 deaths were confirmed in Brazil comprising all 26 states and Federal District. The primary measure in order to contain the spread of SARS-CoV-2 involved social isolation. At that time there were not enough diagnostic tests to identify infected individuals and data were strongly associated with sub notifications. Nevertheless, the effectiveness of this measure largely depends on the individual’s social responsibility. This measure has a severe economic and social impact, as in other countries. In this review, we present an overview and scientific perspectives of the evolution of COVID-19 from Brazilian databases in which climate and economic situations differ from China, European countries, and the USA.
Key words
COVID-19; SARS-CoV-2; Pandemic; Brazil
INTRODUCTION
Coronaviridae is a family of enveloped positive-stranded RNA viruses of vertebrates, introduced into humans from a zoonotic reservoir and which can promote a respiratory syndrome. In the past, the Severe Acute Respiratory Syndrome (SARS), with a fatality rate of around 10% (8,098 cases and 774 deaths), and the Middle East Respiratory Syndrome (MERS), around 34% (2,494 cases and 858 deaths) are the major representants of this virus family (Gorbalenya 2020GORBALENYA AE. 2020. Severe acute respiratory syndrome-related coronavirus–The species and its viruses, a statement of the Coronavirus Study Group. BioRxiv: https://doi.org/10.1101/2020.02.07.937862.
https://doi.org/10.1101/2020.02.07.93786...
, Mahase 2020MAHASE E. 2020. Coronavirus: covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ 368: m641.). Currently, a new virus representative of this family, named SARS-CoV-2, has been promoted a worldwide pandemic outbreak, the 2019-nCoV (disused term) or COVID-19 disease. The human first cases were detected in Wuhan, China, in November 2019, probably from a horseshoe bat contagion in Yunnan Province (Wang & Hu 2013WANG M & HU Z. 2013. Bats as animal reservoirs for the SARS coronavirus: hypothesis proved after 10 years of virus hunting. Virol Sinica 28(6): 315-317., Murdoch & French 2020MURDOCH DR & FRENCH NP. 2020. COVID-19: another infectious disease emerging at the animal-human interface. NZ Med J 133(1510): 12-15., Huang et al. 2020HUANG C ET AL. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506.).
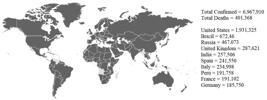
The COVID-19 growth curve in China was built through daily infographics (Figure 1), with an initial plateau in February 29th and new increase of confirmed cases and deaths in April 30th. The evidence of the pandemic could be observed in Figure 2, and 188 countries presented confirmed cases and elevated the number to more than six million people.
Briefing of daily infographics disclosed by China in the National Health Commission of the people’s Republic of China, the last date available was in April 30th (http://en.nhc.gov.cn/index.html). Black vertical bars represent the total deaths by date, and the dotted line represents the number of cases by time.
Total worldwide confirmed cases and the most 10 countries affected by COVID-19 (The map was captured from the CDC website and while the data are contained on the National Center for Immunization and Respiratory Diseases (NCIRD) and Johns Hopkins University websites). Data recovered in June 6th, 7 p.m. Brazilia time).
VIRUS CHARACTERIZATION AND CELL INTERACTION
SARS-CoV-2 is a positive single-stranded RNA virus ((+)ssRNA) of approximately 29802 nucleotides (Wu et al. 2020WU F ET AL. 2020. A new coronavirus associated with human respiratory disease in China. Nature 579(7798): 265-269.). The virus genome consists of fourteen functional ORFs encoding three classes of proteins. Two large polyproteins (pp), pp1a and pp1ab, which are divided into sixteen non-structural proteins (nsps) necessary for viral RNA synthesis and blocking the innate immune response (nsp1; nsp18-2’-O-metil Transferase; nsp3-deubiquitinase). Four structural proteins (S, E, M, and N), are essential for viral assembly and eight accessory proteins, which provide a selective advantage in the infected host (Decroly et al. 2011DECROLY E ET AL. 2011. Crystal structure and functional analysis of the SARS-coronavirus RNA cap 2‐-O-methyltransferase nsp10/nsp16 complex. PLoS pathogens 7.5(5): e1002059., Sevajol et al. 2014SEVAJOL M, SUBISSI L, DECROLY E & IMBERTM BCI. 2014. Insights into RNA synthesis, capping, and proofreading mechanisms of SARS-coronavirus. Virus Res 194: 90-99., Lehmann et al. 2015LEHMANN KC ET AL. 2015. Discovery of an essential nucleotidylating activity associated with a newly delineated conserved domain in the RNA polymerase- containing protein of all nidoviruses. Nucl Acid Res 43(17): 8416-8434.). For the synthesis of viral RNA within host cells, it is necessary to translate and assemble viral replicase complexes (Ziebuhr 2005ZIEBUHR J. 2005. The coronavirus replicase. In: Coronavirus replication and reverse genetics. Springer, Berlin, Heidelberg, p. 57-94., Ahn et al. 2012AHN DG, CHOI JK, TAYLOR DR & OH JW. 2012. Biochemical characterization of a recombinant SARS coronavirus nsp12 RNA-dependent RNA polymerase capable of copying viral RNA templates. Archi Virol 157(11): 2095-2104.), producing genomic and sub-genomic RNAs. Subgenomic RNAs serve as mRNAs of structural protein genes and the non-structural ones, which reside downstream of the polyproteins of the replicase (Jaimes et al. 2020JAIMES JA, ANDRE NM, MILLET JK & WHITTAKER GR. 2020. Structural modeling of 2019-novel coronavirus (nCoV) spike protein reveals a proteolytically-sensitive activation loop as a distinguishing feature compared to SARS-CoV and related SARS-like coronaviruses. arXiv preprint 2002: 06196. https://doi.org/10.1101/2020.02.10.942185.). All positive sense subgenomic RNAs are 3’ co-terminals with complete viral genome. Genomic and subgenomic RNAs are produced through negative chain intermediates (Kirchdoerfer & Ward 2019KIRCHDOERFER RN & WARD AB. 2019. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nature comm 10(1): 1-9.).
After successful replication and subgenomic RNA synthesis, structural proteins S, E, and M are translated and joined into the endoplasmic reticulum (Hu et al. 2017HU B ET AL. 2017. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus.PLoS pathogens 13(11): e1006698., 2018HU D ET AL. 2018. Genomic characterization and infectivity of a novel SARS-like coronavirus in Chinese bats. Emerg Microb Infect 7.1: 1-10., Shi et al. 2018SHI M ET AL. 2018. The evolutionary history of vertebrate RNA viruses. Nature 556(7700): 197-202.). Then, these proteins enter along the secretory pathway into the intermediate compartments of the endoplasmic-Golgi reticulum (Kim et al. 2020KIM Y, JEDRZEJCZAK R, MALTSEVA NI, ENDRES M, GODZIK A, MICHALSKA K & JOACHIMIAK A. 2020. Crystal structure of Nsp15 endoribonuclease NendoU from SARS-CoV-2. bioRxiv: https://doi.org/10.1101/2020.03.02.968388.
https://doi.org/10.1101/2020.03.02.96838...
), they find viral genomes encapsulated by protein N and bind to form a complete virus. After assembly, viruses are transported to the cell surface in vesicles and released by exocytosis (Tang et al. 2020TANG X ET AL. 2020. On the origin and continuing evolution of SARS-CoV-2. Nat Sci Rev: https://doi.org/10.1093/nsr/nwaa036.
https://doi.org/10.1093/nsr/nwaa036...
).
Some data have shown that COVID-19 has killed more people than SARS and MERS combined (Mahase 2020MAHASE E. 2020. Coronavirus: covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ 368: m641.). Clinical signs and symptoms are clear now, after the experience of Chinese colleagues during the outbreak in China. Many studies are still correlating mechanisms of action used by the virus to understand aspects of SARS and MERS. Studies show that the mechanism of interacting of the virus with human cells is associated with the angiotensin-converting enzyme 2 (ACE2) glycoprotein receptor, which binds with the S1 domain of the SARS-CoV S protein (Kuba et al. 2005KUBA K ET AL. 2005. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nature Medicine 11(8): 875-879., Prabakaran et al. 2004PRABAKARAN S ET AL. 2004. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psych 9(7): 684-697., Li et al. 2003LI W ET AL. 2003. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426(6965): 450-454.). Regarding the new coronavirus, studies show that SARS-CoV-2 has a greater capacity to spread in the population. This advantage seems to be conferred due to the high affinity of the spike protein (RBD portion) to its human ACE2 receptor (Wan et al. 2020). Thus, this high-affinity binding allows rapid infection of the population through the airways and mucous membranes, through direct contact or by contact with saliva or contaminated surfaces. After infection of the initial cells, the viruses use protein S to mediate cell-cell fusion in order to infect neighboring cells, giving rise to multinucleated giant cells. Thus, the virus is able to spread in the infected organism without being detected or neutralized by specific antibodies (Hwang et al. 2006HWANG WC, LIN Y, SANTELLI E, SUI J, JAROSZEWSKI L, FARZAN M, MARASCO W & LIDDINGTON R. 2006. Structural basis of neutralization by a human anti-severe acute respiratory syndrome spike protein antibody, 80R. J Biol Chem 281(45): 34610-34616., Letko et al. 2020LETKO M, MARZI A & MUNSTER V. 2020. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nature Microbiol: 1-8.).
CLINICAL ASPECTS, ILLNESS EVOLUTION AND DEATHS
COVID-19 replicates efficiently in the upper respiratory tract and appears to cause common colds in the winter season (Heymann & Shindo 2020HEYMANN DL & SHINDO N. 2020. COVID-19: what is next for public health? The Lancet 395(10224): 542-545.). So, symptoms like fever, cough, fatigue, dyspnea, and headache can be present in both common and severe forms (Tian et al. 2020TIAN X, LI C, HUANG A, XIA S, LU S, SHI Z, LU L, JIANG S, YANG Z, WU Y & YING T. 2020. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microb Infect 9(1): 382-385.). Laboratory studies also can show leucopenia (Li et al. 2020LI X, ZAI J, ZHAO Q, NIE Q, LI Y, FOLEY BT & CHAILLON A. 2020. Evolutionary history, potential intermediate animal host, and cross‐species analyses of SARS‐CoV‐2. J Med Virol: https://doi.org/10.1002/jmv.25731.
https://doi.org/10.1002/jmv.25731...
). The severe form may present a clinical picture ranging from shortness of breath, paroxysmal cough, and productive sputum to multifocal nodular and peripheral ground-glass opacities involving the lung lobes (Kong & Agarwal 2020KONG W & AGARWAL PP. 2020. Chest imaging appearance of COVID-19 infection. Radiol Cardioth Imag 2(1): e200028.). Unfortunately, it seems that hematological and biochemical exams do not show significant changes and can not use alone as diagnosis tools (Ming-Yen et al. 2020MING-YEN N ET AL. 2020. Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiol Cardioth Imag 2(1): e200034.).
Zhou et al. (2020)ZHOU F ET AL. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet 28: 1054-1062. analyzing 191 patients who tested positive for SARS-Cov-2 in two Wuhan hospitals, found a death rate of around 28.27% (n=54) in hospitalized patients. According to these authors, factors such as age, lymphopenia, leukocytosis, and altered levels of biochemical and immunological biomarkers, are associated with the severity of illness and death. However, they also related that severe lymphopenia was common in patients who died. As previously mentioned, these findings are similar to the study published by Tan et al. (2020)TAN L, WANG Q, ZHANG D, DING J, HUANG Q, TANG YQ, WANG Q & MIAO H. 2020. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. medRxiv 33: https://doi.org/10.1038/s41392-020-0148-4., which showed lymphopenia in 5 patients who died.
BRAZILIAN ILLNESS EVOLUTION
The first case in Brazil occurred in February 26th, in São Paulo state. A man, 61 years old, arrived from Italy and presented the characteristic symptoms of the disease. Two days later, around 182 suspect cases occurred in 16 brazilian states. The initial recommendations from the Brazilian Ministry of Health were based on WHO instructions to prevent contagion of the virus in the population are shown in Figure 3. After the flowchart adoption for health professionals, the evaluation criteria was changed, and new statistic data started to be published daily (Brazil 2020aBRAZIL. 2020a. Ministry of Health. Brasil amplia diagnóstico para o coronavírus. https://www.saude.gov.br/noticias/agencia-saude/46472-brasil-amplia-diagnostico-para-o-coronavirus (accessed 19 March 2020).
https://www.saude.gov.br/noticias/agenci...
). From April, the contagion was widely distributed and it was difficult to detect the virus propagation area and control the virus spread in Brazil.
Flowchart with initial recommendations of the Brazilian Ministry of Health to the investigation of new cases by health professionals and sanitary vigilance (Brazilian Ministry of Health site, March 18th).
Although in March 18th those criteria were published by the Brazilian government, the current instructions were modified since the confirmed cases started to be originated through community transmission. Thus, only serious cases associated with hospitalization or quarantine reclusion have been tested with specific kits (RT-PCR investigation and the ELISA kit). In this sense, asymptomatic, light and moderate cases were not investigated and notified from February to April. The last Brazilian panorama to date shows the increase of cases in May 30th, with 514,849 confirmed cases and 29,314 deaths. From this date, Brazil such as Russia, United Kingdom, and the United States, trespass the Chinese numbers, and the prevision to the next two months (June and July) has been tough for the population. The evolution of cases can be seen in Figure 4, and Figure 5 shows the numbers of Brazilian cases by state (Brazil 2020aBRAZIL. 2020a. Ministry of Health. Brasil amplia diagnóstico para o coronavírus. https://www.saude.gov.br/noticias/agencia-saude/46472-brasil-amplia-diagnostico-para-o-coronavirus (accessed 19 March 2020).
https://www.saude.gov.br/noticias/agenci...
).
Infographic showing the evolution of the cases in Brazil. All numbers are associated with confirmed cases through the PCR test. The last data was recovered from the Brazilian Ministry of Health in May 30th which also computed 29,314 deaths by COVID-19.
Total cases investigated in Brazil according to Ministry of Health (May 30th, 6 p.m. Brazilia time). Map adapted using data from State Health Secretaries of Brazil.
The initial structure for the care of COVID-19 suspected patients or patients who presented moderate symptoms of the disease is the Family Health Unit, which consists of a public health network (Brazilian Unique Health System) with clinics, ambulatory and hospitals, located in different neighborhoods of the country’s cities. On the other hand, patients who can pay for the health service (Brazilian Supplementary Health), use private clinics, ambulatory and hospitals. In addition, the major recommendation for the population is that health units should only hospitalize patients who have severe symptoms of the disease. In this way, a telephone service center was created to answer questions about the symptoms in order to guide the population to those should or should not seek hospital care. In this way, according to the clinical situation of each patient, hospitalization could be indicated for the ICU or ward beds. Until May 2020, there were 64 reference hospitals distributed in all five Brazilian regions, 25 in the North, 15 in the Northeast, 4 in the Midwest, 5 in the Southeast, and 15 in the Southern region. Those hospitals were chosen because they have ample capacity to serve the population, with specialized professionals in risk situations for public health. Moreover, also until May, another 304 hospitals across Brazil were in the verification process, in order to become reference centers in COVID-19 mitigation phase. Data from April showed that more than twenty field hospitals were built in the country (Brazil 2020bBRAZIL. 2020b. Ministry of Health. Estados terão R$ 432 milhões para enfrentar o COVID-19. https://www.saude.gov.br/noticias/agencia-14saude/46547-estados-terao-r-432-milhoes-para-enfrentar-covid-19 (accessed 19 March 2020).
https://www.saude.gov.br/noticias/agenci...
, c).
ECONOMIC ASPECTS OF THE COVID-19 PANDEMIC IN BRAZIL
COVID-19 has shown huge economic downfalls and a severe impact on public health worldwide. Financial markets fell on different stock exchanges. Official data from Economy Ministry (2020) shows that from March 9th to 13th, São Paulo stock market accumulated a drop of just over 15%. It is the worst weekly drop since October 2008 financial crisis (De Barros & Santos 2019DE BARROS FRPM & SANTOS VGBCB. 2019. Análise do protecionismo brasileiro pós-crise de 2008: barreiras comerciais e planos de incentivos à indústria. Rev Econ Pol Desenv 6(21): 1-14., Rodrigues & Clemente 2019RODRIGUES RDSA & CLEMENTE A. 2019. Efeitos da corrupção nas bolsas de valores na Crise Financeira de 2008. Ver Contab Organiz 13: 51-63.). In March 16th, financial markets fell on the world’s major indices. As a result, the circuit breaker mechanism, in São Paulo and New York cities, temporarily paralyzed the trading session in attempt to decelerate the pace of falling stocks. In this context, the Brazilian government has taken action to try to mitigate the increasing effects of the pandemic on the economy.
The government’s first reaction, after the beginning of COVID-19 in Brazil was to reinforce the need to approve reforms that alleviate government spending, such as balancing public accounts in order to create an improvement in the Brazilian business environment. This action would increase investor confidence and the possibility of investing money in the country. Brazilian economic team released US$ 29,1 billions, which would be distributed to support the economy in several Brazilian sectors. Exclusive financial support to public health was around US$ 1 billion, of which 94.11% (US$ 948 millions) was under Ministry of Brazilian Health control and 5.89% (US$ 590 thousands) were released directly to the Federal University Hospitals, linked to the Ministry of Brazilian Education. Moreover, in March 16th, the Brazilian economic team announced the release of more than US$ 890 millions to the Unified Public Health System, from the Mandatory Insurance for Personal Damage Caused by Motor Vehicles by Land that remains available in public coffers. Imported medical products also experienced a decrease in import taxes until the end of 2020. The last financial support (from US$ 120 to US$ 240), for poor people and in risk situation, also named informal or autonomous workers was distributed in April. Table I shows other government actions, to support companies and consumers (Brazil 2020dBRAZIL. 2020d. Ministry of Economy. Confira as medidas tomadas pelo Ministério da Economia em função do Covid-19 (Coronavírus). http://www.economia.gov.br/noticias/2020/marco/confira-as-medidas-tomadas-pelo-ministerio-da-economia-em-funcao-do-covid-19-coronavirus (accessed 19 March 2020).
http://www.economia.gov.br/noticias/2020...
, eBRAZIL. 2020e. Ministry of Economy. Ministério da Economia anuncia medidas para diminuir o impacto do coronavírus no país. http://www.economia.gov.br/noticias/2020/marco/ministerio-da-economia-anuncia-medidas-para-diminuir-o-impacto-do-coronavirus-no-pais (accessed 19 March 2020).
http://www.economia.gov.br/noticias/2020...
).
Brazilian government actions adopted to mitigate the increasing effects of the COVID-19 pandemic on the country economy.
Brazil adopted drastic measures to block the virus advance. In March 18th, São Paulo was the most affected state in Brazil. The government determined the closure of malls, fitness centers, stores and offices that operated within the metropolitan region of the city until April 5th (Brazil 2020fBRAZIL. 2020f. State Government of São Paulo. Governo recomenda fechamento de shoppings e academias da Grande SP até fim de abril. São Paulo. http://www.saopaulo.sp.gov.br/spnoticias/governo-recomenda-fechamento-hoppings-academias/ (accessed 19 March 2020).
http://www.saopaulo.sp.gov.br/spnoticias...
, gBRAZIL. 2020g. City Hall of São Paulo. Situação de Emergência: Prefeitura de São Paulo adota medidas para evitar disseminação do coronavírus. São Paulo. http://www.capital.sp.gov.br/noticia/situacao-de-emergencia-prefeitura-de-sao-paulo-adota-medidas-para-evitar-disseminacao-do-coronavirus (accessed 19 March 2020).
http://www.capital.sp.gov.br/noticia/sit...
). Likewise, the government of Pernambuco, in March 19th, announced the closure of malls, beauty salons, restaurants and coffee shops across the state (Brazil 2020hBRAZIL. 2020h. State Government of Pernambuco. Governo de Pernambuco anuncia novas medidas restritivas de combate ao Covid-19. Pernambuco. http://www.pe.gov.br/mobile/blog/2020/03/19/governo-de-pernambuco-anuncia-novas-medidas-restritivas-de-combate-ao-covid-19 (accessed 19 March 2020).
http://www.pe.gov.br/mobile/blog/2020/03...
). Thus, these measures were adopted by several other states in Brazil, such as Bahia (Brazil 2020iBRAZIL. 2020i. State Government of Bahia. Entenda as medidas de combate ao coronavírus na Bahia. http://www.ba.gov.br/noticias/entenda-medidas-de-combate-ao-coronavirus-na-bahia-1 (accessed 19 March 2020).
http://www.ba.gov.br/noticias/entenda-me...
), Ceará (Brazil 2020jBRAZIL. 2020j. State Government of Ceará. Governo do Ceará determina novas medidas de enfrentamento ao coronavírus. https://www.ceara.gov.br/2020/03/19/governo-do-ceara-determina-novas-medidas-de-enfrentamento-ao-coronavirus/ (accessed 19 March 2020).
https://www.ceara.gov.br/2020/03/19/gove...
), Rio de Janeiro (Brazil 2020kBRAZIL. 2020k. State Government of Rio de Janeiro. Medidas restritivas isolam capital do Rio de Janeiro a partir de sábado, dia 21. Rio de Janeiro. http://www.rj.gov.br/NoticiaDetalhe.aspx?id_noticia=5586&pl=medidas-restritivas-isolam-capital-do-rio-de-janeiro-a-partir-de-s%C3%A1bado,-dia-21 (accessed 19 March 2020).
http://www.rj.gov.br/NoticiaDetalhe.aspx...
), and Santa Catarina (Brazil 2020lBRAZIL. 2020l. State Government of Santa Catarina. Coronavírus em SC: Em portaria, Governo do Estado detalha medidas restritivas no enfrentamento à doença. https://www.sc.gov.br/noticias/temas/coronavirus/coronavirus-em-sc-governo-do-estado-detalha-medidas-restritivas-no-enfrentamento-a-doenca (accessed 19 March 2020).
https://www.sc.gov.br/noticias/temas/cor...
). The Senate also unanimously approved, in its first remote deliberative session, a legislative decree, which recognizes the state of public calamity in the country due to the COVID-19 pandemic. The government could be able to breach until December 31st in the current year the fiscal target, and it could release even more resources to fight the pandemic. The instructions until April 20th, indicated that all social isolation measures and commerce closure would be extended until May. Thus, all people in all states should stay in their homes and in some cases possible taxes could be applied.
CHINA’S TEACHINGS AND PROSPECTS FOR THE MANUFACTURE OF IMMUNOBIOLOGICALS
Chineses discovered the virus and sequenced its entire genome a week later from the first identified case. Compared with other cases, such as SARS sequencing in 2003 that lasted months or HIV that lasted years, sequencing SARS-CoV-2 had a quick and effective response (Lu et al. 2020LU R ET AL. 2020. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet 395(10224): 565-574.). Obtaining the virus genome was essential to start developing test kits for diagnosis, vaccines strategies, and other treatments (Prompetchara et al. 2020PROMPETCHARA E, KETLOY C & PALAGA T. 2020. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pacific J Allergy Immunol 38: 1-9., Shanker et al. 2020SHANKER A, BHANU D & ALLURI A. 2020. Analysis of Whole Genome Sequences and Homology Modelling of a 3C Like Peptidase and a Non-Structural Protein of the Novel Coronavirus COVID-19 Shows Protein Ligand Interaction with an Aza-Peptide and a Noncovalent Lead Inhibitor with Possible Antiviral Properties. OSF Preprints: https://doi.org/10.31219/osf.io/2zuea.
https://doi.org/10.31219/osf.io/2zuea...
).
China also showed that the clinical features of the first 41 patients in Wuhan who tested positive for SARS-CoV-2 symptoms included diseases of the lower respiratory tract, fever, dry cough, and dyspnea (Huang et al. 2020HUANG C ET AL. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506.). Such manifestations are similar to those diseases caused by other Coronoviridae family representants that cause Severe Acute Respiratory Syndrome (SARS) and the Middle East Respiratory Syndrome (MERS) (Zaki et al. 2012ZAKI AM, VAN BOHEEMEN S, BESTEBROER TM, OSTERHAUS AD & FOUCHIER RA. 2012. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. New Engl J Med 367(19): 1814-1820., De Groot et al. 2013DE GROOT RJ ET AL. 2013. Commentary: Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol 87(14): 7790-7792.). The vast majority of patients after the third day of symptoms showed abnormal characteristics on computed tomography (CT), such as focal ground-glass opacity associated with smooth interlobular and intralobular septal thickening in the right lower lobes and also, a small amount of pleural effusion in some cases (Li et al. 2020LI X, ZAI J, ZHAO Q, NIE Q, LI Y, FOLEY BT & CHAILLON A. 2020. Evolutionary history, potential intermediate animal host, and cross‐species analyses of SARS‐CoV‐2. J Med Virol: https://doi.org/10.1002/jmv.25731.
https://doi.org/10.1002/jmv.25731...
, Wu et al. 2020WU F ET AL. 2020. A new coronavirus associated with human respiratory disease in China. Nature 579(7798): 265-269.). However, different radiological patterns were observed at several times through the course of the disease. For example, the time between the onset of symptoms and the development of acute respiratory distress syndrome (ARDS) was around nine days among patients with COVID-19 pneumonia. These findings confirm the importance of early recognition of the disease is essential for the clinical cure of patients (Zhao et al. 2003ZHAO Z, LIANG C, ZHANG J, ZHANG R & HE H. 2003. Clinical and imaging findings in patients with severe acute respiratory syndrome. Chinese Med J 116(7): 1104-1105., Xu et al. 2020aXU K ET AL. 2020a. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Med Sci 49(1): https://doi.org/10.3785/j.issn.1008-9292.2020.02.02.).
When a new outbreak begins, the primary strategy is specific, reliable, accurate, and detect methods for tracking infected and uninfected people (Ai et al. 2020AI T, YANG Z, HOU H, ZHAN C, CHEN C, LV Q, SUN Z & XIA L. 2020. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology, 200642. https://doi.org/10.1148/radiol.2020200642.
https://doi.org/10.1148/radiol.202020064...
). In the early days of the outbreak in Wuhan, the health system had no test available kits and the screening depended on the laboratory analysis of the nucleic acid sequencing of the virus, which is time-consuming. Thus, second related by China’s Government, China’s National Medical Products Administration took steps to accelerate the work of biotechnology companies to develop detection kits. Therefore, in January 13th, the first kit was released, with enough supplies available just for two weeks. Independent of speculative information about possible China’s Government omission or non-revelation of the true face of its cases, the Chinese experience taught and reinforced to the world the importance of listening to experts in science and public health and made it clear how Chinese people are essential to global health (Anderson et al. 2020ANDERSON RM, HEESTERBEEK H, KLINKENBERG D & HOLLINGSWORTH TD. 2020. How will country-based mitigation measures influence the course of the COVID-19 epidemic? The Lancet 395: P932-934., WHO 2020WHO - WORLD HEALTH ORGANIZATION. 2020. Clinical management of severe acute respiratory infection when novel coronavirus (COVID-19) infection is suspected: interim guidance. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed 19 March 2020).
https://www.who.int/publications-detail/...
).
The systematic and proactive organization of Chinese risks, based on collaboration between government, health experts and WHO, has helped the world during the pandemic through the release of disease-related clinical data (Chinazzi et al. 2020CHINAZZI M ET AL. 2020. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. https://doi.org/10.1126/science.aba9757.
https://doi.org/10.1126/science.aba9757...
, Xu et al. 2020bXU Z ET AL. 2020b. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine 8: 420-422.). Data of more than 40.000 cases in China could shown that 80% of patients infected with COVID-19 did not need medical intervention (asymptomatic), while the other 20% needed treatment in hospitals.
The obtention of immunobiological tools for the infection is associated with insights learned from the outbreak of SARS-CoV and MERS-CoV (Prompetchara et al. 2020PROMPETCHARA E, KETLOY C & PALAGA T. 2020. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pacific J Allergy Immunol 38: 1-9.). As previously mentioned, the immunological findings to date are too preliminary to draw definitive conclusions. Zhou et al. (2020)ZHOU F ET AL. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet 28: 1054-1062., investigating 99 cases, showed an increase in total neutrophils (38%), a reduction in total lymphocytes (35%), and an increase of serum IL-6 (52%) and C-reactive protein (84%). These results were reinforced by Tan et al. (2020)TAN L, WANG Q, ZHANG D, DING J, HUANG Q, TANG YQ, WANG Q & MIAO H. 2020. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. medRxiv 33: https://doi.org/10.1038/s41392-020-0148-4. and Huang et al. (2020)HUANG C ET AL. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506., observing the patients who needed ICU care due to COVID-19, showed the increase of the different cytokines such as IL-2, IL-7, IL-10, G-CSF, IP-10, MCP-1, MIP-1A, and TNF-α. It has also been reported that SARS-CoV directly infects macrophages and T cells, and have a weak antibody response (Perlman & Dandekar 2005PERLMAN S & DANDEKAR AA. 2005. Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol 5(12): 917-927.). Finally, Prompetchara et al. (2020)PROMPETCHARA E, KETLOY C & PALAGA T. 2020. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pacific J Allergy Immunol 38: 1-9. indicated that COVID-19 can induce a “cytokine storm” which can initiate viral sepsis and inflammatory-induced lung injury. Moreover, it can lead to other complications, including pneumonitis, acute respiratory distress syndrome (ARDS), respiratory failure, shock, organ failure, and potentially death.
Thus, SARS-CoV immune response (in severe disease) is associated with strong T cell responses, higher titers of neutralizing antibody, and more serum Th2 cytokines (IL-4, IL-5, IL-10) (Li et al. 2008LI CK ET AL. 2008. T cell responses to whole SARS coronavirus in humans. J Immunol 181(8): 5490-500., Prompetchara et al. 2020PROMPETCHARA E, KETLOY C & PALAGA T. 2020. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pacific J Allergy Immunol 38: 1-9.). Likewise, in MERS-CoV infection, the early increase of CD8+ T cells correlates with disease severity, and at the convalescent phase, dominant Th1 type helper T cells are observed (Shin et al. 2019SHIN HS ET AL. 2019. Immune responses to Middle East respiratory syndrome coronavirus during the acute and convalescent phases of human infection. Clin Infect Dis 68(6): 984-992., Prompetchara et al. 2020PROMPETCHARA E, KETLOY C & PALAGA T. 2020. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pacific J Allergy Immunol 38: 1-9.). Regarding the humoral response, according to Thevarajan et al. (2020)THEVARAJAN I ET AL. 2020. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med 26: 453-455., both immunoglobulin M (IgM) and immunoglobulin G (IgG) are antibodies that bind to the SARS-CoV-2 and they were detected in human blood before symptomatic recovery. These immunological changes persisted for at least 7 days following full resolution of symptoms. Briefly, if the Th1 response appears to be the key to the successful control of SARS-CoV and MERS-CoV, it is likely to be true also for SARS-CoV-2. The enhance of this Th1 response is a feasible way to make an efficient vaccine.
Data from May 2020 showed that more than 120 vaccines have been developed around the world and eight of them already in clinical trials. Specifically in Brazil, researchers from the Immunology Laboratory of the Heart Institute (Medicine Faculty of the São Paulo University) have developed a vaccine based on Virus-Like Particles (VLP). The group of Biotecnology Research Centre (CTVacinas) formed by researchers from Federal University of Minas Gerais, René Rachou Institute and Hearth Institute of São Paulo has developed an attenuated-virus vaccine, based in H1N1 virus inoculated with genetic information of SARS-CoV-2 (Brazil 2020mBRAZIL. 2020m. Journal of USP. Cientistas brasileiros já trabalham numa candidata à vacina contra novo coronavirus. https://jornal.usp.br/ciencias/ciencias-da-saude/cientistas-brasileiros-ja-trabalham-numa-candidata-a-vacina-contra-novo-coronavirus/ (accessed 19 March 2020).
https://jornal.usp.br/ciencias/ciencias-...
,nBRAZIL. 2020n. Journal of USP. Vacina contra coronavírus em desenvolvimento na USP é diferente da Americana. https://jornal.usp.br/ciencias/ciencias-da-saude/vacina-contra-coronavirus-em-desenvolvimento-na-usp-e-diferente-da-americana/ (accessed 19 March 2020).
https://jornal.usp.br/ciencias/ciencias-...
).
COMPARISON WITH THE MOST AFFECTED COUNTRIES NUMBERS
Although we have learned more about the biology of SARS-CoV-2 infection and the consequent development of COVID-2019, a vaccine should only be available to the population within 10 to 18 months (in the best scenery). Regarding the treatment of COVID-2019, the development of new drugs seems to be less feasible due to the time required for their development, testing, approval, and large-scale production. Thus, there is a great effort to evaluate the effectiveness of existing drugs approved for use in other diseases, for the treatment of individuals with COVID-2019. In this sense, there are encouraging results, but nothing conclusive to date.
Brazil, like many other European countries and even the USA, took long to implement preventive measures regarding the entry of SARS-CoV-2 in its territory. Even after confirmation, it seems to have had a delay in the beginning of the social isolation. Brazil has currently gone through the exponential phase of the COVID-19 cases (514,849 confirmed cases and 29,314 deaths in May 30th). Until April, Brazil had a number of cases similar to those in Italy at the beginning of the outbreak. Italy adopted quarantine measures when 7,375 cases and 366 deaths had already been registered in the country (Figure 6). It was observed that countries had their epidemic times started when they had at least 100 cases each (it was considered the 1st day). Figure 6 also shows that as earlier the quarantine begins as slower the progress of the cases. Spain, Italy, France, and the United States initiated social isolation when there were already 6391 (13th day), 7375 (15th day), 7730 (18th day), and 43781 (22nd day) cases, respectively.
Graphic shows the relationship between the speed of the exponential increase in cases of COVID-19 between countries and the beginning of the adoption of social isolation measures (points highlighted). Source: https://www.worldometers.info/coronavirus/.
PRELIMINARY CONCLUSIONS AND FUTURE PERSPECTIVES
The collapse of the health system in Brazil was not different from the world scenery. Data obtained on May 30th (Table II) shows the hard reality of the country and could help some states and municipals in the adoption of new strategies. However, the pressure of the economy, companies, and employers leaded governors and mays to the flexibility of the quarantine from June. In this sense, the future of contagion is uncertain.
Estimative of occupied hospital beds in May 30th, 2020 in Brazilian public hospitals. Data were found in the Health Secretaries sites of each state at the above date.
Although many inforces were adopted to face the disease, great part of the population did not adhere to the social isolation or used a Personal Protective Equipment (PPE) in public places. brazilian media has argumented that possibilitly its due to the brazilian president’s behavior, speech, and actions. The brazilian president showed up himself in brazilian media, without using PPE in public places, disrespecting the social isolation measures imposed by the state governors. In addition, the president has posted in his personal social media different opinions against OMS recommendations.
The brazilian behavior gained international notoriety and The Lancet Journal (May 9th, 2020) showed a repudiation note in response to him. According to the Journal the brazilian President “not only continues to sow confusion by openly flouting and discouraging the sensible measures of physical distancing and lockdown brought in by state governors and city mayors but has also lost two important and influential ministers in the past 3 weeks” (Lancet 2020LANCET. 2020. COVID-19 in Brazil: “So what?”. The Lancet 9(395): 1461. https://doi.org/10.1016/S0140-6736(20)31095-3.).
The social financial support, to informal workers and poor people promoted daily extensive bank rows increasing the virus exposition. In addition, several of health professionals have gotting sick promoting a decrease in the hospital’s attending.
Epidemiology experts from the Brazilian Ministry of Health initially indicated that the peak of the infection could occur in April, with the plateau expectation in May/June, remaining until July and decreasing in August/September. Recent data shows that the probable peak will be in June/July, remaining until August and without decreasing prevision.
Up to the end of this manuscript written (June 7th), the curve of confirmed cases had been increased and health services like reference hospitals and field hospitals, in different states of the country, showed only 10% of the total capacity to attend the patients with COVID-19. The perspective of some sanitary authorities up to the end of the first semester of 2020 is the collapse of hospitals, morgues, and cemeteries, and many sick and depressed health professionals. However, with the science advance is possible to find anytime a new drug, combined treatments, and efficient immunotherapies to change this panorama until the vaccine discovery.
REFERENCES
- AHN DG, CHOI JK, TAYLOR DR & OH JW. 2012. Biochemical characterization of a recombinant SARS coronavirus nsp12 RNA-dependent RNA polymerase capable of copying viral RNA templates. Archi Virol 157(11): 2095-2104.
- AI T, YANG Z, HOU H, ZHAN C, CHEN C, LV Q, SUN Z & XIA L. 2020. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology, 200642. https://doi.org/10.1148/radiol.2020200642
» https://doi.org/10.1148/radiol.2020200642 - ANDERSON RM, HEESTERBEEK H, KLINKENBERG D & HOLLINGSWORTH TD. 2020. How will country-based mitigation measures influence the course of the COVID-19 epidemic? The Lancet 395: P932-934.
- BRAZIL. 2020a. Ministry of Health. Brasil amplia diagnóstico para o coronavírus. https://www.saude.gov.br/noticias/agencia-saude/46472-brasil-amplia-diagnostico-para-o-coronavirus (accessed 19 March 2020).
» https://www.saude.gov.br/noticias/agencia-saude/46472-brasil-amplia-diagnostico-para-o-coronavirus - BRAZIL. 2020b. Ministry of Health. Estados terão R$ 432 milhões para enfrentar o COVID-19. https://www.saude.gov.br/noticias/agencia-14saude/46547-estados-terao-r-432-milhoes-para-enfrentar-covid-19 (accessed 19 March 2020).
» https://www.saude.gov.br/noticias/agencia-14saude/46547-estados-terao-r-432-milhoes-para-enfrentar-covid-19 - BRAZIL. 2020c. Ministry of Health. Brasil confirma primeiro caso da doença. https://www.saude.gov.br/noticias/agencia-saude/46435-brasil-confirma-primeiro-caso-de-novo-coronavirus (accessed 19 March 2020).
» https://www.saude.gov.br/noticias/agencia-saude/46435-brasil-confirma-primeiro-caso-de-novo-coronavirus - BRAZIL. 2020d. Ministry of Economy. Confira as medidas tomadas pelo Ministério da Economia em função do Covid-19 (Coronavírus). http://www.economia.gov.br/noticias/2020/marco/confira-as-medidas-tomadas-pelo-ministerio-da-economia-em-funcao-do-covid-19-coronavirus (accessed 19 March 2020).
» http://www.economia.gov.br/noticias/2020/marco/confira-as-medidas-tomadas-pelo-ministerio-da-economia-em-funcao-do-covid-19-coronavirus - BRAZIL. 2020e. Ministry of Economy. Ministério da Economia anuncia medidas para diminuir o impacto do coronavírus no país. http://www.economia.gov.br/noticias/2020/marco/ministerio-da-economia-anuncia-medidas-para-diminuir-o-impacto-do-coronavirus-no-pais (accessed 19 March 2020).
» http://www.economia.gov.br/noticias/2020/marco/ministerio-da-economia-anuncia-medidas-para-diminuir-o-impacto-do-coronavirus-no-pais - BRAZIL. 2020f. State Government of São Paulo. Governo recomenda fechamento de shoppings e academias da Grande SP até fim de abril. São Paulo. http://www.saopaulo.sp.gov.br/spnoticias/governo-recomenda-fechamento-hoppings-academias/ (accessed 19 March 2020).
» http://www.saopaulo.sp.gov.br/spnoticias/governo-recomenda-fechamento-hoppings-academias/ - BRAZIL. 2020g. City Hall of São Paulo. Situação de Emergência: Prefeitura de São Paulo adota medidas para evitar disseminação do coronavírus. São Paulo. http://www.capital.sp.gov.br/noticia/situacao-de-emergencia-prefeitura-de-sao-paulo-adota-medidas-para-evitar-disseminacao-do-coronavirus (accessed 19 March 2020).
» http://www.capital.sp.gov.br/noticia/situacao-de-emergencia-prefeitura-de-sao-paulo-adota-medidas-para-evitar-disseminacao-do-coronavirus - BRAZIL. 2020h. State Government of Pernambuco. Governo de Pernambuco anuncia novas medidas restritivas de combate ao Covid-19. Pernambuco. http://www.pe.gov.br/mobile/blog/2020/03/19/governo-de-pernambuco-anuncia-novas-medidas-restritivas-de-combate-ao-covid-19 (accessed 19 March 2020).
» http://www.pe.gov.br/mobile/blog/2020/03/19/governo-de-pernambuco-anuncia-novas-medidas-restritivas-de-combate-ao-covid-19 - BRAZIL. 2020i. State Government of Bahia. Entenda as medidas de combate ao coronavírus na Bahia. http://www.ba.gov.br/noticias/entenda-medidas-de-combate-ao-coronavirus-na-bahia-1 (accessed 19 March 2020).
» http://www.ba.gov.br/noticias/entenda-medidas-de-combate-ao-coronavirus-na-bahia-1 - BRAZIL. 2020j. State Government of Ceará. Governo do Ceará determina novas medidas de enfrentamento ao coronavírus. https://www.ceara.gov.br/2020/03/19/governo-do-ceara-determina-novas-medidas-de-enfrentamento-ao-coronavirus/ (accessed 19 March 2020).
» https://www.ceara.gov.br/2020/03/19/governo-do-ceara-determina-novas-medidas-de-enfrentamento-ao-coronavirus/ - BRAZIL. 2020k. State Government of Rio de Janeiro. Medidas restritivas isolam capital do Rio de Janeiro a partir de sábado, dia 21. Rio de Janeiro. http://www.rj.gov.br/NoticiaDetalhe.aspx?id_noticia=5586&pl=medidas-restritivas-isolam-capital-do-rio-de-janeiro-a-partir-de-s%C3%A1bado,-dia-21 (accessed 19 March 2020).
» http://www.rj.gov.br/NoticiaDetalhe.aspx?id_noticia=5586&pl=medidas-restritivas-isolam-capital-do-rio-de-janeiro-a-partir-de-s%C3%A1bado,-dia-21 - BRAZIL. 2020l. State Government of Santa Catarina. Coronavírus em SC: Em portaria, Governo do Estado detalha medidas restritivas no enfrentamento à doença. https://www.sc.gov.br/noticias/temas/coronavirus/coronavirus-em-sc-governo-do-estado-detalha-medidas-restritivas-no-enfrentamento-a-doenca (accessed 19 March 2020).
» https://www.sc.gov.br/noticias/temas/coronavirus/coronavirus-em-sc-governo-do-estado-detalha-medidas-restritivas-no-enfrentamento-a-doenca - BRAZIL. 2020m. Journal of USP. Cientistas brasileiros já trabalham numa candidata à vacina contra novo coronavirus. https://jornal.usp.br/ciencias/ciencias-da-saude/cientistas-brasileiros-ja-trabalham-numa-candidata-a-vacina-contra-novo-coronavirus/ (accessed 19 March 2020).
» https://jornal.usp.br/ciencias/ciencias-da-saude/cientistas-brasileiros-ja-trabalham-numa-candidata-a-vacina-contra-novo-coronavirus/ - BRAZIL. 2020n. Journal of USP. Vacina contra coronavírus em desenvolvimento na USP é diferente da Americana. https://jornal.usp.br/ciencias/ciencias-da-saude/vacina-contra-coronavirus-em-desenvolvimento-na-usp-e-diferente-da-americana/ (accessed 19 March 2020).
» https://jornal.usp.br/ciencias/ciencias-da-saude/vacina-contra-coronavirus-em-desenvolvimento-na-usp-e-diferente-da-americana/ - CHINAZZI M ET AL. 2020. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. https://doi.org/10.1126/science.aba9757
» https://doi.org/10.1126/science.aba9757 - DE BARROS FRPM & SANTOS VGBCB. 2019. Análise do protecionismo brasileiro pós-crise de 2008: barreiras comerciais e planos de incentivos à indústria. Rev Econ Pol Desenv 6(21): 1-14.
- DECROLY E ET AL. 2011. Crystal structure and functional analysis of the SARS-coronavirus RNA cap 2‐-O-methyltransferase nsp10/nsp16 complex. PLoS pathogens 7.5(5): e1002059.
- DE GROOT RJ ET AL. 2013. Commentary: Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol 87(14): 7790-7792.
- GAO J, TIAN Z & YANG X. 2020. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioScience Trends: https://doi.org/10.5582/bst.2020.01047
» https://doi.org/10.5582/bst.2020.01047 - GORBALENYA AE. 2020. Severe acute respiratory syndrome-related coronavirus–The species and its viruses, a statement of the Coronavirus Study Group. BioRxiv: https://doi.org/10.1101/2020.02.07.937862
» https://doi.org/10.1101/2020.02.07.937862 - HEYMANN DL & SHINDO N. 2020. COVID-19: what is next for public health? The Lancet 395(10224): 542-545.
- HUANG C ET AL. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506.
- HU B ET AL. 2017. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus.PLoS pathogens 13(11): e1006698.
- HU D ET AL. 2018. Genomic characterization and infectivity of a novel SARS-like coronavirus in Chinese bats. Emerg Microb Infect 7.1: 1-10.
- HWANG WC, LIN Y, SANTELLI E, SUI J, JAROSZEWSKI L, FARZAN M, MARASCO W & LIDDINGTON R. 2006. Structural basis of neutralization by a human anti-severe acute respiratory syndrome spike protein antibody, 80R. J Biol Chem 281(45): 34610-34616.
- JAIMES JA, ANDRE NM, MILLET JK & WHITTAKER GR. 2020. Structural modeling of 2019-novel coronavirus (nCoV) spike protein reveals a proteolytically-sensitive activation loop as a distinguishing feature compared to SARS-CoV and related SARS-like coronaviruses. arXiv preprint 2002: 06196. https://doi.org/10.1101/2020.02.10.942185.
- KIM Y, JEDRZEJCZAK R, MALTSEVA NI, ENDRES M, GODZIK A, MICHALSKA K & JOACHIMIAK A. 2020. Crystal structure of Nsp15 endoribonuclease NendoU from SARS-CoV-2. bioRxiv: https://doi.org/10.1101/2020.03.02.968388
» https://doi.org/10.1101/2020.03.02.968388 - KIRCHDOERFER RN & WARD AB. 2019. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nature comm 10(1): 1-9.
- KONG W & AGARWAL PP. 2020. Chest imaging appearance of COVID-19 infection. Radiol Cardioth Imag 2(1): e200028.
- KUBA K ET AL. 2005. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nature Medicine 11(8): 875-879.
- LANCET. 2020. COVID-19 in Brazil: “So what?”. The Lancet 9(395): 1461. https://doi.org/10.1016/S0140-6736(20)31095-3.
- LEHMANN KC ET AL. 2015. Discovery of an essential nucleotidylating activity associated with a newly delineated conserved domain in the RNA polymerase- containing protein of all nidoviruses. Nucl Acid Res 43(17): 8416-8434.
- LETKO M, MARZI A & MUNSTER V. 2020. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nature Microbiol: 1-8.
- LI CK ET AL. 2008. T cell responses to whole SARS coronavirus in humans. J Immunol 181(8): 5490-500.
- LI W ET AL. 2003. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426(6965): 450-454.
- LI X, ZAI J, ZHAO Q, NIE Q, LI Y, FOLEY BT & CHAILLON A. 2020. Evolutionary history, potential intermediate animal host, and cross‐species analyses of SARS‐CoV‐2. J Med Virol: https://doi.org/10.1002/jmv.25731
» https://doi.org/10.1002/jmv.25731 - LU R ET AL. 2020. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet 395(10224): 565-574.
- MAHASE E. 2020. Coronavirus: covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ 368: m641.
- MING-YEN N ET AL. 2020. Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiol Cardioth Imag 2(1): e200034.
- MURDOCH DR & FRENCH NP. 2020. COVID-19: another infectious disease emerging at the animal-human interface. NZ Med J 133(1510): 12-15.
- PERLMAN S & DANDEKAR AA. 2005. Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol 5(12): 917-927.
- PRABAKARAN S ET AL. 2004. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psych 9(7): 684-697.
- PROMPETCHARA E, KETLOY C & PALAGA T. 2020. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pacific J Allergy Immunol 38: 1-9.
- RODRIGUES RDSA & CLEMENTE A. 2019. Efeitos da corrupção nas bolsas de valores na Crise Financeira de 2008. Ver Contab Organiz 13: 51-63.
- SEVAJOL M, SUBISSI L, DECROLY E & IMBERTM BCI. 2014. Insights into RNA synthesis, capping, and proofreading mechanisms of SARS-coronavirus. Virus Res 194: 90-99.
- SHANKER A, BHANU D & ALLURI A. 2020. Analysis of Whole Genome Sequences and Homology Modelling of a 3C Like Peptidase and a Non-Structural Protein of the Novel Coronavirus COVID-19 Shows Protein Ligand Interaction with an Aza-Peptide and a Noncovalent Lead Inhibitor with Possible Antiviral Properties. OSF Preprints: https://doi.org/10.31219/osf.io/2zuea
» https://doi.org/10.31219/osf.io/2zuea - SHI M ET AL. 2018. The evolutionary history of vertebrate RNA viruses. Nature 556(7700): 197-202.
- SHIN HS ET AL. 2019. Immune responses to Middle East respiratory syndrome coronavirus during the acute and convalescent phases of human infection. Clin Infect Dis 68(6): 984-992.
- TANG X ET AL. 2020. On the origin and continuing evolution of SARS-CoV-2. Nat Sci Rev: https://doi.org/10.1093/nsr/nwaa036
» https://doi.org/10.1093/nsr/nwaa036 - TAN L, WANG Q, ZHANG D, DING J, HUANG Q, TANG YQ, WANG Q & MIAO H. 2020. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. medRxiv 33: https://doi.org/10.1038/s41392-020-0148-4.
- THEVARAJAN I ET AL. 2020. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med 26: 453-455.
- TIAN X, LI C, HUANG A, XIA S, LU S, SHI Z, LU L, JIANG S, YANG Z, WU Y & YING T. 2020. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microb Infect 9(1): 382-385.
- WANG M & HU Z. 2013. Bats as animal reservoirs for the SARS coronavirus: hypothesis proved after 10 years of virus hunting. Virol Sinica 28(6): 315-317.
- WHO - WORLD HEALTH ORGANIZATION. 2020. Clinical management of severe acute respiratory infection when novel coronavirus (COVID-19) infection is suspected: interim guidance. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed 19 March 2020).
» https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected - WU F ET AL. 2020. A new coronavirus associated with human respiratory disease in China. Nature 579(7798): 265-269.
- XU K ET AL. 2020a. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Med Sci 49(1): https://doi.org/10.3785/j.issn.1008-9292.2020.02.02.
- XU Z ET AL. 2020b. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine 8: 420-422.
- ZAKI AM, VAN BOHEEMEN S, BESTEBROER TM, OSTERHAUS AD & FOUCHIER RA. 2012. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. New Engl J Med 367(19): 1814-1820.
- ZHAO Z, LIANG C, ZHANG J, ZHANG R & HE H. 2003. Clinical and imaging findings in patients with severe acute respiratory syndrome. Chinese Med J 116(7): 1104-1105.
- ZHOU F ET AL. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet 28: 1054-1062.
- ZIEBUHR J. 2005. The coronavirus replicase. In: Coronavirus replication and reverse genetics. Springer, Berlin, Heidelberg, p. 57-94.
Publication Dates
-
Publication in this collection
24 Aug 2020 -
Date of issue
2020
History
-
Received
8 May 2020 -
Accepted
22 June 2020