Abstract
This study examines the distribution of primary and secondary space holders integrated in morphological and feeder functional groups as well as the species richness over the seasons on northern Patagonian rocky shores. We also evaluated whether the temporal changes in species richness, assemblage structure and species turnover were related to seasonal changes. These patterns were studied by non-destructive samplings between December 2013 and March 2015. Thirty taxa were identified, including six algal morphological functional and three invertebrate feeder groups. The cover of seaweeds and invertebrates was positively correlated with changes in the environmental factors. The marked seasonality in this Patagonian zone changed between two contrasting periods. Species richness and assemblage structure were associated with seasonal changes and were influenced by the more abundant morphological and feeder functional groups. We suggest that habitat-formers, such as articulated calcareous and suspensor feeder groups, can expand and retract over time-scales of months to seasons, generating available space for the colonization and growth of other organisms. We conclude that seasonal changes and the dynamics of articulated calcareous and suspensor feeder groups drive changes in the algal and invertebrate abundances, in turn driving changes in species richness and assemblage structure.
Key words
Spatial and temporal scales; Rocky shores; Seaweeds; Invertebrates
INTRODUCTION
Seaweeds as primary-space holders are major components of intertidal and subtidal communities in intertidal rocky systems; they contribute significantly to primary marine production and form nursery habitats for a diverse benthic fauna (Lüning 1990LÜNING K. 1990. Seaweeds: Their environment, biogeography, and ecophysiology. Wiley-Interscience Publication, New York, USA, 527 p.). In rocky shore benthic communities, the primary-space holders include primary producers and filter feeders (Menge & Sutherland 1987MENGE BA & SUTHERLAND JP. 1987. Community regulation: variation in disturbance, competition, and predation in relation to environmental stress and recruitment. Amer Naturalist 130: 730-757.) and the secondary-space holders include herbivores and carnivores, which are mobile consumers, but many secondary-space holders are also primary producers or filter feeders (Bruno et al. 2003BRUNO JF, STACHOWICZ JJ & BERTNESS MD. 2003. Inclusion of facilitation into ecological theory. Trends Ecol Evol 18: 119-125.). Species richness expressed as the number of species in a community is one of the main traits of communities because it influences stability, productivity and susceptibility to invasion (Scrosati et al. 2011SCROSATI RA, VAN GENNE B, HEAVEN CS & WATT CA. 2011. Species richness and diversity in different functional groups across environmental stress gradients: a model for marine rocky shores. Ecography 34: 151-161.). Also, temporal variability in primary-space holders richness and their associated secondary-space holders and mobile species can cause variability in a regional species pool (Menge & Sutherland 1987MENGE BA & SUTHERLAND JP. 1987. Community regulation: variation in disturbance, competition, and predation in relation to environmental stress and recruitment. Amer Naturalist 130: 730-757., Bruno et al. 2003BRUNO JF, STACHOWICZ JJ & BERTNESS MD. 2003. Inclusion of facilitation into ecological theory. Trends Ecol Evol 18: 119-125.). Hence, the species richness in functional groups influences the community functioning (Cardinale et al. 2006CARDINALE BJ, SRIVASTAVA DS, DUFFY JE, WRIGHT JP, DOWNING AL, SANKARAN M & JOUSEAU C. 2006. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443: 989-992., Duffy et al. 2007DUFFY JE, CARDINALE BJ, FRANCE KE, MCINTYRE PB, THéBAULT E & LOREAU M. 2007. The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecol Lett 10: 522-538., Bruno & Cardinale 2008BRUNO JF & CARDINALE BJ. 2008. Cascading effects of predator richness. Front Ecol Environ 6: 539-546.).
From an environmental point of view, different abiotic factors such as desiccation, seawater temperature and solar radiation emerge as the main drivers of ecological processes and patterns, depending on the spatial and temporal scales in marine ecosystems (Levin 1992LEVIN SA. 1992. The problem of pattern and scale in ecology. Ecology 73: 1943-1967., Willig et al. 2003WILLIG MR, KAUFMAN DM & STEVENS RD. 2003. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu Rev Ecol Evol S 34: 273-309.). Determination at different scales, ranging from local patchiness to variation over biogeographical gradients of species richness, diversity and composition of intertidal marine communities, may provide information on community structure and functioning (Kraufvelin et al. 2010KRAUFVELIN P, LINDHOLM A, PEDERSEN MF, KIRKERUD LA & BONSDORFF E. 2010. Biomass, diversity and production of rocky shore macroalgae at two nutrient enrichment and wave action levels. Mar Biol 157: 29-47., Hooper et al. 2012HOOPER DU, ADAIR EC, CARDINALE BJ, BYRNES JEK, HUNGATE BA, MATULICH KL, GONZALEZ A, DUFFY JE, GAMFELDT L & CONNOR MI. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486: 105-108., Cardinale et al. 2013CARDINALE BJ, GROSS K, FRITSCHIE K, FLOMBAUM P, FOX JW, RIXEN C, VAN RUIJVEN J, REICH PB, SCHEREN-LORENZEN M & WILSEY BJ. 2013. Biodiversity simultaneously enhances the production and stability of community biomass, but the effects are independent. Ecology 94: 1697-1707.).
Because the sessile condition of primary-space holders they are constrained to the effects of long-term exposure to environmental stress, resulting in changes in the structure of the assemblages (Eriksson et al. 2002ERIKSSON O, COUSINS SAO, BRUUN HH & DíAZ S. 2002. Land-use history and fragmentation of traditionally managed grasslands in Scandinavia. J Veg Sci 13: 743-748., Karez et al. 2004KAREZ R, ENGELBERT S, KRAUFVELIN P, PEDERSEN MF & SOMMER U. 2004. Biomass response and changes in composition of ephemeral macroalgal assemblages along an experimental gradient of nutrient enrichment. Aquat Bot 78: 103-117., Kraufvelin et al. 2006KRAUFVELIN P, MOY FE, CHRISTIE H & BOKN TL. 2006. Nutrient addition to experimental rocky shore communities revisited: delayed responses, rapid recovery. Ecosystems 9: 1076-1093., Pinedo et al. 2007PINEDO S, GARCíA M, SATTA MP, TORRES M & BALLESTEROS E. 2007. Rocky-shore communities as indicators of water quality: A case study in the Northwestern Mediterranean. Mar Pollut Bull 55: 126-135.). For this reason, seaweeds are considered useful descriptors of environmental characteristics of coastal habitats, and are being used as ecological quality bio-indicators under the Water Framework Directive (Directive 2000Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. OJ L 327: 1-51./60/EC). Thus, in order to capture the variability of benthic assemblages and relate it to their environment, biodiversity assessments usually rely on proxy measurements, such as structural components of assemblages based on species level taxonomical identifications. However, the enumeration of all species is a time-consuming task requiring expertise, so extrapolative and other techniques are always sought to optimize biodiversity assessment efficiencies (Gaspar et al. 2017GASPAR R, PEREIRA L & NETO JM. 2017. Intertidal zonation and latitudinal gradients on macroalgal assemblages: Species, functional groups and thallus morphology approaches. Ecol Indic 81: 90-103.). Thus, in order to optimize time and resources taken in identifying species-level taxa, and to provide insights into the functional structure of benthic communities, species can be grouped into different functional groups, based on their ecological and morphological attributes in the case of seaweeds, and the feeding type in invertebrates (Litter & Litter 1980LITTER MM & LITTER DS. 1980. The evolution of thallus form and survival strategies in benthic marine macroalgae: field and laboratory tests of a functional form model. Amer Naturalist 116: 25-44., Steneck & Dethier 1994STENECK RL & DETHIER MN. 1994. A functional group approach to the structure of algal dominated communities. Oikos 69: 476-498., Balata et al. 2011BALATA D, PIAZZI L & RINDI F. 2011. Testing a new classification of morphological functional groups of marine macroalgae for the detection of responses to stress. Mar Biol 158: 2459-2469.).
Macroalgal functional groups have been widely used, categorizing species differing in morphological features and in how these form-based features are supposedly linked to different ecological functions (Steneck & Dethier 1994STENECK RL & DETHIER MN. 1994. A functional group approach to the structure of algal dominated communities. Oikos 69: 476-498.). Moreover, biological traits, usually measured at the individual level and used comparatively across species, may be seen as well as a surrogate measure of the (species-level measured) biodiversity (McGill et al. 2006MCGILL BJ, ENQUIST BJ, WEIHER E & WESTOBY M. 2006. Rebuilding community ecology from functional traits. Trends Ecol Evol 21: 178-185.).
Despite the ecological and economic importance of Patagonian coasts, its benthic diversity remains poorly understood, except for some specific sites (Torres & Caille 2009TORRES A & CAILLE G. 2009. The hard-bottom intertidal communities before and after removal of an anthropogenic disturbance: a case study in the coast of Puerto Madryn (Patagonia, Argentina). Rev Biol Mar Oceanog 44: 517-521., Irigoyen et al. 2010IRIGOYEN AJ, TROBBIANI G, SGARLATTA MP & RAFFO MP. 2010. Effects of the alien algae Undaria pinnatifida (Phaeophyceae, Laminariales) on the diversity and abundance of benthic macrofauna in Golfo Nuevo (Patagonia, Argentina): potential implications for local food webs. Biol Invasion 1: 1521-1532., Sueiro et al. 2011SUEIRO MC, BORTOLUS A & SCHWINDT E. 2011. Habitat complexity and community composition: relationships between different ecosystem engineers and the associated macroinvertebrate assemblages. Helgoland Mar Res 65: 467-477.). In addition, there are difficulties related to the assessment of benthic communities, such as marine invertebrates and seaweeds, which thrive in intertidal rocky shores. Such difficulties include: a) high natural variability across a range of spatial and temporal scales, b) their diversity assessment implying high sampling and laboratory processing effort and good taxonomical expertise, and c) insufficient knowledge about their structural and functional characteristics. Considering these difficulties, the objective of the present work was to study how different functional groups, including seaweeds trait-based thallus morphologies and invertebrate feeding types, respond to temporal gradient in the lower intertidal zone along the Punta Este shore in Western Patagonia.
MATERIALS AND METHODS
Study area
This study was performed on the northern Patagonian coast of Argentina at Golfo Nuevo, on the rocky shore of Punta Este (42°78’S, 64°95’W) (Fig. 1). Golfo Nuevo is a semi enclosed basin of low hydrodynamics, located in the transition zone between the cold-temperate and warm-temperate biogeographic regions of the south-western Atlantic Ocean. The region is characterized by extreme weather conditions, with a predominance of strong winds from the west and low humidity (Paruelo et al. 1998PARUELO JM, BELTRAN A, JOBBAGY SALA OE & GOLLUSCIO RA. 1998. The climate of Patagonia: general patterns and controls on biotic processes. Ecol Aust 8: 85-101.). These strong, dry winds, combined with low local rainfall, make the Patagonian intertidal zone the place with the highest desiccation stress recorded for rocky shore communities (Bertness et al. 2006BERTNESS MD, CRAIN CM, SILLIMAN BR, BAZTERRICA MC, REYNA MV, HIDALGO F & FARINA JK. 2006. The community structure of western Atlantic Patagonian rocky shores. Ecol Monogr 76: 439-460.). The tide regime is semidiurnal, and the mean amplitude is 3.8 m, reaching 5.7 m in spring. Water temperature and salinity fluctuate between 10 to 19.5 °C and 33.7 to 33.9% in summer and winter, respectively.
Variation in daylength (hours), solar radiation (Wh/m2 day) and surface seawater temperature (°C) monitored between December 2013 and March 2015.
Sampling and proceeding
Non-invasive samplings using photography and observational techniques as well as percentage cover estimates of invertebrates and seaweeds were performed between December 2013 and March 2015. Samplings were made every 15 days, at low tide in the lower intertidal zone. The low-intertidal level at Punta Este has a mean height of 2.05 m above sea level and was defined as the zone dominated by dense coralline algal cover. At the lower intertidal level, six quadrats of 0.5 × 0.5 m (i.e., 0.25 m2) were marked exactly. Each replicate was individually georeferenced and later photographed every fifteen days during the entire sampling period. The quadrats were located in three different positions in the intertidal zone, western, middle and eastern zones, since the air exposure time from west to east is different during low tide, the quadrats located in eastern zone being more exposed. Post hoc species sample analyses indicated that on average 75% ± 5.0 SE of the species pool was sampled each time.
Whenever necessary, multi-layered species assemblages were taken into account by setting aside canopy species for secondary photographic records of understory species.
In each quadrat divided into 25 equal 0.25 m2 frame sections we measured each species percentage cover using the digital processing software ImageJ. For data analyses, it is important to have abundance data expressed in the same measurement unit for every species. We chose percentage cover to quantify abundance because alternative measures (e.g. individual density) cannot always be reliably determined for clonal organisms (Scrosati 2005SCROSATI RA. 2005. Review of studies on biomass-density relationships (including self-thinning lines) in seaweeds: Main contributions and persisting misconceptions. Phycol Res 53: 224-233.) or (e.g. biomass) would have implied destructive sampling of numerous shore areas. No organisms were removed from the quadrats. Cover values were obtained from the projection of three-dimensional structures to the plane of the sampling frame. Total percentage cover for a quadrat could exceed 100% because of the assemblage multi-layered structure. When the cover was less than 1% in total for a given species, we recorded it as 0.5% (Dethier et al. 1993DETHIER MN, GRAHAM ES, COHEN S & TEAR LM. 1993. Visual versus random point percent cover estimations: objective is not always better. Mar Ecol Prog Ser 96: 93-100., Scrosati 2016). In each photograph, all taxa present in the quadrat were identified.
Taxa assignments
To identify the invertebrates and seaweeds, samples near to the marked quadrats were removed from the substrate and placed in bags. The invertebrates were fixed in 10% formalin and later transferred to 70% ethanol. The algae samples were squeezed by hand to remove excess seawater and subsequently transported to the laboratory in closed plastic bags. Samples were stored overnight at 5 °C. The following day, each specimen was washed thoroughly with seawater to remove adhering sand and the fronds were stored in FAA (ethyl alcohol: formaldehyde: acetic acid at 8:1:1). Invertebrates and algae were identified to the lowest possible taxonomic level. The algae taxa were assigned to different traits: a) growth (fast or slow growth), thallus longevity (annual or perennial) and succession (opportunistic or late successional); and b) thallus morphology (filamentous and leaf-like, fleshy, thick, or calcareous upright, and calcareous and non-calcareous crusts) (Orfanidis et al. 2011ORFANIDIS S, PANAYOTIDIS P & UGLAND KI. 2011. Ecological Evaluation Index continuous formula (EEI-c) application: a step forward for functional groups, the formula and reference condition values. Mediterr Mar Sci 12: 199-231.). Also, algae taxa were assigned according to the different morphological-functional groups, namely, filamentous, foliose, corticated foliose, corticated, leathery, articulated calcareous, and crustose (Steneck & Dethier 1994STENECK RL & DETHIER MN. 1994. A functional group approach to the structure of algal dominated communities. Oikos 69: 476-498.).
Each invertebrate organism was assigned to a functional group according to its feeding type: suspension feeder, predator - scavenger and grazer. The mytillids Perumytilus purpuratus, Brachidontes rodriguezii, Mytilus edulis and Aulacomya atra were counted and grouped together under the name “mussels” because of their difficult identification in the photographs.
Environmental data
Surface seawater temperature and daylength were measured daily at the sampling location. Radiation data were provided by Automatic Meteorological Station of Climatology Laboratory of CENPAT-CONICET. Monthly average values are reported.
DATA ANALYSIS
The information on species cover was used to construct a comprehensive table where each species is classified as very frequent (recorded 19-32 times), frequent (recorded 10-18 times), or rare (recorded 1-9 times). Frequency of occurrence of each taxon was calculated by counting the number of sampling times in which each species was recorded. Species turnover was calculated by counting how many species were lost or gained from all quadrats between successive sampling times.
Multivariate analysis of morphological and feeder functional group were performed using the statistical package PRIMER 6 with the PERMANOVA add-on (Clarke & Gorley 2006CLARKE KR & GORLEY RN. 2006. PRIMER V6: Manual/Tutorial. PRIMER-E, Plymouth, 192 p., Anderson et al. 2008ANDERSON MJ, GORLEY RN & CLARKE KR 2008. PERMANOVA for PRIMER: Guide to Software and Statistical Methods. PRIMER-E, Plymouth, UK, 214 p.). Multivariate relationships were assessed by correlating the similarity matrices (RELATE) of environmental (Euclidean distance) data to each other and to model matrices for cyclicity, testing for seasonality and gradients following individual environmental variables. Cover data based on species and functional groups were used to construct resemblance matrices with a Bray-Curtis similarity index.
Differences in functional group and species richness were assessed by analysing species percent cover with a Permutational Multivariate Analysis of Variance. The analysis had three fixed factors: months, seasons and position and was performed on Bray-Curtis matrix of square-root transformed data, using 9999 permutations of residuals under a reduced model. Pair-wise comparisons were then performed for significant factors with more than two levels. The different seasons were defined by the following months: autumn (March, April and May), winter (June, July and August), spring (September, October and November) and summer (December, January and February). SIMPER test was used to determine the relative contribution of each functional group to the average Bray–Curtis dissimilarity between positions and seasons. This method determines which morphological and feeder group and taxa were responsible for any differences.
In order to relate environmental variables to the community, BEST/BIOENV procedure (Clarke & Gorley 2006CLARKE KR & GORLEY RN. 2006. PRIMER V6: Manual/Tutorial. PRIMER-E, Plymouth, 192 p.) was applied. Environmental variables were correlated with each other in order to detect multicollinearity and exclude it in the analysis since it would not provide additional information. Before applying the method, the environmental variables were log-transformed and were categorised with Euclidean similarities while biological data were square root transformed and categorised using Bray–Curtis similarities. Then data matrices were analysed with BIOENV, estimating Spearman rank correlation between these two matrices.
The relationship between environmental and biological variables (algal and invertebrate assemblages, species richness and abundance of articulated calcareous and suspensor feeder groups) was assessed by Pearson correlation. Principal component analysis (PCA) was used to identify the main environmental gradients across the samples time. The PCA ordination was performed on a correlation matrix after logarithmic transformation to partially reduce the large differences in raw values among environmental variables. Daylength variable was not included in the PCA because it was strongly correlated with radiation and therefore was considered redundant. A subsequent mapping of the biological data from cluster analysis onto the environmental ordination allowed for a visual assessment of the correspondence between major biological patterns and dominant environmental gradients.
RESULTS
Benthic community assemblage
A total of 32 invertebrate and algae taxa were identified from 192 photographic samples. Seaweeds were represented by 18 taxa and the invertebrates by 14 (Tables I and II, respectively). Algal taxa were assigned to six thallus morphology types, two types of growth and successional traits, and seven different morphological functional groups. The annual, opportunistic and fast-growing species were featured as fleshy, filamentous and leaf-like morphologies. The fleshy morphology was represented by five brown algae, while filamentous morphology was observed in brown, green and red algae. The leaf- like morphology was only represented by the genus Ulva. Fleshy species comprise the fleshy corticated and fleshy corticated foliose groups, and were only represented by brown algal species. The filamentous functional group exhibited filamentous morphology and was mainly integrated by red algae. Thick, crustose non-calcareous and calcareous upright morphologies corresponded to slow-growing, late succession and perennial species. These categories, consisting of three taxa, Undaria pinnatifida, Ralfsia verrucosa, and Corallina sp., represented three different functional groups, leathery, crustose, and articulated calcareous, respectively. The leathery functional group was represented by the introduced kelp, U. pinnatifida, which can reach high abundance in the subtidal zone of Punta Este.
Algal taxa assignments in term of growth/thallus longevity/succession, thallus morphology and morphological functional group.
Table II shows the invertebrate taxa assignments in term of feeder functional group. Three different feeder functional groups were represented by the invertebrates found in the lower intertidal zone. The suspension feeder group was integrated by the highest number of taxa; it is three molluscan species, the introduced Balanus glandula and a non-identified species of sponge. The grazer feeder group was constituted by molluscan species. The third group, predator scavenger, was represented by two taxa, the molluscan Buccinanops deformis and the cnidarian Antholoba achates.
The abundance (% cover) and occurrence of taxa from each group (invertebrates and algae) are listed in Table III. 56.2% of all taxa were very frequent, 37.5% were rare and only 6.2% of the taxa were frequent. Even though the rare species represented 37.5% of the benthic assemblage, its cover did not exceed 0.12%, whereas the two frequent species covered a greater area of more than 0.57%. The very frequent species assemblages had the greatest contribution to the percentage cover, reaching 73.8%. All the morphological and feeder functional groups recorded in this intertidal zone were represented in the very frequent species. The remaining 25.5% cover was represented by bare substrate.
Occurrence expressed as the presence at any sampling time or number of samples of rare species (recorded 1-9 times), frequent species (recorded 10-18 times) and very frequent species (recorded 19-32 times). Biomass is cover/0.25 m2 ± standard error; single occurrence denoted by (-), no error is reported.
Temporal variations of environmental parameters, species richness and benthic covers
Environmental parameters
Physical environmental parameters showed a clear seasonal pattern, typical of the transitional north Patagonian coastal area. Seawater temperature, daylength and solar radiation peaked in summer (December to February) (Fig. 1). Both summer periods considered in this study were similar over the two consecutive years (ρcyclicity=0.649, p<0.001) (Fig. 2).
Species richness and benthic covers
The total species richness estimated in the sampling time ranged from 14 to 18 taxa. The number of species was variable when comparing both months and seasons (Months: PERMANOVA pseudo-F(11, 191)=1.927, p=0.037; seasons: PERMANOVA pseudo-F(3, 191)=5.310, p=0.001). The lowest species richness was observed during June and July (Fig. 3). The highest mean species richness difference was noticed between summer and winter (p=0.0003), reaching highest richness during summer period.
Total number of species found in all six quadrants (0.25 m2) considering a total area analysed of 1.5 m2 per sampling time (black bars) and cumulative total species richness (line).
Regarding the spatial distribution of species richness, a distribution pattern was not detected by comparing the quadrat positions (PERMANOVA pseudo-F(2, 191)=0.077; p=0.925).
The curve of the cumulative species richness showed that during the first six months 18 to 22 taxa were found out of a total of 32, corresponding to from 56.2 - 68.7% of the benthic assemblage. From June onwards, more than 90% of benthic assemblage was observed, gradually reaching the total number of species of the assembly (Fig. 3).
The turn-over of species between successive sampling times was generally low, ranging between 6.6% and 36% of total species richness. The highest number of species gained was reported in May, whereas the highest number of species lost was observed in four months: January, April, August and December 2014 (Fig. 4).
Species richness and cover (%) of algal and invertebrate taxa gained or lost between successive sample times between December 2013 and March 2015.
Regarding the benthic fauna, the highest invertebrate cover gained was observed in November, reaching values near to 9%. Another two high cover increases were noted in December 2013 and April 2014, ranging between 6% and 8%, respectively. The largest loss of invertebrate cover was observed in three months: March, May and June 2014. These cover losses reached values higher than 6% and 9%. Regarding the benthic flora, the highest algal cover gained was observed in May, jointly with the highest gained species number, indicating that the higher percentage was due to the increase in the number of species in the assemblage, especially large-size species, such as U. pinnatifida, reaching c. 8%, a little lower than that observed in the invertebrate group. Other periods with high increases in algal cover were March and June 2014. The three months with a large loss of cover were: April and November 2014, and January 2015. The first two months coincided with periods characterized by high increases in the cover of invertebrates (Fig. 4). However, most of these events could not be confirmed seasonally due a lack of replication in the second year.
Among the algal functional groups, the articulated calcareous group exhibited the highest cover, reaching 62.7/0.25 m2. The crustose and corticated groups had intermediate covers, while the remaining functional groups were less abundant. In general, these morphological functional groups cover varied between 0.6 and 6.6/0.25 m2, representing very low values compared to the calcareous group. The suspensor feeder group presented a considerable cover compared to the other two feeder groups, reaching values of 22.7/0.25 m2, whereas the predator group reached values of 0.6/0.25 m2 and the grazer group 1.4/0.25 m2 (Fig. 5).
Distribution in abundance (cover %) of macroalgal morphological and invertebrate feeder functional groups during the whole sampling period.
Seaweeds and invertebrates covers differed between positions (invertebrates: PERMANOVA pseudo-F(2,191)=13.863; p=0.0001; seaweeds: PERMANOVA pseudo-F(2,191)=8.465; p=0.0001). The total invertebrates cover was higher in the western position quadrat, whereas the total seaweeds cover was higher in the central quadrats.
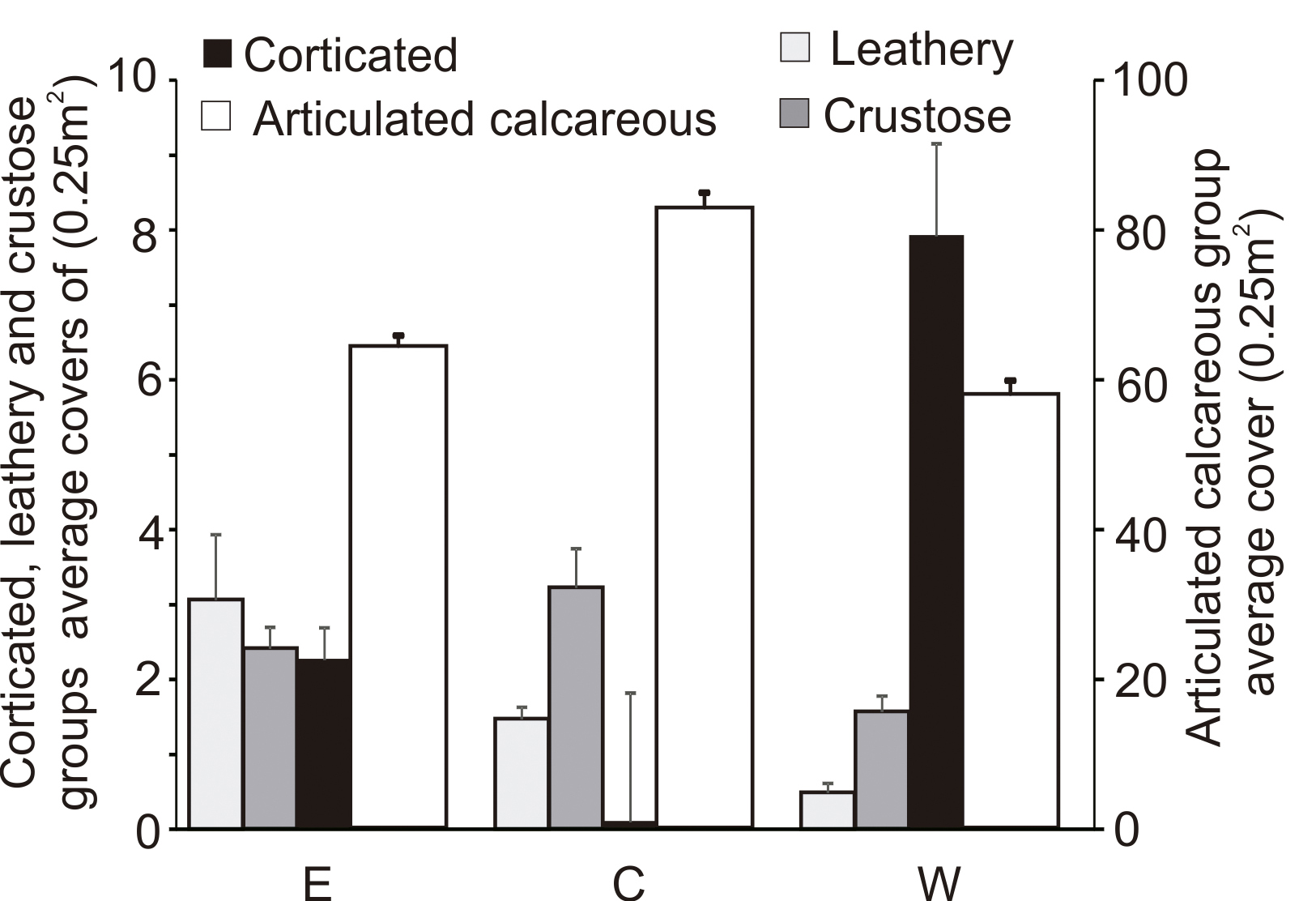
Regarding invertebrates, the suspensor group had a considerably greater coverage compared to the remaining invertebrate groups and was more abundant in the west (PERMANOVA pseudo-F(2,191)=52.655; p=0.0001). The cover of the predator-scavenger group was higher in the central quadrat (PERMANOVA pseudo-F(2, 191)=4.463; p=0.005), whereas the grazers were more abundant in the east (PERMANOVA pseudo-F(2, 191)=2.670; p=0.025). Hence, an interesting decrease of grazers was observed from east to west (Fig. 6). The SIMPER test showed a greater dissimilarity between the quadrats located in the central and western positions. This dissimilarity was determined by the higher abundance of the suspensor feeder group, represented by the “mussel” group, in the western position. The lower presence of grazer in that position also contributed to this dissimilarity (Table IV).
Distribution of abundance (cover %) of feeder groups in quadrats located at three different sampling sites (E: east; C: centre; W: west).
SIMPER test showing morphological and feeder functional groups that most contribute to dissimilities.
Regarding seaweeds, in the western quadrats all groups showed the lowest covers, with the exception of corticated group, which had the highest cover in that position (PERMANOVA pseudo-F(2, 191)=7.241; p=0.0001) (Fig. 7). The SIMPER test showed that the greatest dissimilarity occurred between the central and eastern positions; such dissimilarity was given by a higher cover of corticated, articulated calcareous and crustose functional groups in the central position, mainly determined by higher covers of Leathesia marina, Corallina sp. and Ralfsia verrucosa, respectively (Table IV).
Distribution of abundance (cover %) of algal morphological functional groups in quadrats located at three different sampling sites (E: east; C: centre; W: west).
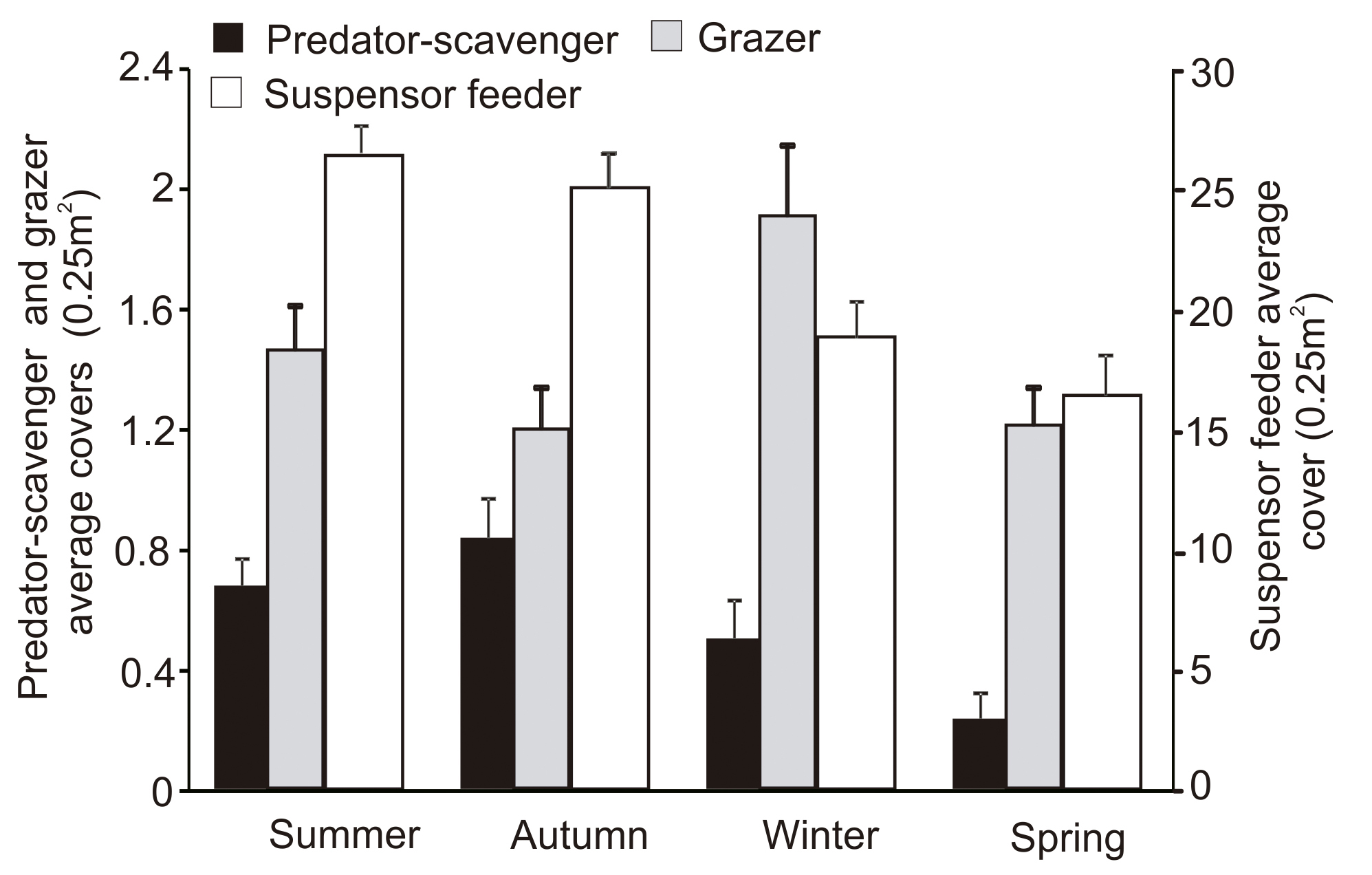
Feeder groups cover varied between seasons (PERMANOVA pseudo-F(3, 191)=11.243; p=0.0001). The highest invertebrate covers were observed during summer and autumn. The suspensor was the most abundant group and dominated during all four seasons, being however more abundant in summer and autumn (PERMANOVA pseudo-F(3, 191)=13.106; p=0.0001). Winter was also characterized by the highest grazer cover (PERMANOVA pseudo-F(3, 191)=2.038; p=0.037), while in spring the predator group was the least represented (PERMANOVA pseudo-F(3,191)=12.759; p=0.0001) (Fig. 8). SIMPER test indicated that the greatest dissimilarity was given between autumn and spring. The higher cover of both suspensor feeder and predator scavenger groups in autumn was responsible for such dissimilarity (Table IV). This dissimilarity was given by the mussels and Antholoba achates, respectively.
The algal morphological functional groups varied between seasons (PERMANOVA pseudo-F(3, 191)=17.836; p=0.0001). Articulated calcareous group, represented by Corallina sp., had the highest cover during all the period examined, being statistically different between seasons; i.e., more abundant in winter and spring (PERMANOVA pseudo-F(3, 191)=18.249; p=0.0001). The corticated group, integrated by four brown algae, was statistically more abundant during summer time (PERMANOVA pseudo-F(3, 191)=24.508; p=0.0001). The crustose group, represented by the brown algae R. verrucosa, was more abundant in autumn (PERMANOVA pseudo-F(3, 191)=3.177; p=0.010) and the leathery morphological group was more abundant in winter (PERMANOVA pseudo-F(3, 191)=9.222; p=0.0001) (Fig. 9). The greatest dissimilarity indicated by the SIMPER analysis was observed between summer and winter. These differences were given by the corticated, articulated calcareous and leathery groups (Table IV). Summer was characterized by high abundance of Leathesia marina and the lowest cover of Corallina sp and U. pinnatifida.
Distribution of abundance (cover %) of algal morphological functional groups over the seasons.
Relationships of morpho-functional and feeder benthic groups with environmental factors
BEST/BIOENV analysis revealed that the most important variables structuring morphological and feeder functional groups were seawater temperature and radiation (ρ=0.866, p=0.01). Several permutations were performed to test if there were any changes in the BIOENV outcome. In particular, this test gave the following correlation when the variables were considered separately: daylength (ρ=0.840), temperature and daylength (ρ=0.642), and temperature (ρ=0.272).
In the PCA analysis, the first two axes explained 75.6% of the joint variation of environmental factors and biological variables. PC 1 was related mainly to the seasonal variability of the samples and morphological and feeder groups. Samples from warmer months (December - March) were grouped on the more positive side of the PCA axis I, whereas samples from the cold period (April - October) were on the more negative side of PCA axis I. In addition, the right side of PC 1 was characterized by high species richness, high seawater temperatures, high radiation and high cover of algal assemblages and suspensor group. The left side of PC 1 grouped the colder months and was characterized by high cover articulated calcareous (Fig. 10).
PCA biplot: association between biological and environmental variables. Biological variables: species richness, algal and invertebrate assemblages, suspensor and articulated calcareous groups. Environmental variables: seawater temperature and radiation. Months sampled were indicated by abbreviations: January (Jan), February (Feb), March (Mar), April (Apr), May, June (Jun), July (Jul), August (Aug), September (Sep), October (Oct), November (Nov) and December (Dec). The letters A and B indicate successive sampling times in the same month.
Interesting results were observed when the correlation between environmental parameters and benthic attributes were performed. An inverse relationship was seen between the articulated calcareous group and seawater temperature (ρ=-0.854), radiation (ρ=-0.688), species richness (ρ=-0.781), presence of suspensor feeder group (ρ=-0.746) and the algal assemblage (ρ=-0.873). On the other hand, a positive relationship was observed between the suspensor feeder group and seawater temperature (ρ=0.806) and radiation (ρ=0.709).
DISCUSSION
Two main biogeographical provinces have been proposed for the Atlantic coast of Argentina: the Magellan province, with cold-water covering southern Patagonia, and the Argentinean province, with warmer waters extending from North Patagonia northwards (Lutz et al. 2003LUTZ VA ET AL. 2003. Perspectives of marine biodiversity studies in Argentina. Gayana 673: 71-382.). Punta Este is located to the north of this latter province. In general, the species richness is relatively poor in this area and the intertidal rocky shores are characterized by a diminished seaweed flora, mainly due to harsh physical conditions (Bolton 1994BOLTON JJ. 1994. Global seaweed diversity: patterns and anomalies. Bot Mar 37: 241-245., Paruelo et al. 1998PARUELO JM, BELTRAN A, JOBBAGY SALA OE & GOLLUSCIO RA. 1998. The climate of Patagonia: general patterns and controls on biotic processes. Ecol Aust 8: 85-101., Bertness et al. 2006BERTNESS MD, CRAIN CM, SILLIMAN BR, BAZTERRICA MC, REYNA MV, HIDALGO F & FARINA JK. 2006. The community structure of western Atlantic Patagonian rocky shores. Ecol Monogr 76: 439-460.). Moreover, it is a zone characterized by the presence of calcareous algae covering the low tidal level in wave-protected sites (Bertness et al. 2006BERTNESS MD, CRAIN CM, SILLIMAN BR, BAZTERRICA MC, REYNA MV, HIDALGO F & FARINA JK. 2006. The community structure of western Atlantic Patagonian rocky shores. Ecol Monogr 76: 439-460.). In this study we coincidentally observed low species richness and the highest covers of the articulated calcareous group in the Punta Este low intertidal zone, as well as the presence of ephemeral occurrence endemic species, such as M. major. Rocky intertidal communities on Argentinean coasts are characterized by the dominance of mytillids, particularly B. rodriguezii in the warm temperate region and P. purpuratus in the cold temperate sector, coexisting in the same beds along the transition zone (41-43º S) (Trovant et al. 2013TROVANT B, RUZZANTE DE, BASSO NG & ORENSANZ JM. 2013. Distinctness, phylogenetic relations and biogeography of intertidal mussels (Brachidontes, Mytilidae) from the south-western Atlantic. JMBA 93: 1843-1855., Van der Molen et al. 2013VAN DER MOLEN S, MáRQUEZ F, IDASZKIN YL & ADAMI M. 2013. Use of shell-shape to discriminate between Brachidontes rodriguezii and Brachidontes purpuratus species (Mytilidae) in the transition zone of their distributions (South-western Atlantic). JMBA 93: 803-808.). In the low intertidal zone of Punta Este, we noted the presence of P. purpuratus and B. rodriguezii forming a conspicuous mussel bed, mixed with the articulated calcareous group. This region was also characterized by a high abundance of the grazer gastropod T. patagonica and predator species, this high grazer abundance was coincident with the highest abundance of the leathery morphological functional group, integrated exclusively by U. pinnatifida. Rechimont et al. (2013)RECHIMONT ME ET AL. 2013. Benthic diversity and assemblage structure of a north Patagonian rocky shore: a monitoring legacy of the NaGISA project. JMBA 93(8): 2049-2058. also associated the dominance of grazers with U. pinnatifida.
Ecological studies on seaweeds from the southeast South American region are scarce; thus, seaweed distribution, abundance patterns and functional roles in these ecosystems are poorly known. In particular, seaweeds benthic assemblages are considered to be variable in terms of distribution and abundance. For such studies, it is essential to choose a non-destructive sampling method that guarantees accurate results of the intertidal assemblages. Assignment and use of morphological and feeder functional groups can considerably reduce the time and resources needed to monitor wide geographical areas and, simultaneously, it allows comparisons to be made between regions with different species assemblages (Vandewalle et al. 2010VANDEWALLE M ET AL. 2010. Functional traits as indicators for monitoring biodiversity response to land use changes across ecosystems and organisms. Biodivers Conserv 19: 2921-2947.). Moreover, trait-based approaches in morphologies or feeder preferences can improve biodiversity understanding, reflecting the species’ ecological roles (Díaz & Cabido 2001DíAZ S & CABIDO M. 2001. Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16: 646-655.). Such approach has also been used by other authors, such as Gaspar et al. (2017)GASPAR R, PEREIRA L & NETO JM. 2017. Intertidal zonation and latitudinal gradients on macroalgal assemblages: Species, functional groups and thallus morphology approaches. Ecol Indic 81: 90-103., and proven to be efficient in detecting differences in the algal and invertebrate assemblage patterns in terms of species composition and species cover measured at small scales.
A variation in the assemblage structure (species composition and species cover) throughout the year was observed. In fact, it was more marked than the variability at horizontal fine-scale; i.e., between eastern, central and western sites. Such horizontal fine-scale variability was only seen in the distribution of feeder groups, as the eastern quadrats presented higher grazer cover than the central and western ones. These results are difficult to interpret, but cannot be ignored because the high sample size and statistical results show a strong significant difference between the three invertebrate functional group covers. A possible explanation of this variation could be that the grazer group prefers sites with greater air exposure. In fact, air exposure time between the three quadrats hardly differs, but the eastern quadrats were slightly more exposed than western ones. Horizontal fine-scale variation in species assemblages has been reported as a common attribute of marine benthic habitats and can often occur at fine resolutions, i.e. with a small (10s to 100s of centimetres) to middle scale (10s to 100s of meters) variation (Valdivia et al. 2011VALDIVIA N, SCROSATI RA, MOLIS M & KNOX AS. 2011. Variation in community structure across vertical intertidal stress gradients: how does it compare with horizontal variation at different scales? PLoS ONE 6: e24062., Fraschetti et al. 2005FRASCHETTI S, TERLIZZI A & BENEDETTI-CECCHI L. 2005. Patterns of distribution of marine assemblages from rocky shores: evidence of relevant scales of variation. Mar Ecol Prog Ser 296: 13-29.).
Both biological and physical factors, such as substrate availability, recruitment, grazing, competition, wave and aerial exposure, irradiance, temperature or nutrient exchange, have been mentioned as being potentially responsible for the variability at these spatial scales (Benedetti-Cecchi 2001BENEDETTI-CECCHI L. 2001. Variability in abundance of algae and invertebrates at different spatial scales on rocky sea shores. Mar Ecol Prog Ser 215: 79-92., Lobban & Harrison 1994LOBBAN C & HARRISON PJ. 1994. Seaweed Ecology and Physiology. Cambridge University Press, New York, USA, 366 p., Choi & Kim 2004CHOI TS & KIM KY. 2004. Spatial pattern of intertidal macroalgal assemblages associated with tidal levels. Hydrobiologia 512: 49-56.).
In this study, we determined that intertidal seaweed communities are strongly regulated by physical and biological factors which vary according to season, detecting temporal dynamics of both algal and invertebrate assemblages. Regarding the physical factors, given the co-varying nature of seawater temperature, daylength and solar radiation, it is difficult to separate their individual effect contribution. With respect to biological factors, the abundance of grazers was low; indicating low grazing pressure. Similarly, Bertness et al. (2006)BERTNESS MD, CRAIN CM, SILLIMAN BR, BAZTERRICA MC, REYNA MV, HIDALGO F & FARINA JK. 2006. The community structure of western Atlantic Patagonian rocky shores. Ecol Monogr 76: 439-460. found low grazing pressure on the western Patagonian rocky shores due to the absence of common predaceous crabs and snails and the limited impact of limpets.
However, competition was an important biological factor at Punta Este since temporal dynamics in algal richness, seaweeds assemblage and invertebrate structures and species turnover was a function of the complex interplay between the environmental factors and the abundance of Corallina sp. On the one hand, the presence of articulated calcareous groups directly affected the area of available substrate for colonization by other seaweeds, thereby regulating the species richness. On the other hand, the articulated group only favoured the development of crustose algae, such as L. marina and C. sinuosa, which only grow attached to Corallina thalli.
Articulated calcareous cover was directly correlated with environmental variation, whereas species richness of algae was associated with temporal changes in the calcareous groups. Seasonal change in the cover of Corallina sp. was negatively correlated with all of the environmental variables considered, and in particular with seawater temperature. In contrast to temporal patterns in calcareous cover, species richness was not well correlated with any of the environmental factors, suggesting that they are not the main driver of species seasonality per se.
Species losses and gains over time were very variable. This variability probably reflects the reproduction, recruitment and growth of many species, particularly those with ephemeral life cycles. However, we agree with Wernberg et al. (2005)WERNBERG T, KENDRICK GA & TOOHEY BD. 2005. Modification of the physical environment by an Ecklonia radiata (Laminariales) canopy and implications for associated foliose algae. Aquat Ecol 39: 419-430. who affirmed that the broad range in individual responses to seasonal environmental changes of understory algae and the presence of a canopy layer make it very difficult to identify specific factors or threshold values that control temporal patterns in species richness.
Mussel beds characterized by suspension-feeders are critical in the structure and function of the intertidal community (Silliman et al. 2011SILLIMAN BR ET AL. 2011. Whole-community facilitation regulates biodiversity on Patagonian rocky shores. PLoS ONE 6: e24502.). This feeding type is favoured in this environment due to plankton abundance in the water around the rocky shores and wave movement that contributes to nutrient circulation (Bertness 1999BERTNESS MD. 1999. Rocky shores. In: The ecology of Atlantic rocky shorelines. Sinauer Associates, Sunderland, p. 177-247.).
In several coastal ecosystems, diversity decline is evident due to mussel dominance, but at the same time, beds development creates a secondary substratum enriched in fauna and epibenthic assemblages (Dittman & Robles 1991DITTMAN D & ROBLES C. 1991. Effect of algal epiphytes on the mussel Mytilus californianus. Ecology 72: 286-296., Lohse 1993LOHSE DP. 1993. The importance of secondary substratum in a rocky intertidal community. J Exp Mar Biol Ecol 166: 1-17., Ragnarsson & Raffaelli 1999RAGNARSSON SA & RAFFAELLI D. 1999. Effects of the mussel Mytilus edulis L. on the invertebrate fauna of sediments. J Exp Mar Biol Ecol 241: 31-43.). However, in this study, species richness was not correlated with suspensor feeder group dominance.
It has already been confirmed, that the turf forming coralline alga also provides a more benign habitat for recruitment and survival of several tiny invertebrate species (Kelaher et al. 2007KELAHER BP, CASTILLA JC, PRADO L, YORK P, SCHWINDT E & BORTOLUS A. 2007. Spatial variation in molluscan assemblages from coralline turf of Argentinean Patagonia. J Mollus Stud 73: 139-146.); nonetheless, articulated calcareous group abundance did not favour mussel beds. Our result indicated that there is competition for the substrate between the articulated calcareous and the suspensor feeder groups.
This study represents an important baseline on morphological and-feeder functional groups since studies incorporating both algae and invertebrates in Patagonian Argentina are very scarce. Under this point of view, this study also represents a species-level baseline in the literature, so that future researchers will have solid data for comparison if benthic composition changes in response to global climate change, or other anthropogenic factors. As a future perspective, it would be appropriate to implement the same method, but on a larger scale at different sites on the Patagonian coast.
We conclude that the dominating interactions at the lower intertidal level and small-scale spatial variation jointly with abiotic factors promote a better environment, preventing other species from extreme physical stress, thus leading to community development. We consider that this pattern is common to a wide range of natural systems where assemblages (algae and invertebrates) are influenced by complex sets of physical and biological processes, such as those operating in the marine ecosystems. However, additional experimental investigations are required in order to have a clear picture of the mechanisms underlying assemblage patterns on the rocky shores of Punta Este and along the Patagonian coast.
ACKNOWLEDGMENTS
M.C.G., C.F. and E.R.P. are researchers of Consejo Nacional de Investigaciones Científicas y Tecnológicas) CONICET and J.F.E. is an associate technician of Centro Nacional Patagónico (CENPAT). Support was provided by grants from the Secretaría de Ciencia y Tecnología de la Universidad Nacional del Sur (PGI CSU-24B/234) and CONICET (PIP-11220130100070CO, P-UE IADO 22920160100057CO). We also thank Rosemary Scoffield, MSc for reading the manuscript.
REFERENCES
- ANDERSON MJ, GORLEY RN & CLARKE KR 2008. PERMANOVA for PRIMER: Guide to Software and Statistical Methods. PRIMER-E, Plymouth, UK, 214 p.
- BALATA D, PIAZZI L & RINDI F. 2011. Testing a new classification of morphological functional groups of marine macroalgae for the detection of responses to stress. Mar Biol 158: 2459-2469.
- BENEDETTI-CECCHI L. 2001. Variability in abundance of algae and invertebrates at different spatial scales on rocky sea shores. Mar Ecol Prog Ser 215: 79-92.
- BERTNESS MD. 1999. Rocky shores. In: The ecology of Atlantic rocky shorelines. Sinauer Associates, Sunderland, p. 177-247.
- BERTNESS MD, CRAIN CM, SILLIMAN BR, BAZTERRICA MC, REYNA MV, HIDALGO F & FARINA JK. 2006. The community structure of western Atlantic Patagonian rocky shores. Ecol Monogr 76: 439-460.
- BOLTON JJ. 1994. Global seaweed diversity: patterns and anomalies. Bot Mar 37: 241-245.
- BRUNO JF & CARDINALE BJ. 2008. Cascading effects of predator richness. Front Ecol Environ 6: 539-546.
- BRUNO JF, STACHOWICZ JJ & BERTNESS MD. 2003. Inclusion of facilitation into ecological theory. Trends Ecol Evol 18: 119-125.
- CARDINALE BJ, GROSS K, FRITSCHIE K, FLOMBAUM P, FOX JW, RIXEN C, VAN RUIJVEN J, REICH PB, SCHEREN-LORENZEN M & WILSEY BJ. 2013. Biodiversity simultaneously enhances the production and stability of community biomass, but the effects are independent. Ecology 94: 1697-1707.
- CARDINALE BJ, SRIVASTAVA DS, DUFFY JE, WRIGHT JP, DOWNING AL, SANKARAN M & JOUSEAU C. 2006. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443: 989-992.
- CHOI TS & KIM KY. 2004. Spatial pattern of intertidal macroalgal assemblages associated with tidal levels. Hydrobiologia 512: 49-56.
- CLARKE KR & GORLEY RN. 2006. PRIMER V6: Manual/Tutorial. PRIMER-E, Plymouth, 192 p.
- DETHIER MN, GRAHAM ES, COHEN S & TEAR LM. 1993. Visual versus random point percent cover estimations: objective is not always better. Mar Ecol Prog Ser 96: 93-100.
- DíAZ S & CABIDO M. 2001. Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16: 646-655.
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. OJ L 327: 1-51.
- DITTMAN D & ROBLES C. 1991. Effect of algal epiphytes on the mussel Mytilus californianus. Ecology 72: 286-296.
- DUFFY JE, CARDINALE BJ, FRANCE KE, MCINTYRE PB, THéBAULT E & LOREAU M. 2007. The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecol Lett 10: 522-538.
- ERIKSSON O, COUSINS SAO, BRUUN HH & DíAZ S. 2002. Land-use history and fragmentation of traditionally managed grasslands in Scandinavia. J Veg Sci 13: 743-748.
- FRASCHETTI S, TERLIZZI A & BENEDETTI-CECCHI L. 2005. Patterns of distribution of marine assemblages from rocky shores: evidence of relevant scales of variation. Mar Ecol Prog Ser 296: 13-29.
- GASPAR R, PEREIRA L & NETO JM. 2017. Intertidal zonation and latitudinal gradients on macroalgal assemblages: Species, functional groups and thallus morphology approaches. Ecol Indic 81: 90-103.
- HOOPER DU, ADAIR EC, CARDINALE BJ, BYRNES JEK, HUNGATE BA, MATULICH KL, GONZALEZ A, DUFFY JE, GAMFELDT L & CONNOR MI. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486: 105-108.
- IRIGOYEN AJ, TROBBIANI G, SGARLATTA MP & RAFFO MP. 2010. Effects of the alien algae Undaria pinnatifida (Phaeophyceae, Laminariales) on the diversity and abundance of benthic macrofauna in Golfo Nuevo (Patagonia, Argentina): potential implications for local food webs. Biol Invasion 1: 1521-1532.
- KAREZ R, ENGELBERT S, KRAUFVELIN P, PEDERSEN MF & SOMMER U. 2004. Biomass response and changes in composition of ephemeral macroalgal assemblages along an experimental gradient of nutrient enrichment. Aquat Bot 78: 103-117.
- KELAHER BP, CASTILLA JC, PRADO L, YORK P, SCHWINDT E & BORTOLUS A. 2007. Spatial variation in molluscan assemblages from coralline turf of Argentinean Patagonia. J Mollus Stud 73: 139-146.
- KRAUFVELIN P, LINDHOLM A, PEDERSEN MF, KIRKERUD LA & BONSDORFF E. 2010. Biomass, diversity and production of rocky shore macroalgae at two nutrient enrichment and wave action levels. Mar Biol 157: 29-47.
- KRAUFVELIN P, MOY FE, CHRISTIE H & BOKN TL. 2006. Nutrient addition to experimental rocky shore communities revisited: delayed responses, rapid recovery. Ecosystems 9: 1076-1093.
- LEVIN SA. 1992. The problem of pattern and scale in ecology. Ecology 73: 1943-1967.
- LITTER MM & LITTER DS. 1980. The evolution of thallus form and survival strategies in benthic marine macroalgae: field and laboratory tests of a functional form model. Amer Naturalist 116: 25-44.
- LOBBAN C & HARRISON PJ. 1994. Seaweed Ecology and Physiology. Cambridge University Press, New York, USA, 366 p.
- LOHSE DP. 1993. The importance of secondary substratum in a rocky intertidal community. J Exp Mar Biol Ecol 166: 1-17.
- LÜNING K. 1990. Seaweeds: Their environment, biogeography, and ecophysiology. Wiley-Interscience Publication, New York, USA, 527 p.
- LUTZ VA ET AL. 2003. Perspectives of marine biodiversity studies in Argentina. Gayana 673: 71-382.
- MCGILL BJ, ENQUIST BJ, WEIHER E & WESTOBY M. 2006. Rebuilding community ecology from functional traits. Trends Ecol Evol 21: 178-185.
- MENGE BA & SUTHERLAND JP. 1987. Community regulation: variation in disturbance, competition, and predation in relation to environmental stress and recruitment. Amer Naturalist 130: 730-757.
- ORFANIDIS S, PANAYOTIDIS P & UGLAND KI. 2011. Ecological Evaluation Index continuous formula (EEI-c) application: a step forward for functional groups, the formula and reference condition values. Mediterr Mar Sci 12: 199-231.
- PARUELO JM, BELTRAN A, JOBBAGY SALA OE & GOLLUSCIO RA. 1998. The climate of Patagonia: general patterns and controls on biotic processes. Ecol Aust 8: 85-101.
- PINEDO S, GARCíA M, SATTA MP, TORRES M & BALLESTEROS E. 2007. Rocky-shore communities as indicators of water quality: A case study in the Northwestern Mediterranean. Mar Pollut Bull 55: 126-135.
- RAGNARSSON SA & RAFFAELLI D. 1999. Effects of the mussel Mytilus edulis L. on the invertebrate fauna of sediments. J Exp Mar Biol Ecol 241: 31-43.
- RECHIMONT ME ET AL. 2013. Benthic diversity and assemblage structure of a north Patagonian rocky shore: a monitoring legacy of the NaGISA project. JMBA 93(8): 2049-2058.
- SCROSATI RA. 2005. Review of studies on biomass-density relationships (including self-thinning lines) in seaweeds: Main contributions and persisting misconceptions. Phycol Res 53: 224-233.
- SCROSATI RA. 2006. The clonal seaweed Chondrus crispus as a foundation species. Algae 31(1): 41-48.
- SCROSATI RA, VAN GENNE B, HEAVEN CS & WATT CA. 2011. Species richness and diversity in different functional groups across environmental stress gradients: a model for marine rocky shores. Ecography 34: 151-161.
- SILLIMAN BR ET AL. 2011. Whole-community facilitation regulates biodiversity on Patagonian rocky shores. PLoS ONE 6: e24502.
- STENECK RL & DETHIER MN. 1994. A functional group approach to the structure of algal dominated communities. Oikos 69: 476-498.
- SUEIRO MC, BORTOLUS A & SCHWINDT E. 2011. Habitat complexity and community composition: relationships between different ecosystem engineers and the associated macroinvertebrate assemblages. Helgoland Mar Res 65: 467-477.
- TORRES A & CAILLE G. 2009. The hard-bottom intertidal communities before and after removal of an anthropogenic disturbance: a case study in the coast of Puerto Madryn (Patagonia, Argentina). Rev Biol Mar Oceanog 44: 517-521.
- TROVANT B, RUZZANTE DE, BASSO NG & ORENSANZ JM. 2013. Distinctness, phylogenetic relations and biogeography of intertidal mussels (Brachidontes, Mytilidae) from the south-western Atlantic. JMBA 93: 1843-1855.
- VALDIVIA N, SCROSATI RA, MOLIS M & KNOX AS. 2011. Variation in community structure across vertical intertidal stress gradients: how does it compare with horizontal variation at different scales? PLoS ONE 6: e24062.
- VAN DER MOLEN S, MáRQUEZ F, IDASZKIN YL & ADAMI M. 2013. Use of shell-shape to discriminate between Brachidontes rodriguezii and Brachidontes purpuratus species (Mytilidae) in the transition zone of their distributions (South-western Atlantic). JMBA 93: 803-808.
- VANDEWALLE M ET AL. 2010. Functional traits as indicators for monitoring biodiversity response to land use changes across ecosystems and organisms. Biodivers Conserv 19: 2921-2947.
- WERNBERG T, KENDRICK GA & TOOHEY BD. 2005. Modification of the physical environment by an Ecklonia radiata (Laminariales) canopy and implications for associated foliose algae. Aquat Ecol 39: 419-430.
- WILLIG MR, KAUFMAN DM & STEVENS RD. 2003. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu Rev Ecol Evol S 34: 273-309.
Publication Dates
-
Publication in this collection
28 June 2021 -
Date of issue
2021
History
-
Received
16 May 2019 -
Accepted
6 Nov 2019